-
PDF
- Split View
-
Views
-
Cite
Cite
Timm Seewöster, Falco Kosich, Philipp Sommer, Livio Bertagnolli, Gerhard Hindricks, Jelena Kornej, Prediction of low-voltage areas using modified APPLE score, EP Europace, Volume 23, Issue 4, April 2021, Pages 575–580, https://doi.org/10.1093/europace/euaa311
Close - Share Icon Share
Abstract
The presence of low-voltage areas (LVAs) in patients with atrial fibrillation (AF) reflects left atrial (LA) electroanatomical substrate, which is essential for individualized AF management. However, echocardiographic anteroposterior LA diameter included into previous LVAs prediction scores does not mirror LA size accurately and impaired left ventricular ejection fraction (LV-EF) is not directly associated with atrial myopathy. Therefore, we aimed to compare a modified (m)APPLE score, which included LA volume (LAV) and LA emptying fraction (LA-EF) with the regular APPLE score for the prediction of LVAs.
In patients undergoing first AF catheter ablation, LVAs were determined peri-interventionally using high-density maps and defined as signal amplitude <0.5 mV. All patients underwent cardiovascular magnetic resonance imaging before intervention. The APPLE (one point for Age ≥ 65 years, Persistent AF, imPaired eGFR ≤ 60 mL/min/1.73 m2, LA diameter ≥ 43 mm, and LVEF < 50%) and (m)APPLE (last two variables changed by LAV ≥ 39 mL/m2, and LA-EF < 31%) scores were calculated at baseline. The study population included 219 patients [median age 65 (interquartile range 57–72) years, 41% females, 59% persistent AF, 25% LVAs]. Both scores were significantly associated with LVAs [OR 1.817, 95% CI 1.376–2.399 for APPLE and 2.288, 95% CI 1.650–3.172 for (m)APPLE]. Using receiver operating characteristic curves analysis, the (m)APPLE score [area under the curve (AUC) 0.779, 95% CI 0.702–0.855] showed better LVAs prediction than the APPLE score (AUC 0.704, 95% CI 0.623–0.784), however, without statistically significant difference (P = 0.233).
The modified (m)APPLE score demonstrated good prognostic value for LVAs prediction and was comparable with the regular APPLE score.
In our study, we compared the APPLE and modified (m)APPLE scores and investigated their impact on pre-procedural low-voltage areas (LVAs) prediction.
We found that both scores had similar ability for LVAs prediction in AF patients with a trend of better prediction using the (m)APPLE score.
Due to the inclusion of CMR-derived parameters describing LA size and function, the modified (m)APPLE score demonstrated good prognostic value for LVAs prediction.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical routine and it is associated with severe morbidity and increased mortality.1 The presence of low-voltage areas (LVAs) in AF patients reflects electroanatomical remodelling. It is a surrogate parameter of AF progression and it defines advanced stages of atrial cardiomyopathy.
LVAs can be found in approximately 20–25% of all AF patients, and up to one-third of AF patients with LVAs have impaired rhythm outcomes after catheter ablation.2 That means a more intensified treatment concept due to extensive ablation strategies and more frequent follow-up. In some patients, life-long antiarrhythmic drug intake is necessary and arrhythmia recurrences can lead to higher health costs due to hospital re-admissions.3
Recently, we showed that cardiovascular magnetic resonance imaging (CMR) measured increased LA volume (LAV) and impaired LA emptying fraction (LA-EF) are associated with LVAs presence.4,5 These non-invasive prognostic LVAs parameters could be taken into account by the electrophysiologist before catheter ablation. Thus, the clinician would be able to individualize the therapeutic ablation approach, the follow-up management, and optimize patient-shaped AF treatment.
Several scores were evaluated to predict LVAs before catheter ablation. Previously we reported that arrhythmia recurrences and LVAs can be predicted using the APPLE score.6 However, echocardiographic anteroposterior LA diameter does not mirror the accurate three-dimensional LA size and LV-EF <50% is not necessarily associated with atrial cardiomyopathy. Therefore, the aim of current analysis was to compare predictive value of modified (m)APPLE score, which included such CMR variables as LAV and LA-EF, with the regular APPLE score, which used echocardiographic parameters, for LVAs presence.
