-
PDF
- Split View
-
Views
-
Cite
Cite
Simone Savastano, Veronica Dusi, Enrico Baldi, Roberto Rordorf, Antonio Sanzo, Rita Camporotondo, Rosa Fracchia, Sara Compagnoni, Laura Frigerio, Luigi Oltrona Visconti, Gaetano Maria De Ferrari, Anatomical-based percutaneous left stellate ganglion block in patients with drug-refractory electrical storm and structural heart disease: a single-centre case series, EP Europace, Volume 23, Issue 4, April 2021, Pages 581–586, https://doi.org/10.1093/europace/euaa319
Close - Share Icon Share
Abstract
The adoption of percutaneous stellate ganglion blockade for the treatment of drug-refractory electrical storm (ES) has been increasingly reported; however, the time of onset of the anti-arrhythmic effects, the safety of a purely anatomical approach in conscious patients and the additional benefit of repeated procedures remain unclear.
This study included consecutive patients undergoing percutaneous left stellate ganglion blockade (PLSGB) in our centre for drug-refractory ES. Lidocaine, bupivacaine, or a combination of both were injected in the vicinity of the left stellate ganglion. Overall, 18 PLSGBs were performed in 11 patients (age 69 ± 13 years; 63.6% men, left ventricular ejection fraction 31.6 ± 16%). Seven patients received only one PLSGB; three underwent two procedures and one required three PLSGB and two continuous infusions to control ventricular arrhythmias (VAs). All PLSGBs were performed with an anatomical approach; lidocaine, alone, or in combination was used in 77.7% of the procedures. The median burden of VAs 1 h after each block was zero compared with five in the hour before (P < 0.001); 83% of the patients were free from VAs; the efficacy at 24 h increased with repeated blocks. The anti-arrhythmic efficacy of PLSGB was not related to anisocoria. No procedure-related complications were reported.
Anatomical-based PLSGB is a safe and rapidly effective treatment for refractory ES; repeated blocks provide additional benefits. Percutaneous left stellate ganglion blockade should be considered for stabilizing patients to allow further ES management.
Demonstration of safety and efficacy of anatomical-based percutaneous left stellate ganglion block in patients with refractory electrical storm.
First analytical approach with per-procedure and per-patient analysis.
First demonstration of additional benefit of repeated left-sided blocks.
Introduction
Electrical storm (ES) is commonly defined as the occurrence of three or more episodes of sustained or treated ventricular arrhythmia (VA) over 24 h.1 The management of ES is challenging, with the risk of haemodynamic deterioration, heart failure progression,2 and also high mortality rates,1 suggesting the paramount importance of effective and timely anti-arrhythmic strategies. Anti-arrhythmic drugs (AADs), deep sedation, general anaesthesia, and haemodynamic support as well as catheter ablation are not always effective or available and mortality rates in patients with ES remain high.3 The autonomic nervous system plays a pivotal role in ventricular arrhythmogenesis4 and neural modulation, via a number of avenues, is gaining increasing attention.4,5Anti-arrhythmic strategies, directly targeting neuromodulation and suitable for an acute clinical setting, include thoracic epidural anaesthesia, spinal cord stimulation, percutaneous stellate ganglion block (PSGB), and renal denervation.4 Percutaneous left stellate ganglion blockade has several practical advantages over the other approaches: it can be performed by a trained cardiologist at bedside, in supine position, and in an emergency setting, including on patients with haemodynamic instability. Available clinical data suggest a good anti-arrhythmic efficacy independently of the substrate with no major safety concerns.5–8 Yet, the few studies published so far have mostly evaluated the anti-arrhythmic impact of a single procedure of PSGB (eventually bilateral) in a relatively long timeframe (24–72 h). However, in such a lengthy time interval, several factors other than (or in addition to) PSGB may have contributed to reducing the arrhythmic burden, also taking into account that the half-life of the anaesthetic drugs typically used for ganglion block (bupivacaine, ropivacaine, and lidocaine) is only a few hours. Moreover, the potential for an additional anti-arrhythmic benefit of repeated blocks in the same patient has not been assessed.
The aim of our study was to evaluate the safety and efficacy of left PSGB (PLSGB) with a short-term per-procedure analysis, to quantify its impact on the management of unstable patients and with a per-patient analysis to assess the role of repeated blocks.
Methods
Type of study
This was a prospective single-centre interventional study approved by the Ethical Committee of the Fondazione IRCCS Policlinico San Matteo in Pavia (proc 20190046932).
