-
PDF
- Split View
-
Views
-
Cite
Cite
Xiao-Gang Guo, Xu Liu, Gong-Bu Zhou, Qi Sun, Jian-Du Yang, Bin Luo, Feifan Ouyang, Jian Ma, Shu Zhang, Clinical, electrocardiographic, and electrophysiological characteristics of left upper septal fascicular ventricular tachycardia, EP Europace, Volume 20, Issue 4, April 2018, Pages 673–681, https://doi.org/10.1093/europace/euw429
Close - Share Icon Share
Abstract
We sought to investigate the clinical, electrocardiographic, and electrophysiological characteristics of left upper septal fascicular ventricular tachycardia (LUS-VT).
Eleven consecutive patients with LUS-VT were identified among 196 patients with left fascicular ventricular tachycardia (VT). Clinical VTs presented as paroxysmal in 8 patients and incessant in 3 patients. Six patients had previous left posterior fascicular VT ablation history. All VTs had narrow QRS complexes with QRS duration of 101.1 ± 9.2 ms. The frontal QRS axis was normal or right deviation. Precordial morphology was either right bundle branch block type or similar to that of sinus rhythm. A retrograde His with H-V interval of 21.9 ± 7.2 ms was recorded during VT. The earliest Purkinje potential (PP) to QRS interval during VT averaged 35.7 ± 4.5 ms. Clear diastolic potentials (DPs) with high frequency and low amplitude were found in only one patient. Ten patients were managed successfully by 11 ablation sessions, and 1 patient declined ablation. Successful targets at the left upper septum were sites with the earliest PP (9 cases) or with DP (1 case) during VT. After ablation, 2 cases (10%) developed new left anterior hemiblock or incomplete left bundle branch block. No VT recurred during a median follow-up period of 3.2 (range 1.0–12.7) years.
LUS-VT presented as narrow QRS complex tachycardia. Some LUS-VTs occurred after ablation targeting left posterior fascicular VT. The VTs can be managed successfully by focal ablation at the left upper septum with a mild risk of fascicular injury.
Introduction
What’s new?
This report involved the biggest single-center cohort of patients with left upper septal fascicular ventricular tachycardia (VT).
All LUS-VTs presented as narrow QRS complex tachycardia with precordial QRS morphology of right bundle branch block type or similar to that of sinus rhythm. Some LUS-VTs occurred after ablation targeting left posterior fascicular VT.
The activation of presystolic PP was centrifugal during VT with the earliest PP recorded at the left bundle branch. Clear DPs with high frequency and low amplitude were found in only one patient and was confined to the a small area at the left upper septum. Some patients had a second VT morphology induced after left ventricular endocardial mapping.
The VTs were managed successfully by focal ablation at the left upper septum where the earliest Purkinje potential or diastolic potential was recorded during VT, with a mild risk of fascicular injury.
Methods
Study population
Between January 2003 and May 2015, 196 patients with idiopathic left fascicular VT were managed at our center. Among them, 11 patients with idiopathic left VT presented as the left upper septal type and were included in this retrospective analysis. All patients had structurally normal heart structure. The characteristics of LUS-VT, on which our inclusion of patients was based, were firstly described by Nogami et al.1 as follows: (1) VT ECG of narrow QRS configuration and normal or right axis deviation (i.e. inferior axis); (2) Induction and/or entrainment with ventricular and/or atrial pacing, dependence of tachycardia on left ventricular fascicular activation, and Purkinje potentials preceding ventricular activation during VT; (3) Verapamil sensitive termination or slowing of tachycardia.
Electrophysiological study
The study was approved by institutional review committee at Fuwai Hospital, and all patients gave written informed consent. After withdrawal of antiarrhythmic drugs for at least 5 half-lives (amiodarone for at least 3 weeks), all patients underwent electrophysiological study in fasting state under minimal sedation.