Methods
The study population was described previously.6 Briefly, patients presenting for catheter ablation due to symptomatic AF from October 2015 to April 2017 were included in the study. Paroxysmal and persistent AF were defined according to current guidelines.7 Exclusion criteria were pregnancy, age <18 or >75 years, valvular AF, cancer, acute or systemic inflammatory diseases, and acute hyperthyreotic state. The study was approved by the local Ethical Committee (Medical Faculty, University of Leipzig), and patients provided written informed consent for participation. Cardiovascular magnetic resonance imaging was performed prior to the AF catheter ablation and used for LA anatomy measurements.
Cardiovascular magnetic resonance
Patients underwent 1.5 T CMR (Ingenia, Philips Medical) before AF catheter ablation.4 Briefly, a contrast-enhanced MR angiography of the left atrium and the pulmonary veins (PV) was acquired during breath-hold without ECG gating using real-time bolus tracking. Cardiovascular magnetic resonance imaging data were reviewed and total LAV was determined after exclusion of the atrial appendage and the PV. LA-EF was calculated using a biplane model based on cine 4- and 2-chamber views. In patients with AF during CMR, the atrial emptying fraction was measured for five cycles and the mean value was calculated.
Scores
The APPLE score [one point for Age ≥ 65 years, Persistent AF, imPaired eGFR ≤ 60 mL/min/1.73 m2, Left atrial (LA) diameter ≥ 43 mm, and EF < 50%]8 and the (m)APPLE score (one point for Age ≥ 65 years, Persistent AF, imPaired eGFR ≤ 60 mL/min/1.73 m2, median indexed LAV ≥39 mL/m2, and median biplane LA-EF <31%) were calculated using patients’ baseline characteristics before catheter ablation.6 Statistical medians had been chosen as cut-offs for LAV and LA-EF within the (m)APPLE score (Table 1).
| . | Total study population n = 219 . | Low-voltage areas . | P-value . | |
|---|---|---|---|---|
| No (n = 164) . | Yes (n = 55) . | |||
| Age (years) | 65 (57–72) | 62 (55–70) | 69 (64–75) | <0.001 |
| Females | 89 (41) | 57 (35) | 32 (58) | 0.002 |
| Persistent AF | 130 (59) | 84 (51) | 46 (84) | <0.001 |
| Low-voltage areas | 55 (25) | — | 100% | |
| BMI (kg/m2) | 30 (26–33) | 29 (26–33) | 30 (26–33) | 0.277 |
| eGFR (mL/min/1.73 m2) | 78 (64–89) | 80 (68–93) | 69 (60–83) | 0.001 |
| LAV (mL/BSA) (CMR) | 39 (30–50) | 37 (28–45) | 49 (36–56) | <0.001 |
| LA-EF (biplane) (%) (CMR) | 31 (21–55) | 39 (23–60) | 19 (15–28) | <0.001 |
| LA diameter (mm) (echo) | 44 (39–48) | 43 (38–48) | 45 (42–49) | 0.042 |
| LV-EF (biplane) (%) (echo) | 56 (47–61) | 56 (47–61) | 55 (47–59) | 0.374 |
| APPLE | 2 (1–3) | 2 (1–3) | 3 (2–4) | <0.001 |
| (m)APPLEa | 2 (1–3) | 2 (1–3) | 3 (3–4) | <0.001 |
| . | Total study population n = 219 . | Low-voltage areas . | P-value . | |
|---|---|---|---|---|
| No (n = 164) . | Yes (n = 55) . | |||
| Age (years) | 65 (57–72) | 62 (55–70) | 69 (64–75) | <0.001 |
| Females | 89 (41) | 57 (35) | 32 (58) | 0.002 |
| Persistent AF | 130 (59) | 84 (51) | 46 (84) | <0.001 |
| Low-voltage areas | 55 (25) | — | 100% | |
| BMI (kg/m2) | 30 (26–33) | 29 (26–33) | 30 (26–33) | 0.277 |
| eGFR (mL/min/1.73 m2) | 78 (64–89) | 80 (68–93) | 69 (60–83) | 0.001 |
| LAV (mL/BSA) (CMR) | 39 (30–50) | 37 (28–45) | 49 (36–56) | <0.001 |
| LA-EF (biplane) (%) (CMR) | 31 (21–55) | 39 (23–60) | 19 (15–28) | <0.001 |
| LA diameter (mm) (echo) | 44 (39–48) | 43 (38–48) | 45 (42–49) | 0.042 |
| LV-EF (biplane) (%) (echo) | 56 (47–61) | 56 (47–61) | 55 (47–59) | 0.374 |
| APPLE | 2 (1–3) | 2 (1–3) | 3 (2–4) | <0.001 |
| (m)APPLEa | 2 (1–3) | 2 (1–3) | 3 (3–4) | <0.001 |
Data are presented as median (IQR) or n (%).
AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; CMR, cardiovascular magnetic resonance imaging; eGFR, estimated glomerular filtration rate; EF, emptying fraction; IQR, interquartile range; LAV, left atrial volume; LA, left atrial; LV, left ventricular.
Available in 189 patients.
| . | Total study population n = 219 . | Low-voltage areas . | P-value . | |
|---|---|---|---|---|
| No (n = 164) . | Yes (n = 55) . | |||
| Age (years) | 65 (57–72) | 62 (55–70) | 69 (64–75) | <0.001 |
| Females | 89 (41) | 57 (35) | 32 (58) | 0.002 |
| Persistent AF | 130 (59) | 84 (51) | 46 (84) | <0.001 |
| Low-voltage areas | 55 (25) | — | 100% | |
| BMI (kg/m2) | 30 (26–33) | 29 (26–33) | 30 (26–33) | 0.277 |
| eGFR (mL/min/1.73 m2) | 78 (64–89) | 80 (68–93) | 69 (60–83) | 0.001 |
| LAV (mL/BSA) (CMR) | 39 (30–50) | 37 (28–45) | 49 (36–56) | <0.001 |
| LA-EF (biplane) (%) (CMR) | 31 (21–55) | 39 (23–60) | 19 (15–28) | <0.001 |
| LA diameter (mm) (echo) | 44 (39–48) | 43 (38–48) | 45 (42–49) | 0.042 |
| LV-EF (biplane) (%) (echo) | 56 (47–61) | 56 (47–61) | 55 (47–59) | 0.374 |
| APPLE | 2 (1–3) | 2 (1–3) | 3 (2–4) | <0.001 |
| (m)APPLEa | 2 (1–3) | 2 (1–3) | 3 (3–4) | <0.001 |
| . | Total study population n = 219 . | Low-voltage areas . | P-value . | |
|---|---|---|---|---|
| No (n = 164) . | Yes (n = 55) . | |||
| Age (years) | 65 (57–72) | 62 (55–70) | 69 (64–75) | <0.001 |
| Females | 89 (41) | 57 (35) | 32 (58) | 0.002 |
| Persistent AF | 130 (59) | 84 (51) | 46 (84) | <0.001 |
| Low-voltage areas | 55 (25) | — | 100% | |
| BMI (kg/m2) | 30 (26–33) | 29 (26–33) | 30 (26–33) | 0.277 |
| eGFR (mL/min/1.73 m2) | 78 (64–89) | 80 (68–93) | 69 (60–83) | 0.001 |
| LAV (mL/BSA) (CMR) | 39 (30–50) | 37 (28–45) | 49 (36–56) | <0.001 |
| LA-EF (biplane) (%) (CMR) | 31 (21–55) | 39 (23–60) | 19 (15–28) | <0.001 |
| LA diameter (mm) (echo) | 44 (39–48) | 43 (38–48) | 45 (42–49) | 0.042 |
| LV-EF (biplane) (%) (echo) | 56 (47–61) | 56 (47–61) | 55 (47–59) | 0.374 |
| APPLE | 2 (1–3) | 2 (1–3) | 3 (2–4) | <0.001 |
| (m)APPLEa | 2 (1–3) | 2 (1–3) | 3 (3–4) | <0.001 |
Data are presented as median (IQR) or n (%).
AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; CMR, cardiovascular magnetic resonance imaging; eGFR, estimated glomerular filtration rate; EF, emptying fraction; IQR, interquartile range; LAV, left atrial volume; LA, left atrial; LV, left ventricular.
Available in 189 patients.
Peri-procedural left atrial mapping
The electroanatomical mapping was performed in sinus rhythm as described previously.4 In cases with AF at the beginning of the procedure, AF was electrically cardioverted after transseptal puncture and the mapping was conducted in sinus rhythm. If AF recurred early during mapping, only anatomical map was completed. After PVI, if still needed, another cardioversion was performed and the voltage map was completed in sinus rhythm. Multielectrode spiral mapping catheters [Reflexion Spiral and Advisor, St. Jude Medical (SJM), Saint Paul, MN, USA in NavX Ensite procedures and Carto Lasso, Biosense Webster, Diamond Bar, CA, USA in Carto3 procedures] were used to generate electroanatomical voltage maps of the LA. The cut-off value for LVAs were <0.5 mV for low-voltage and <0.2 mV for dense scar in both mapping systems. Ectopic beats were excluded from the voltage map. The number of points obtained was >1000.
The ablation catheters (irrigated tip catheter and power of 25–40 W) used in NavX Ensite procedures were TactiCath (SJM, Saint Paul, MN, USA) and for Carto3 procedures SmartTouch Thermocool (Biosense Webster, Diamond Bar, CA, USA). End point of catheter ablation was PV isolation, which was verified with a multipolar circular mapping catheter. We defined LVAs not only if electroanatomical mapping demonstrated low/impaired conduction areas, but also if an additional linear ablation (substrate modification) was clinically indicated. Patients with small/negligible LVAs dispersely distributed in the left atrium and not suitable for ablation, who received the PVI only without additional ablation lines, were not included into analyses.
Statistical analysis
Data are presented as mean and standard deviation (SD) for normally distributed or median (interquartile range) for skewed continuous variables and as proportions for categorical variables. The differences between continuous values were assessed using an unpaired t-test or the Mann–Whitney, and a χ2 test for categorical variables.
Logistic regression analysis was used to identify factors associated with LVAs. Receiver operating characteristic curves (ROC) were generated to analyse performance of the APPLE and (m)APPLE scores predicting LVAs, with the area under the curve (AUC) being equivalent to the c-index for determining the predictive value for the parameters. Finally, the c-indices (i.e. areas under the ROC curves) for both scores were compared using DeLong's method.9 A P-value <0.05 was considered statistically significant. All analyses were performed with SPSS statistical software version 23 (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
In total, 219 patients (65 ± 7 years, 41% women, 59% persistent AF) undergoing first AF ablation and with pre-procedurally available CMR were included in this study. LVAs were detected in 55 patients (25%). Clinical characteristics of the study population are summarized in Table 1. Patients with LVAs were significantly older, more frequently females and with persistent AF and had a lower eGFR (all P < 0.001). Furthermore, patients with LVAs had significantly higher indexed LAV, a lower LA-EF (both P < 0.001), and higher LAD (P = 0.042), while LVEF (P = 0.374) and BMI (P = 0.277) were not significantly different among both groups. Both the APPLE and (m)APPLE scores were significantly higher in patients with LVAs (P < 0.001, Table 1).
Variables and scores for low-voltage areas prediction
Clinical variables such as age ≥ 65 years (OR 3.493, 95% CI 1.819–6.706, P < 0.001) and persistent AF (OR 4.868, 95% CI 2.238–10.589, P < 0.001) were significantly associated with LVAs. Also imaging parameters as LA-EF < 31% (OR 4.797, 95% CI 2.392–9.621, P < 0.001), indexed LAV ≥ 39 mL (OR 3.077, 95% CI 1.485–6373, P = 0.002), and LAD ≥ 43 mm (OR 2.473, 95% CI 1.264–4.840, P = 0.008) remained significant predictors for LVAs, while eGFR ≤ 60 mL/min/1.73 m2 (OR 2.047, 95% CI 10.949–4.414, P = 0.068) did not reach the level of significance (Table 2). The sensitivity/specificity for APPLE and (m)APPLE scores ≥2 were 86/40% and 73/49% respectively.