Definitions
Electrical storm was defined as the presence of three or more separate episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) (either sustained or requiring treatment) within 24 h.
Electrical storm was defined as refractory in the case of arrhythmic recurrences, despite intravenous administration of AADs as recommended by guidelines.1 Patients requiring discontinuation of AAD intravenous infusion because of adverse events were also eligible for the study.
An arrhythmic event was defined as an episode of sustained VT or VF treated either with anti-tachycardia pacing or with a direct current-shock by the internal defibrillator or by an external defibrillator.
Patient selection
We enrolled all consecutive patients admitted to the Fondazione IRCCS Policlinico San Matteo from July 2017 to December 2019 who either presented refractory ES or who developed ES during the hospital stay and subsequently received PLSGB to control arrhythmias. Patients younger than 18 years old with a history of heart transplant or of previous surgical cardiac sympathetic denervation were excluded.
Electrical storm management
Electrical storm was managed according to standard clinical practice at our institution, which includes treatment of reversible causes (i.e. correction of ionic imbalances, treatment of acute myocardial ischaemia, and treatment of septic status) administration of AADs and β-blockers and reprogramming of the implantable cardioverter-defibrillator (ICD) to minimize shocks. If required for haemodynamic support during ES, patients received a mechanical circulatory support device (intra-aortic balloon pump or extracorporeal membrane oxygenation). General anaesthesia is not routinely performed in our centre to control VAs, but it is considered for selected patients. Percutaneous left stellate ganglion blockade can be performed urgently 24 h a day by a trained interventional cardiologist or by an anaesthesiologist. Ventricular tachycardia ablation is performed on an urgent basis from Monday to Friday during the day.
Percutaneous left stellate ganglion block protocol and procedure
The operators were trained for both the anatomical-guided and the ultrasonography-guided approach to perform PLSGB and they were free to decide how to proceed. The choice of the type of anaesthetic as well as the decision to possibly repeat the block and/or to leave a catheter in place for continuous infusion was shared between the operator and the clinician caring for the patient. After each block, the operator evaluated (and recorded) the eventual development of anisocoria and ptosis. We chose not to routinely check the temperature of the left arm because of several potential confounding/masking factors modulating the external body temperature in emergency settings of poor peripheral perfusion. Regardless of the technique chosen, if the first PLSGB was unsuccessful in controlling arrhythmias, the possibility of repeating PLSGB was taken into consideration within a variable timeframe according to the anaesthetic being used to perform the block (at least 10 min in the case of lidocaine, at least 30 min in the case of bupivacaine). If PLSGB was successful but arrhythmias recurred later, the block could be repeated according to the physician’s decision. Independently of the onset of anisocoria/ptosis after the first block, the decision to perform repeated blocks always targeted the left stellate ganglion (LSG). Given the emergency conditions, the procedure was performed regardless of any anti-coagulant or anti-platelet therapy.
The anatomical approach, according to the Moore technique,9 consisted in a paratracheal anterior injection of 200 mg of lidocaine (10 mL) or 50 mg of bupivacaine (10 mL) or both (lidocaine 100 mg and 50 mg of bupivacaine—15 mL) at the level of the left-sided Chassaignac’s tubercle. The needle was advanced perpendicularly to the skin up to the bone of the transverse process of C6 and then minimally retracted before injection. The ultrasound-guided lateral approach consisted in an intra-scalenic injection over the longus colli muscle of the same drugs and concentrations used for the anatomical approach. Regardless of the approach, a 22-G echo-reflecting needle was used and an aspiration check was performed before the injection. In the case of the ultrasound-guided approach, a linear 10 mHz probe was used. In case of continuous infusion, a solution of 2% lidocaine was used at 7 mL/h.
Study endpoints
To assess the efficacy of each PLSGB, the primary efficacy endpoint was a per-procedure analysis of the change in arrhythmic burden in the first hour after every procedure compared with the hour before the procedure. Secondary endpoints were: a per-patient analysis of the change in arrhythmic burden in 24 h before the first procedure compared with 24 h after the last PLSGB procedure and the relationship between the reduction of arrhythmic events and anisocoria.
The primary safety endpoint was the occurrence of complications after PLSGB. Local haematoma not requiring surgery or blood transfusion was defined a minor complication. Local haematoma requiring surgery or blood transfusion, vascular damage requiring surgery, intravascular injection of anaesthetic with neurological symptoms, brachial plexus damage, and adverse drugs reactions were considered major complications.