Catheters were introduced into the right ventricular apex, at the HBE region and into the coronary sinus (CS). Twelve-lead surface electrocardiogram (ECG) and intracardiac electrograms were recorded simultaneously by a digital multichannel system (LabSystem PRO, Bard Electrophysiology, Lowell, MA), filtered at 30–500 Hz for bipolar electrograms and at 0.05–500 Hz for unipolar electrograms. The stimulation protocol consisted of programmed ventricular stimulation from the right ventricular apex, right ventricular outflow tract, left ventricular apex and CS at 2 drive cycles lengths with up to 2 extrastimuli and incremental burst pacing at cycle length up to 250 ms. If tachycardia was not induced under baseline condition, the stimulation was repeated after isoproterenol challenge achieving an increase of heart rate by 25%.
Algorithm of Endocardial mapping and target choice
Left ventricular mapping was performed via a retrograde aortic approach with NaviStar ThermoCool catheter (Biosense Webster) with 2 mm inter-electrode distance. Left upper septum, right coronary cusp, and noncoronary cusp where His is anatomically located, left bundle branch and proximal segments of left anterior and posterior fascicle were areas of special interest.
The left ventricular mapping was performed during VT and sinus rhythm to identify the left bundle branch, and the proximal left posterior and anterior fascicle, which presents as a sharp, high-frequency, and low amplitude activation preceding the ventricular activation.9 The site with PPs was tagged and marked on the 3D map using the CARTO system (Biosense Webster). The following electrophysiological features were analyzed: the QRS morphology of VT and sinus rhythm, change of VT morphology during mapping, the VT tachycardia cycle length, QRS duration during VT and sinus rhythm, H-V interval during VT and sinus rhythm (H-VVT and H-VSR), and sinus rhythm QRS change after ablation. Diagnoses of complete and incomplete left bundle branch block (LBBB), left anterior hemiblock, left posterior hemiblock were strictly based on convention.10
In this study, the following electrophysiological parameters were used for guiding radiofrequency (RF) ablation: (1) During tachycardia, diastolic potential (DP) with concomitant PP was initially searched and marked on the 3D map, and RF application was attempted if the DP was found; (2) If DP was not detected or ablation targeting DP failed, the site of the earliest PP during tachycardia was targeted. The PP-to-Ventricular interval during VT and PP-to-Ventricular interval during sinus rhythm was measured from first sharp deflection of PP to the onset of QRS complex during VT. Additionally, entrainment at the site of the earliest PP was attempted to understand the tachycardia mechanism when local ventricle or fascicle can be stably captured.
RFCA
RF was performed with a temperature-controlled mode with upper limit of 55–60°C using a Stokert 70 RF Generator (Biosense Webster). The RF was started at 15 Watts, titrated up to 25 Watts and maintained for 60 s if termination of tachycardia occurred within 10 s and catheter dislodgement and atrioventricular conduction disturbance were not observed. In case that the ablation caused irregular ventricular irritation, RF was performed during CS pacing at pacing cycle length of 400 ms to suppress the irregular ventricular activation induced by RF.
An ablation attempt was regarded as acutely successful by the absence of tachycardia 30 min after ablation despite repetition of the stimulation protocol described above.
Post-procedural management and follow-up
After the procedure, continuous monitoring of ECG was performed for 48 h in all patients. They were treated with oral aspirin (100 mg/d for adults and weight-adjusted dose for children) for 3 months.
ECG was recorded whenever there was a symptom of palpitation. Transthoracic echocardiography and Holter monitoring were performed at an interval of 3 months in the first year after the last successful RFCA procedure, and at an interval of 12 months thereafter.
Statistical analysis
Data were given as mean ± standard deviation or median and range if continuous and as count and ratio if categorical. Because of the small sample size, we performed non-parametric tests between groups. For comparison between groups, the Wilcoxon test was used for the continuous variables and the Fisher’s exact test for the categorical and dichotomous variables. A two-sided P value <0.05 was considered statistically significant. Analyses were performed using SAS ver.9.2 (SAS Institute, Inc., Cary, NC).