Variables included into APPLE and (m)APPLE scores and their association with low voltage areas
| Variables . | OR . | 95% confidence interval . | P-Value . |
|---|---|---|---|
| Age ≥ 65 years | 3.493 | 1.819–6.706 | <0.001 |
| Persistent AF | 4.868 | 2.238–10.589 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 2.047 | 0.949–4.414 | 0.068 |
| LA diameter, ≥43 mm (echo) | 2.473 | 1.264–4.840 | 0.008 |
| LV-EF < 50% (echo) | 1.668 | 0.667–4.180 | 0.275 |
| LAV ≥ 39 mL/BSA (CMR) | 3.077 | 1.485–6373 | 0.002 |
| LA-EF <31% (CMR) | 4.797 | 2.392–9.621 | <0.001 |
| APPLE | 1.817 | 1.376–2.399 | <0.001 |
| (m)APPLEa | 2.288 | 1.650–3.172 | <0.001 |
| Variables . | OR . | 95% confidence interval . | P-Value . |
|---|---|---|---|
| Age ≥ 65 years | 3.493 | 1.819–6.706 | <0.001 |
| Persistent AF | 4.868 | 2.238–10.589 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 2.047 | 0.949–4.414 | 0.068 |
| LA diameter, ≥43 mm (echo) | 2.473 | 1.264–4.840 | 0.008 |
| LV-EF < 50% (echo) | 1.668 | 0.667–4.180 | 0.275 |
| LAV ≥ 39 mL/BSA (CMR) | 3.077 | 1.485–6373 | 0.002 |
| LA-EF <31% (CMR) | 4.797 | 2.392–9.621 | <0.001 |
| APPLE | 1.817 | 1.376–2.399 | <0.001 |
| (m)APPLEa | 2.288 | 1.650–3.172 | <0.001 |
AF, atrial fibrillation; BSA, body surface area; CMR, cardiovascular magnetic resonance; eGFR, estimated glomerular filtration rate; LA, left atrial; LV-EF, left ventricular ejection fraction; LA-EF, left atrial emptying fraction; LAV, left atrial volume.
Available in 189 patients.
Variables included into APPLE and (m)APPLE scores and their association with low voltage areas
| Variables . | OR . | 95% confidence interval . | P-Value . |
|---|---|---|---|
| Age ≥ 65 years | 3.493 | 1.819–6.706 | <0.001 |
| Persistent AF | 4.868 | 2.238–10.589 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 2.047 | 0.949–4.414 | 0.068 |
| LA diameter, ≥43 mm (echo) | 2.473 | 1.264–4.840 | 0.008 |
| LV-EF < 50% (echo) | 1.668 | 0.667–4.180 | 0.275 |
| LAV ≥ 39 mL/BSA (CMR) | 3.077 | 1.485–6373 | 0.002 |
| LA-EF <31% (CMR) | 4.797 | 2.392–9.621 | <0.001 |
| APPLE | 1.817 | 1.376–2.399 | <0.001 |
| (m)APPLEa | 2.288 | 1.650–3.172 | <0.001 |
| Variables . | OR . | 95% confidence interval . | P-Value . |
|---|---|---|---|
| Age ≥ 65 years | 3.493 | 1.819–6.706 | <0.001 |
| Persistent AF | 4.868 | 2.238–10.589 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 2.047 | 0.949–4.414 | 0.068 |
| LA diameter, ≥43 mm (echo) | 2.473 | 1.264–4.840 | 0.008 |
| LV-EF < 50% (echo) | 1.668 | 0.667–4.180 | 0.275 |
| LAV ≥ 39 mL/BSA (CMR) | 3.077 | 1.485–6373 | 0.002 |
| LA-EF <31% (CMR) | 4.797 | 2.392–9.621 | <0.001 |
| APPLE | 1.817 | 1.376–2.399 | <0.001 |
| (m)APPLEa | 2.288 | 1.650–3.172 | <0.001 |
AF, atrial fibrillation; BSA, body surface area; CMR, cardiovascular magnetic resonance; eGFR, estimated glomerular filtration rate; LA, left atrial; LV-EF, left ventricular ejection fraction; LA-EF, left atrial emptying fraction; LAV, left atrial volume.