Statistical analysis
Categorical variables are presented as numbers and percentages and compared with a χ2 test. Continuous variables were tested for normality with the D’Agostino–Pearson test. Normally distributed continuous variables are presented as mean ± standard deviation and compared with Student’s t-test. Non-normally distributed continuous variables are presented as median and 25–75% inter-quartile range (IQR) and compared with the Mann–Whitney U test if independent or with the Wilcoxon test in case of paired variables. A P-value <0.05 was considered statistically significant. Analyses were performed with MedCalc (MedCalc software bvba version 19.1).
Results
Population
The study population includes 11 patients who underwent a total of 18 PLSGB procedures: 7/11 (64%) patients received only one PLSGB, 3/11 (27%) underwent two procedures, and 1 patient (9%) required three PLSGBs and two continuous infusions to keep arrhythmias under control. The three patients who required a second PLSGB experienced on average 4.7 episodes during an average time of 2.7 h between the two procedures. They were all treated with lidocaine for their first PSGB after which 2/3 showed the appearance of anisocoria. For the second PLSGB procedure, all but one was treated with a combination of lidocaine and bupivacaine. They were all treated with an intravenous infusion of lidocaine, amiodarone, or both at the time of their first procedure and the treatment was continued unchanged between the two procedures. None of the patients received major interventions such as ICD reprogramming or catheter ablation. Finally, one patient required three PLSGBs and two continuous infusions; he was a markedly compromised patient with a septic shock and a severe myocardial dysfunction; he experienced on average 7.3 arrhythmic episodes during an average time of 4.8 h between each one of the three procedures. This patient was treated with a combination of lidocaine and bupivacaine for each PLSGB; he never developed anisocoria and he concomitantly received an intravenous infusion of lidocaine, amiodarone, and esmolol. Also, he did not undergo any major intervention between the PLSGB procedures.
An acute myocardial infarction was present in five (45.5%) patients treated with a primary coronary intervention. In four cases, ES occurred and was managed with the PLSGB before the revascularization. One patient presented the ES both before and after the revascularization. The other patients suffered from previously known cardiovascular conditions (dilated cardiomyopathy in two, arrhythmogenic right ventricular cardiomyopathy in one and chronic coronary artery disease in three) complicated by ES. Concerning the haemodynamic stability at presentation, three (27.3%) patients were in shock (two cardiogenic and one septic) and two (18.2%) were in refractory cardiac arrest. No patient received major anti-arrhythmic interventions (such as VT ablation and/or cardiac sympathetic denervation) in 24 h after PSGB. In-hospital death occurred in four (36.4%) patients: two for cardiogenic shock; one for cerebral death and one for massive pulmonary embolism. The mean time from the last PLSGB to the death was 5.5 ± 4.2 days. Tables 1 and 2 summarize the baseline characteristics of treated patients and the pre-block clinical features.