Results
Clinical characteristics
As shown in Table 1, 11 patients (2 women, median age 27 [range 14–54] years) diagnosed as LUS-VT with structurally normal heart were included. Clinical symptom was only palpitation. Clinical VTs presented as paroxysmal in 8 patients and incessant in 3 patients. Five patients presented initially as LUS-VT (Group 1). However, the other 6 patients presented initially as common LPF-VT with QRS morphology of RBBB and left axis deviation (Group 2) and underwent successful ablation, either at our institution or at other hospitals. In Group 2, LUS-VTs was induced or occurred spontaneously immediately after ablation attempts at left posterior fascicles or during the same hospital stay in 3 patients. In the other 3 patients, LUS-VT recurred at a median of 1.0 (range 0.5–5) year after successful elimination of LPF-VT. Sensitivity to intravenous administration of verapamil was documented in 5 out of the 11 patients.
| Group . | Case no. . | Age(years)/ Sex . | Previous LPF-VT ablation . | SR ECG . | SR QRS duration (ms) . | Clinical pattern of VT . | Verapamil Sensitivity . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 14/F | 0 | Small S in leads I and aVL; deep Q in lead III | 75 | Paroxysmal | NA |
| 1 | 2 | 54/M | 0 | Small S in leads I and aVL; deep Q in lead III, small Q in leads II and aVF; I° AVB | 86 | Paroxysmal | + |
| 1 | 3 | 16/F | 0 | Small Q in lead aVL; deep S in leads II, III and aVF | 90 | Incessant | NA |
| 1 | 4 | 27/M | 0 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 98 | Incessant | + |
| 1 | 5 | 30/M | 0 | Small S in leads I and aVL; deep Q in lead III | 82 | Paroxysmal | NA |
| 2 | 6 | 44/M | 2 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 100 | Paroxysmal | + |
| 2 | 7 | 34/M | 2 | Small S in lead I; deep S in lead aVL; small Q in lead II, III and aVF | 90 | Paroxysmal | NA |
| 2 | 8 | 17/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 112 | Paroxysmal | NA |
| 2 | 9 | 25/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 102 | Paroxysmal | NA |
| 2 | 10 | 40/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; I° AVB | 96 | Incessant | + |
| 2 | 11 | 26/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; LPHB | 98 | Paroxysmal | + |
| Group . | Case no. . | Age(years)/ Sex . | Previous LPF-VT ablation . | SR ECG . | SR QRS duration (ms) . | Clinical pattern of VT . | Verapamil Sensitivity . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 14/F | 0 | Small S in leads I and aVL; deep Q in lead III | 75 | Paroxysmal | NA |
| 1 | 2 | 54/M | 0 | Small S in leads I and aVL; deep Q in lead III, small Q in leads II and aVF; I° AVB | 86 | Paroxysmal | + |
| 1 | 3 | 16/F | 0 | Small Q in lead aVL; deep S in leads II, III and aVF | 90 | Incessant | NA |
| 1 | 4 | 27/M | 0 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 98 | Incessant | + |
| 1 | 5 | 30/M | 0 | Small S in leads I and aVL; deep Q in lead III | 82 | Paroxysmal | NA |
| 2 | 6 | 44/M | 2 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 100 | Paroxysmal | + |
| 2 | 7 | 34/M | 2 | Small S in lead I; deep S in lead aVL; small Q in lead II, III and aVF | 90 | Paroxysmal | NA |
| 2 | 8 | 17/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 112 | Paroxysmal | NA |
| 2 | 9 | 25/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 102 | Paroxysmal | NA |
| 2 | 10 | 40/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; I° AVB | 96 | Incessant | + |
| 2 | 11 | 26/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; LPHB | 98 | Paroxysmal | + |
AVB, atrioventricular block; LPHB, left posterior hemiblock; NA, not available; SR, sinus rhythm; VT, ventricular tachycardia.