Available in 189 patients.
Both scores were significantly associated with LVAs [OR 1.817, 95% CI 1.376–2.399, P < 0.001 for APPLE and OR 2.288, 95% CI 1.650–3.172, P < 0.001 for (m)APPLE]. Using ROC analysis, the (m)APPLE score (AUC 0.779, 95% CI 0.702–0.855, P < 0.001) showed better LVAs prediction than the regular APPLE score (AUC 0.704, 95% CI 0.623–0.784, P < 0.001), however, the difference was not significant (P = 0.233) (Figure 1).
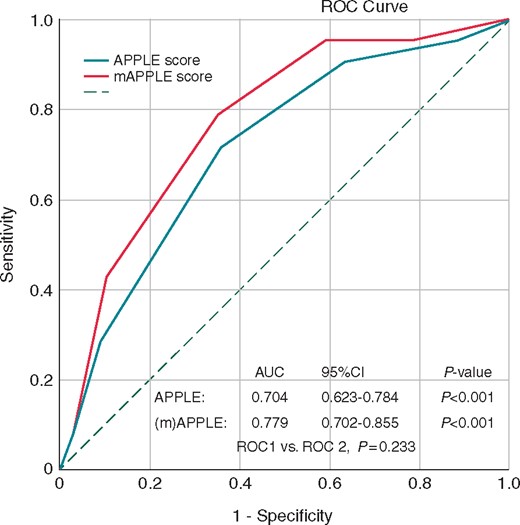
Prediction of low-voltage areas using regular APPLE and modified (m)APPLE score.
Discussion
In our study, we compared the APPLE and modified (m)APPLE scores and investigated their impact on pre-procedural LVAs prediction. We found that both scores had similar ability for LVAs prediction in AF patients with a trend of better prediction using the (m)APPLE score.
Prediction of electroanatomical substrate using clinical scores
The peri-procedural evidence of LVAs is an important marker for AF progression and the severity stage, which directly mirrors advanced LA remodelling. Several studies proved the prognostic impact of LVAs for AF recurrences.3,10,11 In addition, the presence of LVAs influences the choice of ablation modality. There is an evidence that AF patients with LVAs had worse clinical outcomes after cryoballoon ablation.12 Therefore, LVAs prediction scores might be helpful to individualize invasive treatment and improve clinical outcomes after catheter ablation.
There is a link between AF incidence, thromboembolism, and fibrotic atrial cardiomyopathy.13 Several scores were designed to predict AF incidence, thromboembolic events, AF recurrences, or mortality associated with AF,14 yet only a few are able to predict LVAs (Table 3).
Scores developed to predict low voltage areas in patients undergoing catheter ablation
 |
 |
Blue: variables directly associated with atrial myopathy and apricot: variables indirectly associated with atrial myopathy.
HF, heart failure; aHT, arterial hypertension; DM, diabetes mellitus; Sex, female gender; eGFR, estimated glomerular filtration rate; AF type, persistent AF; LAD, left atrial diameter (echo); LVEF, left ventricular ejection fraction (echo); LAV, left atrial volume (CMR); LAFF, left-atrial ejection fraction (CMR); ANP, NT-proANP.
Scores developed to predict low voltage areas in patients undergoing catheter ablation
 |
 |
Blue: variables directly associated with atrial myopathy and apricot: variables indirectly associated with atrial myopathy.