| Age (years) | 69 ± 13.3 |
| Male sex (%) | 7 (63.6) |
| LVEF (%) | 31.6 ± 16.6 |
| Associated diagnosis (%) | |
| Arrhythmogenic right ventricular cardiomyopathy | 1 (9.1) |
| Acute myocardial infarction | 5 (45.5) |
| Dilated cardiomyopathy | 2 (18.2) |
| Chronic coronary artery disease | 3 (27.3) |
| Diabetes (%) | 4 (36.4) |
| ATP/shocks in 24 h before the first block (IQR) | 7 (4–12) |
| Refractory cardiac arrest (%) | 2 (18.2) |
| Cardiogenic shock (%) | 2 (18.2) |
| Septic shock (%) | 1 (9.1) |
| General anaesthesia (%) | 1 (9.1) |
| ICD recipients (%) | 1 (9.1) |
| Oral drugs at admission (%) | |
| β-blockers | 5 (45.4) |
| Class III AADs | 2 (18.2) |
| Anti-coagulant/anti-platelet therapy (%) | |
| None | 4 (36.4) |
| Acetylsalicylic acid | 4 (36.4) |
| DAPT | 2 (18.2) |
| Heparin for ECMO + DAPT | 1 (9.1) |
| In hospital death (%) | 4 (36.4) |
| Cardiogenic shock | 2 (18.2) |
| Cerebral death | 1 (9.1) |
| Massive pulmonary embolism | 1 (9.1) |
| VT ablation (%) | 3 (27.3) |
| Bilateral cardiac sympathetic denervation (%) | 1 (9.1) |
| Age (years) | 69 ± 13.3 |
| Male sex (%) | 7 (63.6) |
| LVEF (%) | 31.6 ± 16.6 |
| Associated diagnosis (%) | |
| Arrhythmogenic right ventricular cardiomyopathy | 1 (9.1) |
| Acute myocardial infarction | 5 (45.5) |
| Dilated cardiomyopathy | 2 (18.2) |
| Chronic coronary artery disease | 3 (27.3) |
| Diabetes (%) | 4 (36.4) |
| ATP/shocks in 24 h before the first block (IQR) | 7 (4–12) |
| Refractory cardiac arrest (%) | 2 (18.2) |
| Cardiogenic shock (%) | 2 (18.2) |
| Septic shock (%) | 1 (9.1) |
| General anaesthesia (%) | 1 (9.1) |
| ICD recipients (%) | 1 (9.1) |
| Oral drugs at admission (%) | |
| β-blockers | 5 (45.4) |
| Class III AADs | 2 (18.2) |
| Anti-coagulant/anti-platelet therapy (%) | |
| None | 4 (36.4) |
| Acetylsalicylic acid | 4 (36.4) |
| DAPT | 2 (18.2) |
| Heparin for ECMO + DAPT | 1 (9.1) |
| In hospital death (%) | 4 (36.4) |
| Cardiogenic shock | 2 (18.2) |
| Cerebral death | 1 (9.1) |
| Massive pulmonary embolism | 1 (9.1) |
| VT ablation (%) | 3 (27.3) |
| Bilateral cardiac sympathetic denervation (%) | 1 (9.1) |
AAD, anti-arrhythmic drugs; DAPT, double anti-platelet therapy; ECMO, extracorporeal membrane oxygenation; ICD implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
| Age (years) | 69 ± 13.3 |
| Male sex (%) | 7 (63.6) |
| LVEF (%) | 31.6 ± 16.6 |
| Associated diagnosis (%) | |
| Arrhythmogenic right ventricular cardiomyopathy | 1 (9.1) |
| Acute myocardial infarction | 5 (45.5) |
| Dilated cardiomyopathy | 2 (18.2) |
| Chronic coronary artery disease | 3 (27.3) |
| Diabetes (%) | 4 (36.4) |
| ATP/shocks in 24 h before the first block (IQR) | 7 (4–12) |
| Refractory cardiac arrest (%) | 2 (18.2) |
| Cardiogenic shock (%) | 2 (18.2) |
| Septic shock (%) | 1 (9.1) |
| General anaesthesia (%) | 1 (9.1) |
| ICD recipients (%) | 1 (9.1) |
| Oral drugs at admission (%) | |
| β-blockers | 5 (45.4) |
| Class III AADs | 2 (18.2) |
| Anti-coagulant/anti-platelet therapy (%) | |
| None | 4 (36.4) |