| Group . | Case no. . | Age(years)/ Sex . | Previous LPF-VT ablation . | SR ECG . | SR QRS duration (ms) . | Clinical pattern of VT . | Verapamil Sensitivity . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 14/F | 0 | Small S in leads I and aVL; deep Q in lead III | 75 | Paroxysmal | NA |
| 1 | 2 | 54/M | 0 | Small S in leads I and aVL; deep Q in lead III, small Q in leads II and aVF; I° AVB | 86 | Paroxysmal | + |
| 1 | 3 | 16/F | 0 | Small Q in lead aVL; deep S in leads II, III and aVF | 90 | Incessant | NA |
| 1 | 4 | 27/M | 0 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 98 | Incessant | + |
| 1 | 5 | 30/M | 0 | Small S in leads I and aVL; deep Q in lead III | 82 | Paroxysmal | NA |
| 2 | 6 | 44/M | 2 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 100 | Paroxysmal | + |
| 2 | 7 | 34/M | 2 | Small S in lead I; deep S in lead aVL; small Q in lead II, III and aVF | 90 | Paroxysmal | NA |
| 2 | 8 | 17/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 112 | Paroxysmal | NA |
| 2 | 9 | 25/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 102 | Paroxysmal | NA |
| 2 | 10 | 40/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; I° AVB | 96 | Incessant | + |
| 2 | 11 | 26/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; LPHB | 98 | Paroxysmal | + |
| Group . | Case no. . | Age(years)/ Sex . | Previous LPF-VT ablation . | SR ECG . | SR QRS duration (ms) . | Clinical pattern of VT . | Verapamil Sensitivity . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 14/F | 0 | Small S in leads I and aVL; deep Q in lead III | 75 | Paroxysmal | NA |
| 1 | 2 | 54/M | 0 | Small S in leads I and aVL; deep Q in lead III, small Q in leads II and aVF; I° AVB | 86 | Paroxysmal | + |
| 1 | 3 | 16/F | 0 | Small Q in lead aVL; deep S in leads II, III and aVF | 90 | Incessant | NA |
| 1 | 4 | 27/M | 0 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 98 | Incessant | + |
| 1 | 5 | 30/M | 0 | Small S in leads I and aVL; deep Q in lead III | 82 | Paroxysmal | NA |
| 2 | 6 | 44/M | 2 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF | 100 | Paroxysmal | + |
| 2 | 7 | 34/M | 2 | Small S in lead I; deep S in lead aVL; small Q in lead II, III and aVF | 90 | Paroxysmal | NA |
| 2 | 8 | 17/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 112 | Paroxysmal | NA |
| 2 | 9 | 25/M | 1 | Deep S in leads I and aVL; small Q in leads II, III and aVF; LPHB | 102 | Paroxysmal | NA |
| 2 | 10 | 40/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; I° AVB | 96 | Incessant | + |
| 2 | 11 | 26/M | 1 | Small S in lead I; deep S in lead aVL; small Q in leads II, III and aVF; LPHB | 98 | Paroxysmal | + |
AVB, atrioventricular block; LPHB, left posterior hemiblock; NA, not available; SR, sinus rhythm; VT, ventricular tachycardia.
ECG characteristics
As a result of previous left ventricular mapping and ablation, S wave in leads I and aVL and small Q wave and tall R wave in leads II, III, and aVF of baseline sinus rhythm ECG was found in all 6 patients of Group 2 (Figure 1C). Three of them met the diagnostic criteria of left posterior hemiblock (Figure 1D). This feature was also shared by Group 1, who had no previous LPF-VT ablation history, but to a less degree, since no fascicular block was present (Figure 1A and B). The QRS duration during sinus rhythm of Group 2 was longer than that of Group 1 (99.7 ± 7.3 ms vs. 86.2 ± 8.6 ms, P = 0.0277). Table 1 summarizes the baseline sinus rhythm ECG characteristics.
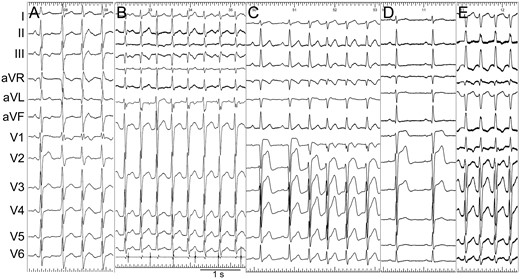
Representative ECG during sinus rhythm and VT. (A) ECG of Case 3. The first QRS was sinus rhythm. (B) ECG of Case 4. The third QRS was captured by sinus rhythm. The lowest tracing was intracardiac recording of CS catheter, which revealed the VA relationship. (C) ECG of Case 10. The first QRS was sinus rhythm. (D and E) Sinus ECG (Panel D) and VT ECG (Panel E) of Case 11.