HF, heart failure; aHT, arterial hypertension; DM, diabetes mellitus; Sex, female gender; eGFR, estimated glomerular filtration rate; AF type, persistent AF; LAD, left atrial diameter (echo); LVEF, left ventricular ejection fraction (echo); LAV, left atrial volume (CMR); LAFF, left-atrial ejection fraction (CMR); ANP, NT-proANP.
The CHADS2 score (one point for Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus and two points for Stroke) was designed for the prediction of thromboembolic events and it is associated with systemic inflammation and cardiovascular prognosis in AF patients.15 In a cohort of 247 patients, Chao et al.16 linked a higher CHADS2 score with decreased atrial voltage and more frequent arrhythmia recurrences after catheter ablation. Another study reported an inverse association between LA voltage and each CHA2DS2-VASc score.17 The CHADS2 score and its contemporary version the CHA2DS2-VASc score are frequently used in clinical routine for the prediction of thromboembolic events7 and are familiar to most clinical professionals. However, due to inclusion of the most important AF risk factors, both scores reflect patients’ cardiovascular health state. Although CHADS2 and CHA2DS2-VASc scores were tested for general decrease in atrial voltage, none of these scores was used for the prediction of LVAs.
The first score developed specifically for the LVAs prediction, is the DR-FLASH score (one point for Diabetes mellitus, Renal dysfunction, persistent Form of AF, LA diameter >45 mm, age >65 years, female Sex, and Hypertension). The probability for the presence of LA substrate increased by a factor of 2.2 with each point in the validation cohort.18 However, this score includes similar clinical parameters as the CHADS2 and CHA2DS2-VASc scores, and reflects more patients’ cardiovascular health than atrial myopathy. Furthermore, the study defined renal impairment as GFR < 90 mL/min, which is critical since patients in a ‘typical’ AF ablation cohort are often older than 60 years. In such patients, eGFR is usually decreased to 70–80 mL/min at the time of AF ablation because age is the main contributor to GFR decline.19
Although the APPLE score had been first developed for prediction of rhythm outcomes after AF catheter ablation and was validated in several external cohorts,14 this score is useful for the LVAs prediction, too.20 Both DR-FLASH and APPLE scores share, however, some similarities. First, both use echocardiographic anteroposterior LA diameter to describe LA size. But, as we demonstrated previously, this insufficiently describes the accurate LA anatomy and is rather an imprecise marker of LA dilatation.4 Also, both scores include persistent AF, which is one of the strongest indicators for an atrial myopathy and consequently advanced atrial remodelling. However, in contrast to DR-FLASH, the APPLE score includes a more appropriate cut-off for impaired renal function (GFR < 60 mL/min/1.73 m2) and also uses the CKD-EPI equation instead of the inaccurate Cockcroft–Gault formula as represented in DR-FLASH.21
APPLE vs. (m)APPLE scores for low-voltage areas prediction
Recently, we reported a significant association between LA structural (size) and functional (LA-EF) changes using CMR with peri-interventionally detected LVAs.4,5 Reduction in left ventricular ejection fraction and a LAV increase can be seen as surrogate parameters for the LA electroanatomical remodelling. Due to underlying diseases, such as hypertension with consequently elevated left ventricular pressure and LA dilatation, initiation and progression of electroanatomical changes lead to atrial fibrosis22 and impairment of LA-EF.23
In current analysis, the regular APPLE score was compared with the modified (m)APPLE score, which includes CMR parameters such as LAV and LA-EF. Although the ability for LVAs prediction was better in (m)APPLE (OR 2.288 and AUC 0.779) than in APPLE score (OR 1.817 and AUC 0.704), it was not significant comparing both scores. One explanation could be relatively modest cohort size. Another reason could be inclusion of similar parameters—three out of five variables were the same, including two main determinants for LVAs—age and persistent AF. Therefore, we were not able to demonstrate significant benefit including CMR derived LA structural and functional parameters (LA-EF and LAV) instead of echocardiographic parameters (LV-EF and LAD). These findings support the use of echocardiography, which also has an advantage of its wide clinical availability. Besides high-volume tertiary centres, CMR is not ubiquitous in clinical routine. Therefore, an advanced research performed in this area and recommendations for CMR usage are often not practical for many electrophysiology labs worldwide.