| Acetylsalicylic acid | 4 (36.4) |
| DAPT | 2 (18.2) |
| Heparin for ECMO + DAPT | 1 (9.1) |
| In hospital death (%) | 4 (36.4) |
| Cardiogenic shock | 2 (18.2) |
| Cerebral death | 1 (9.1) |
| Massive pulmonary embolism | 1 (9.1) |
| VT ablation (%) | 3 (27.3) |
| Bilateral cardiac sympathetic denervation (%) | 1 (9.1) |
| Age (years) | 69 ± 13.3 |
| Male sex (%) | 7 (63.6) |
| LVEF (%) | 31.6 ± 16.6 |
| Associated diagnosis (%) | |
| Arrhythmogenic right ventricular cardiomyopathy | 1 (9.1) |
| Acute myocardial infarction | 5 (45.5) |
| Dilated cardiomyopathy | 2 (18.2) |
| Chronic coronary artery disease | 3 (27.3) |
| Diabetes (%) | 4 (36.4) |
| ATP/shocks in 24 h before the first block (IQR) | 7 (4–12) |
| Refractory cardiac arrest (%) | 2 (18.2) |
| Cardiogenic shock (%) | 2 (18.2) |
| Septic shock (%) | 1 (9.1) |
| General anaesthesia (%) | 1 (9.1) |
| ICD recipients (%) | 1 (9.1) |
| Oral drugs at admission (%) | |
| β-blockers | 5 (45.4) |
| Class III AADs | 2 (18.2) |
| Anti-coagulant/anti-platelet therapy (%) | |
| None | 4 (36.4) |
| Acetylsalicylic acid | 4 (36.4) |
| DAPT | 2 (18.2) |
| Heparin for ECMO + DAPT | 1 (9.1) |
| In hospital death (%) | 4 (36.4) |
| Cardiogenic shock | 2 (18.2) |
| Cerebral death | 1 (9.1) |
| Massive pulmonary embolism | 1 (9.1) |
| VT ablation (%) | 3 (27.3) |
| Bilateral cardiac sympathetic denervation (%) | 1 (9.1) |
AAD, anti-arrhythmic drugs; DAPT, double anti-platelet therapy; ECMO, extracorporeal membrane oxygenation; ICD implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
| Approach (%) | |
| Anatomical | 18 (100) |
| Lateral ultrasound-guided | 0 (0) |
| Type (%) | |
| Bolus | 16 (88.9) |
| Bolus and infusion | 2 (11.1) |
| Anaesthetic used (%) | |
| Lidocaine | 6 (33.3) |
| Bupivacaine | 4 (22.2) |
| Lidocaine + bupivacaine | 8 (44.4) |
| Type of arrhythmias (%) | |
| VT | 5 (27.8) |
| VF | 3 (16.7) |
| VT and VF | 10 (55.6) |
| Arrhythmia cycle length (ms) | 309 ± 126 |
| Pre PLSGB intervention (%) | |
| Intubation | 4 (22.2) |
| Sedation | 4 (22.2) |
| ECMO | 2 (11.1) |
| IABP | 2 (11.1) |
| Pre PLSGB i.v. medication (%) | |
| Amines | 4 (22.2) |
| Amiodarone | 4 (22.2) |
| Lidocaine | 5 (27.8) |
| Amiodarone and lidocaine | 7 (38.9) |
| β-blockers | 1 (5.6) |
| Post-PLSGB anisocoria (%) | 5 (27.8) |
| Approach (%) | |
| Anatomical | 18 (100) |
| Lateral ultrasound-guided | 0 (0) |
| Type (%) | |
| Bolus | 16 (88.9) |
| Bolus and infusion | 2 (11.1) |
| Anaesthetic used (%) | |
| Lidocaine | 6 (33.3) |
| Bupivacaine | 4 (22.2) |
| Lidocaine + bupivacaine | 8 (44.4) |
| Type of arrhythmias (%) | |
| VT | 5 (27.8) |
| VF | 3 (16.7) |
| VT and VF | 10 (55.6) |
| Arrhythmia cycle length (ms) | 309 ± 126 |
| Pre PLSGB intervention (%) | |
| Intubation | 4 (22.2) |
| Sedation | 4 (22.2) |
| ECMO | 2 (11.1) |
| IABP | 2 (11.1) |
| Pre PLSGB i.v. medication (%) | |
| Amines | 4 (22.2) |
| Amiodarone | 4 (22.2) |
| Lidocaine | 5 (27.8) |
| Amiodarone and lidocaine | 7 (38.9) |
| β-blockers | 1 (5.6) |
| Post-PLSGB anisocoria (%) | 5 (27.8) |
ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; PLSGB, percutaneous left ganglion block; VT, ventricular tachycardia; VF, ventricular fibrillation.