All VTs had narrow QRS complexes with mean QRS duration of 101.1 ± 9.2 ms. The frontal QRS axes of VT in Group 1 were all normal, while those in Group 2 were all right axis deviation. Precordial morphologies were either RBBB type with prominant R wave or terminal R wave in V1, or similar to sinus rhythm ECG with subtle differences such as a notch in lead V1. Typical sinus rhythm ECG and VT morphologies were presented in Figure 1.
Electrophysiological features and mapping
In the electrophysiological study, the VT was persistent or incessant in 3 patients, occurred spontenously in 3 pateints, and was induced by atrial or ventricular pacing in the other 5 patients. In all VT, a clear retrograde H potential with distal to proximal activation was documented. The H-V interval during VT was 21.9 ± 7.2 ms (range 10–36 ms), which was siginificantly shorter than the H-V interval during sinus rhythm (50.7 ± 4.6 ms, range 42–58 ms). The detailed electrophysiological parameters (e.g. QRS duration during VT, H-VSR, H-VVT, and tachycardia cycle lenght) are shown in Table 2. There were no differences between the two groups.
| Case no. . | H-VSR . | VT QRS . | H-VVT . | Precordial leads . | Frontal axis . | TCL . | VT induction in electrophysiological lab . | Presence of multiform VTs . |
|---|---|---|---|---|---|---|---|---|
| (ms) . | duration (ms) . | (ms) . | ||||||
| 1 | 42 | 92 | 36 | RBBB type | Normal axis | 327–412 | Spontaneous | No |
| 2 | 58 | 105 | 22 | RBBB type | Normal axis | 384 | Spontaneous | No |
| 3 | 52 | 107 | 18 | RBBB type | Normal axis | 494 | Incessant | No |
| 4 | 54 | 90 | 20 | Similar to SR QRS | Normal axis | 404 | Persistent | No |
| 5 | 50 | 100 | 16 | RBBB type | Normal axis | 338 | Atrial or ventricular pacing | Precordial incomplete RBBB type with frontal LAD |
| 6 | 50 | 108 | 10 | Similar to SR QRS | RAD | 300 | Atrial pacing | Precordial incomplete RBBB type with frontal LAD |
| 7 | 54 | 84 | 28 | RBBB type | RAD | 292 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 8 | 55 | 110 | 25 | RBBB type | RAD | 365 | Atrial or ventricular pacing | Precordial complete RBBB type with frontal RAD |
| 9 | 45 | 100 | 16 | RBBB type | RAD | 286 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 10 | 48 | 114 | 28 | Similar to SR QRS | RAD | 410–610 | Incessant | No |
| 11 | 50 | 102 | 22 | RBBB type | RAD | 370 | Spontaneous | No |
| Case no. . | H-VSR . | VT QRS . | H-VVT . | Precordial leads . | Frontal axis . | TCL . | VT induction in electrophysiological lab . | Presence of multiform VTs . |
|---|---|---|---|---|---|---|---|---|
| (ms) . | duration (ms) . | (ms) . | ||||||
| 1 | 42 | 92 | 36 | RBBB type | Normal axis | 327–412 | Spontaneous | No |
| 2 | 58 | 105 | 22 | RBBB type | Normal axis | 384 | Spontaneous | No |
| 3 | 52 | 107 | 18 | RBBB type | Normal axis | 494 | Incessant | No |
| 4 | 54 | 90 | 20 | Similar to SR QRS | Normal axis | 404 | Persistent | No |
| 5 | 50 | 100 | 16 | RBBB type | Normal axis | 338 | Atrial or ventricular pacing | Precordial incomplete RBBB type with frontal LAD |
| 6 | 50 | 108 | 10 | Similar to SR QRS | RAD | 300 | Atrial pacing | Precordial incomplete RBBB type with frontal LAD |
| 7 | 54 | 84 | 28 | RBBB type | RAD | 292 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 8 | 55 | 110 | 25 | RBBB type | RAD | 365 | Atrial or ventricular pacing | Precordial complete RBBB type with frontal RAD |
| 9 | 45 | 100 | 16 | RBBB type | RAD | 286 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 10 | 48 | 114 | 28 | Similar to SR QRS | RAD | 410–610 | Incessant | No |
| 11 | 50 | 102 | 22 | RBBB type | RAD | 370 | Spontaneous | No |
LAD, left axis deviation; RAD, right axis deviation; RBBB, right bundle branch block; SR, sinus rhythm; TCL, tachycardia cycle length; VT, ventricular tachycardia.