‘Ideal’ score for low-voltage areas prediction
Presence and prediction of LVAs is essential for AF management as the operator has usually an option to choose between cryoballoon or radiofrequency ablation. The main advantages of cryoballoon ablation are short intervention time and effective PVI with good transmural lesions, while such as disadvantage as failing possibility to detect and ablate LVAs should be taken into account. Additional LVAs ablation has a more beneficial effect on rhythm outcomes than PVI ‘only’ in LVAs patients.3,24
So far, there is an unmet clinical need for an ‘ideal’ and simple LVAs score. In a case with LVAs prediction, this could be achieved including parameters, which reflect atrial cardiomyopathy: e.g. ECG variables—P-wave duration, PR interval25; imaging variables—LA function and size4,5; blood biomarkers—NT-proANP, Troponin T.26,27 However, it has to be simple and ‘handy’ with commonly available diagnostic tools. For example, several CMR assessments for the calculation of the ejection fraction are not routinely performed, yet. Thus, they are likely to be limited to large academic centres. Another example is an ECG—cost effective, widely available, and informative diagnostic tool in clinical routine. Magnani et al.25 hypothesized that abnormalities in PR interval and P-wave duration reflect subclinical AF and could be considered as a non-invasive indicator of atrial remodelling. However, these findings are based on epidemiological cohorts’ data and are practically understudied in clinical AF ablation cohorts. Other studies with a non-invasive signal averaged electrocardiograms used a vector composite of filtered orthogonal leads and accurately measured cardiac activation times, including delayed atrial conduction.28 In contrast to analysis using standard 12-lead ECG, the signal averaged electrocardiogram seems to be superior analyzing P-wave indices (especially duration and amplitude) as a risk markers of AF. However, their applicability in a daily routine are limited by time-consuming procedure and advanced technical support. Finally, despite promising results considering NT-proANP as a surrogate parameter for LVAs,29 its usage is limited by non-widely available laboratory assays for clinical routine.
Limitations and future directions
The major limitation of current study is a modest size of study cohort. Also, we could not validate our results in an external cohort, partly because of limited number of AF ablation studies using (i) CMR as a diagnostic imaging tool prior to catheter ablation and (ii) blood biobank. In 30 patients with implantable cardiac devices, CMR was not performed. Instead, these patients underwent computed tomography (CT) scan before AF ablation. However, because the non-ECG gated CT scans were performed, systolic/diastolic parameters and consequently LA-EF could be not measured in these patients. Nevertheless, it would be interesting to analyse the LVAs prediction using modified APPLE score based on CT scan data, if haemodynamic parameters are measured.30 Also, the measurement of late gadolinium enhancement as a marker for LA fibrosis was not performed in this cohort and should be addressed in future studies. Although LVAs presence is one of the important factors describing atrial myopathy, further studies analysing extrapulmonary substrate and triggers (e.g. atrial flutter) should be assessed in the future.
Another limitation is inconsistent follow-up data, which differ significantly compared with our previous research.31 The main explanation for this is the changed follow-up strategy after ablation, when many patients without recurrences or complications related to ablation were followed by general practitioners or cardiologists in the external outpatient setting. However, rhythm outcomes are still of clinical interest and should be addressed in future randomized trials with more accurate and continuous rhythm monitoring (e.g. using implantable loop recorders, mobile health, or digital health technologies).
Conclusion
Modified (m)APPLE score demonstrated good prognostic value for LVAs prediction but was comparable with the regular APPLE score. A simple LVAs prediction score, which could improve individualized AF management, is still lacking and should be addressed in larger clinical studies.
Funding
J.K. received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 838259).
Conflict of interest: P.S. is in the advisory board for Abbott, Biosense Webster, Medtronic und Boston Scientific.
Data availability
The data underlying this article cannot be shared publicly due to patient privacy. The data will be shared on reasonable request to the corresponding author.
References
Author notes
Timm Seewöster and Falco Kosich authors contributed equally to the study.