| Approach (%) | |
| Anatomical | 18 (100) |
| Lateral ultrasound-guided | 0 (0) |
| Type (%) | |
| Bolus | 16 (88.9) |
| Bolus and infusion | 2 (11.1) |
| Anaesthetic used (%) | |
| Lidocaine | 6 (33.3) |
| Bupivacaine | 4 (22.2) |
| Lidocaine + bupivacaine | 8 (44.4) |
| Type of arrhythmias (%) | |
| VT | 5 (27.8) |
| VF | 3 (16.7) |
| VT and VF | 10 (55.6) |
| Arrhythmia cycle length (ms) | 309 ± 126 |
| Pre PLSGB intervention (%) | |
| Intubation | 4 (22.2) |
| Sedation | 4 (22.2) |
| ECMO | 2 (11.1) |
| IABP | 2 (11.1) |
| Pre PLSGB i.v. medication (%) | |
| Amines | 4 (22.2) |
| Amiodarone | 4 (22.2) |
| Lidocaine | 5 (27.8) |
| Amiodarone and lidocaine | 7 (38.9) |
| β-blockers | 1 (5.6) |
| Post-PLSGB anisocoria (%) | 5 (27.8) |
| Approach (%) | |
| Anatomical | 18 (100) |
| Lateral ultrasound-guided | 0 (0) |
| Type (%) | |
| Bolus | 16 (88.9) |
| Bolus and infusion | 2 (11.1) |
| Anaesthetic used (%) | |
| Lidocaine | 6 (33.3) |
| Bupivacaine | 4 (22.2) |
| Lidocaine + bupivacaine | 8 (44.4) |
| Type of arrhythmias (%) | |
| VT | 5 (27.8) |
| VF | 3 (16.7) |
| VT and VF | 10 (55.6) |
| Arrhythmia cycle length (ms) | 309 ± 126 |
| Pre PLSGB intervention (%) | |
| Intubation | 4 (22.2) |
| Sedation | 4 (22.2) |
| ECMO | 2 (11.1) |
| IABP | 2 (11.1) |
| Pre PLSGB i.v. medication (%) | |
| Amines | 4 (22.2) |
| Amiodarone | 4 (22.2) |
| Lidocaine | 5 (27.8) |
| Amiodarone and lidocaine | 7 (38.9) |
| β-blockers | 1 (5.6) |
| Post-PLSGB anisocoria (%) | 5 (27.8) |
ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; PLSGB, percutaneous left ganglion block; VT, ventricular tachycardia; VF, ventricular fibrillation.
Primary endpoints
The per-procedure analysis showed a statistically significant reduction of the arrhythmic events in the first hour after PLSGB compared with the hour before [0 (IQR 0–0) vs. 5 (IQR 1–10) P < 0.001] (Figure 1). A complete suppression of VAs at 1 h after PLSGB occurred in 83% of the procedures.
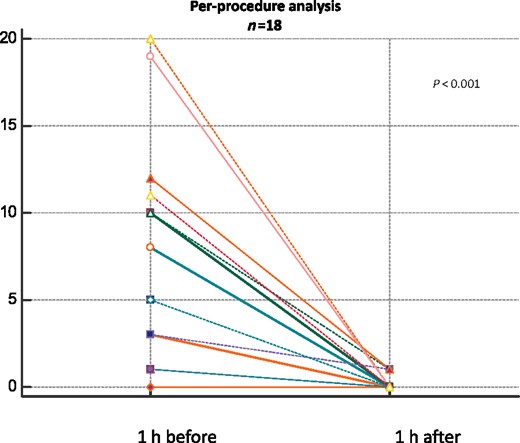
Per-procedure analysis (n = 18) showing the overall number of arrhythmic events in the hour after each PLSGB compared with the hour before.
Regarding the safety endpoint, all the procedures were performed with an anatomical approach and neither major nor minor complications occurred. Most PLSGBs (14/18) were performed on conscious patients with no need for specific sedation/analgesia.
Secondary endpoints
The per-patient analysis comparing the arrhythmic burden 24 h before the first PLSGB and after the last procedure showed a significant reduction [7 (IQR 4.3–12) vs. 1 (IQR 0–1.8) P = 0.002] (Figure 2).
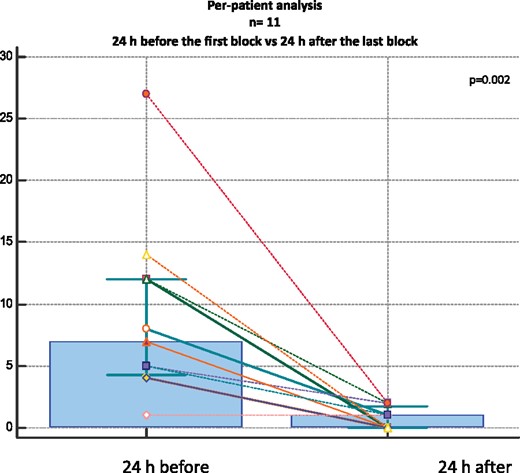
Per-patient analysis of the overall population (n = 11) comparing the number of treated arrhythmic events in 24 h before the first PLSGB to 24 h after the last procedure.
Interestingly a statistically significant reduction was found even when comparing the arrhythmic burden in 24 h before and after the first procedure [7 (IQR 4.3–12) vs. 1 (IQR 0–1.75) P = 0.04], and before the first and after the second block [7 (IQR 4.3–12) vs. 1 (IQR 0–1.8) P = 0.02] (Supplementary material online, Figure S1).