| Case no. . | H-VSR . | VT QRS . | H-VVT . | Precordial leads . | Frontal axis . | TCL . | VT induction in electrophysiological lab . | Presence of multiform VTs . |
|---|---|---|---|---|---|---|---|---|
| (ms) . | duration (ms) . | (ms) . | ||||||
| 1 | 42 | 92 | 36 | RBBB type | Normal axis | 327–412 | Spontaneous | No |
| 2 | 58 | 105 | 22 | RBBB type | Normal axis | 384 | Spontaneous | No |
| 3 | 52 | 107 | 18 | RBBB type | Normal axis | 494 | Incessant | No |
| 4 | 54 | 90 | 20 | Similar to SR QRS | Normal axis | 404 | Persistent | No |
| 5 | 50 | 100 | 16 | RBBB type | Normal axis | 338 | Atrial or ventricular pacing | Precordial incomplete RBBB type with frontal LAD |
| 6 | 50 | 108 | 10 | Similar to SR QRS | RAD | 300 | Atrial pacing | Precordial incomplete RBBB type with frontal LAD |
| 7 | 54 | 84 | 28 | RBBB type | RAD | 292 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 8 | 55 | 110 | 25 | RBBB type | RAD | 365 | Atrial or ventricular pacing | Precordial complete RBBB type with frontal RAD |
| 9 | 45 | 100 | 16 | RBBB type | RAD | 286 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 10 | 48 | 114 | 28 | Similar to SR QRS | RAD | 410–610 | Incessant | No |
| 11 | 50 | 102 | 22 | RBBB type | RAD | 370 | Spontaneous | No |
| Case no. . | H-VSR . | VT QRS . | H-VVT . | Precordial leads . | Frontal axis . | TCL . | VT induction in electrophysiological lab . | Presence of multiform VTs . |
|---|---|---|---|---|---|---|---|---|
| (ms) . | duration (ms) . | (ms) . | ||||||
| 1 | 42 | 92 | 36 | RBBB type | Normal axis | 327–412 | Spontaneous | No |
| 2 | 58 | 105 | 22 | RBBB type | Normal axis | 384 | Spontaneous | No |
| 3 | 52 | 107 | 18 | RBBB type | Normal axis | 494 | Incessant | No |
| 4 | 54 | 90 | 20 | Similar to SR QRS | Normal axis | 404 | Persistent | No |
| 5 | 50 | 100 | 16 | RBBB type | Normal axis | 338 | Atrial or ventricular pacing | Precordial incomplete RBBB type with frontal LAD |
| 6 | 50 | 108 | 10 | Similar to SR QRS | RAD | 300 | Atrial pacing | Precordial incomplete RBBB type with frontal LAD |
| 7 | 54 | 84 | 28 | RBBB type | RAD | 292 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 8 | 55 | 110 | 25 | RBBB type | RAD | 365 | Atrial or ventricular pacing | Precordial complete RBBB type with frontal RAD |
| 9 | 45 | 100 | 16 | RBBB type | RAD | 286 | Ventricular pacing | Precordial similar to SR QRS with frontal LAD |
| 10 | 48 | 114 | 28 | Similar to SR QRS | RAD | 410–610 | Incessant | No |
| 11 | 50 | 102 | 22 | RBBB type | RAD | 370 | Spontaneous | No |
LAD, left axis deviation; RAD, right axis deviation; RBBB, right bundle branch block; SR, sinus rhythm; TCL, tachycardia cycle length; VT, ventricular tachycardia.