The appearance of anisocoria (±ptosis) was observed only in a minority of PLSGB procedures (5/18, 28%) and was not related to PLSGB efficacy. Indeed, 2/5 patients with anisocoria compared with 1/13 without had VA recurrences in the hour after PLSGB (P = 0.09).
Discussion
The main findings of our paper are (i) PLSGB is highly effective in reducing the arrhythmic burden in patients with refractory ES; (ii) the anti-arrhythmic efficacy of PLSGB mostly performed with lidocaine (alone or in combination with bupivacaine) has a rapid onset and is not related to the appearance of anisocoria; (iii) PLSGB can be safely and effectively repeated over time to control VAs, eventually as continuous infusion with additional benefit; and (iv) the anatomical approach according to Moore’s technique was preferred by the operators and caused no complications, highlighting an easy and safe procedure worthy of being spread among cardiologists.
The arrhythmogenic role of sympathetic nervous system activation is well established. Pre-clinical studies consistently showed that LSG stimulation not only enhances automaticity10 and triggered activity11 but also shortens ventricular repolarization length in a spatially and temporally inhomogeneous way12 and modulates conduction velocity in the border zone of post-infarct scars.13 This results in an increased susceptibility to both scar-related and functional reentrant-related VAs. Left stellate ganglion contribution was found to be predominant over the right on the left ventricle innervations. Accordingly, LSG block increased the VF threshold14 and reduced ischaemia-related arrhythmias.15 As opposed to dogs and cats, LSG in humans provides a partial ipsilateral cardiac sympathetic innervation, and the limited data available so far suggest a good anti-arrhythmic efficacy of LSGB in the acute clinical setting of refractory ES. This probably reflects the importance of acutely interrupting the vicious circle of sympathetic hyperactivity by acting on the afferent as well the efferent component of the subcortical sympathetic reflex arch (both travelling along the stellate ganglion). Yet, it must be acknowledged that the largest review5 and single-centre case series8,16 published so far assessed the anti-arrhythmic efficacy of the procedure by comparing 24, 48, and 72 h before and after PSGB. Although this way of presenting the results may appear enduring, suggesting a long-lasting effect of the block; it may prevent the understanding of the real impact of PSGB and its time of onset and offset. Indeed, other anti-arrhythmic interventions could have been provided in such a long timeframe and spontaneous reduction of arrhythmias could have occurred, given the often-transient nature of ES. Since ES can be self-limited in time, the longer the period of observation, the higher the likelihood of a spontaneous reduction of the arrhythmic burden. Finally, since neural block lasts as long as local anaesthetics are present in meaningful concentrations, the rationale for judging the efficacy long after their pharmacological effects appears questionable.
For all these reasons, we decided to present both a per-procedure analysis comparing arrhythmia burden the hour before PLSGB with the hour after and a per-patient analysis comparing 24 h before and after the block. As a matter of fact, the present study provided an analytical approach. By doing so, we were able to show a significant acute reduction after PLSGB (83% free from VAs at 1 h) supporting the use of this procedure to stabilize critical patients. At variance with other experiences reported in the literature in our single-centre case series, we decided to perform only PLSGB, eventually repeating it in case of arrhythmia recurrence. Tian et al.8 reported on 30 patients receiving echo or fluoro-guided PSGB: 15 underwent only PLSGB and 15 underwent additional right PSGB because of VA recurrence in the first 10 min after PLSGB. Right PSGB did not result in any additional benefit in controlling arrhythmias compared with only PLSGB, supporting our unilateral approach to the LSG. In another recent paper, Fudim et al.16 reported data on 20 patients treated with stellate ganglion block for refractory VT/VF with a priori bilateral approach under ultrasound control. Several pathophysiological issues support our PLSGB-only approach. First, the acknowledged quantitative predominance of the left-sided cardiac sympathetic innervations over those of the right15 and the fact that in the case of effective block of right-sided cardiac fibres, after an anatomically ineffective (not reaching cardiac fibres) left block, there is a potential for a proarrhythmic effect, supported by pre-clinical data showing a decrease in the VF threshold after an isolated right stellate ganglion block.14 Secondly, the expected additional benefits of right stellate ganglion block after an anatomically effective PLSGB are limited and possibly absent, as also suggested by preliminary clinical data already available in 20176 and recently confirmed.8 Cases of phrenic nerve block and/or respiratory distress after right PSGB are possible, albeit rare. Finally, unlike the bilateral approach, PLSGB appears safer, easier and requires little material, a major issue considering the critical clinical status of the patients to which it is addressed. Therefore, we believe our results strongly suggest that in the case of arrhythmia recurrence after a first procedure, PLSGB can be repeated safely and effectively, a conclusion of great clinical interest.
Moreover, most blocks in the study by Tian and Fudim,8,16 as well as in the largest PSGB review published so far,5 were performed using bupivacaine, which is known to have slower onset of action (5–10 min) compared with lidocaine, therefore leading to question whether VAs occurred in the first 10 min after the first PLSGB were true failures of the procedures. On the other hand, lidocaine acts faster (2–5 min), but its pure pharmacodynamic effects only last up to 2 h. Accordingly, four patients in our case-series required repeated blocks in the first 24 h. Repeated blocks in the same person have already been reported both in the following hours5–7 and in the following days alternating side of block and followed by the use of radiofrequency,17 but we are the first to provide a quantification of their additional benefit on VA control.
Concerning the characteristics of the study population, our case-series has some peculiarities. First, none of our patients suffered a purely electrical disorder. The inclusion of patients with channelopathies in previous studies might have led to an overestimation of the true efficacy of PLSGB in the setting of structural heart disease, due to the expected particularly good response in these patients.18 Secondly, the rate of patients under general anaesthesia before PLSGB is lower compared with the study by Tian et al.,8 the study by Fudim et al.16 and the review by Meng et al.,5 suggesting that PLSGB could be considered to control arrhythmias before intubation to avoid the risks and the practical aspects of general anaesthesia. This point is again of great clinical utility since it suggests that PLSGB can be performed to manage refractory ES at the bedside in a cardiological environment. Further supporting this assumption is the choice of the operators in favour of the anatomical approach for all PLSGB procedures in our study. On the contrary, in most of the published case series an imaging technique was used to guide the procedure, represented either by fluoroscopy or by ultrasonography.5,6,8,16 Our findings show that the anatomical approach, described >60 years ago, was the preferred approach in this study probably because it is easier and faster, and it can be safely performed at bedside by a trained cardiologist also in conscious patients, without the need of specific expertise in echo- or fluoroscopy-guided procedures. Indeed, this is the largest series reporting the use of the old anatomical approach in a contemporary population with a high percentage of patients on anti-platelet and/or anti-coagulant treatments. In our cohort, ∼70% of the patients were taking aspirin; 30% were in dual anti-platelet therapy and one patient had high level of anticoagulation because of the use of an extracorporeal membrane oxygenator.
Finally, we confirmed that the development of anisocoria is probably not related to the anti-arrhythmic efficacy of PLSGB, as postulated by Schwartz et al.19 since the 1970s. This finding may be explained by the anatomical distribution of neuronal fibres in the stellate ganglion: ocular fibres mostly run in the upper part (C8) while cardiac fibres mostly run in the lower part (T1). Yet, several authors used anisocoria as a marker of an anatomically effective block of cardiac fibres, eventually suggesting an association between the development of ocular signs and the anti-arrhythmic efficacy of PLSGB.20 While an occasional association is indeed possible (albeit not supported by our data), clinicians must be aware that it is not the rule. Therefore, PLSGB can be repeated shortly after even in the case of anisocoria.
Limitations
The present study has several limitations. These include a limited sample size, which did not allow us to properly assess the relationship between acute (1 h) and subacute (24 h) efficacy of PLSGB and survival outcomes. Nevertheless, this is the second largest single-centre case series on PLSGB in ES and the largest in Europe. Additionally, we did not evaluate markers of an anatomically correct and effective block. Some studies assessed the change in temperature of the ipsilateral side of the arm, an evaluation which is not suitable in the emergency setting.
Finally, although our results strongly suggest that the anatomical-based approach is safe and effective, a head to head comparison with an imaging-guided approach has never been performed. More data are required to definitely address which is the most suitable approach for PLSGB.
Conclusions
Anatomical-based percutaneous LSG block is a safe, well-tolerated and effective means to treat refractory VAs in patients with structural heart disease. It has a rapid onset of anti-arrhythmic efficacy and can be repeated if needed. This approach should be considered, before general anaesthesia, to stabilize patients unresponsive to first-line conventional treatments.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
We would like to thank Dr Diego Beltrutti for his help in teaching this technique.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.