| Case no. . | Ablation target . | PP-QRS during VT at ablation target . | Ablation sessions . | SR ECG change after ablation . |
|---|---|---|---|---|
| 1 | Earliest PP during VT | 35 | 2 | More RAD (small S in lead I, deep S in lead aVL, small Q in leads II, III and aVF)R amplitude reduction in leads V2-V3 |
| 2 | Earliest PP during VT | 37 | 1 | No change |
| 3 | Earliest PP during VT | 30 | 1 | R amplitude reduction in leads V2-V3 |
| 4 | DP during VT | 44 | 1 | R amplitude reduction in leads V2-V3 |
| 5 | Earliest PP during VT | 40 | 1 | No change |
| 6 | Earliest PP during VT | 32 | 1 | Incomplete LBBB* |
| 7 | Earliest PP during VT | 40 | 1 | LAHB, R amplitude reduction in leads V2-V4* |
| 8 | Earliest PP during VT | 33 | 1 | No change |
| 9 | Earliest PP during VT | 30 | 1 | No change* |
| 10 | No ablation | 34 | 0 | NA |
| 11 | Earliest PP during VT | 38 | 1 | No change* |
| Case no. . | Ablation target . | PP-QRS during VT at ablation target . | Ablation sessions . | SR ECG change after ablation . |
|---|---|---|---|---|
| 1 | Earliest PP during VT | 35 | 2 | More RAD (small S in lead I, deep S in lead aVL, small Q in leads II, III and aVF)R amplitude reduction in leads V2-V3 |
| 2 | Earliest PP during VT | 37 | 1 | No change |
| 3 | Earliest PP during VT | 30 | 1 | R amplitude reduction in leads V2-V3 |
| 4 | DP during VT | 44 | 1 | R amplitude reduction in leads V2-V3 |
| 5 | Earliest PP during VT | 40 | 1 | No change |
| 6 | Earliest PP during VT | 32 | 1 | Incomplete LBBB* |
| 7 | Earliest PP during VT | 40 | 1 | LAHB, R amplitude reduction in leads V2-V4* |
| 8 | Earliest PP during VT | 33 | 1 | No change |
| 9 | Earliest PP during VT | 30 | 1 | No change* |
| 10 | No ablation | 34 | 0 | NA |
| 11 | Earliest PP during VT | 38 | 1 | No change* |
A reduction of R amplitude reduction in leads V2-V3 and late precordial R/S transition at lead V4 had already been present before LUS-VT ablation.
DP, diastolic potential; LBBB, incomplete left bundle branch block; LAD, left axis deviation; LAHB, left anterior hemiblock; NA, not associated; PP, Purkinje potential; SR, sinus rhythm; VT, ventricular tachycardia.
| Case no. . | Ablation target . | PP-QRS during VT at ablation target . | Ablation sessions . | SR ECG change after ablation . |
|---|---|---|---|---|
| 1 | Earliest PP during VT | 35 | 2 | More RAD (small S in lead I, deep S in lead aVL, small Q in leads II, III and aVF)R amplitude reduction in leads V2-V3 |
| 2 | Earliest PP during VT | 37 | 1 | No change |
| 3 | Earliest PP during VT | 30 | 1 | R amplitude reduction in leads V2-V3 |
| 4 | DP during VT | 44 | 1 | R amplitude reduction in leads V2-V3 |
| 5 | Earliest PP during VT | 40 | 1 | No change |
| 6 | Earliest PP during VT | 32 | 1 | Incomplete LBBB* |
| 7 | Earliest PP during VT | 40 | 1 | LAHB, R amplitude reduction in leads V2-V4* |
| 8 | Earliest PP during VT | 33 | 1 | No change |
| 9 | Earliest PP during VT | 30 | 1 | No change* |
| 10 | No ablation | 34 | 0 | NA |
| 11 | Earliest PP during VT | 38 | 1 | No change* |
| Case no. . | Ablation target . | PP-QRS during VT at ablation target . | Ablation sessions . | SR ECG change after ablation . |
|---|---|---|---|---|
| 1 | Earliest PP during VT | 35 | 2 | More RAD (small S in lead I, deep S in lead aVL, small Q in leads II, III and aVF)R amplitude reduction in leads V2-V3 |
| 2 | Earliest PP during VT | 37 | 1 | No change |
| 3 | Earliest PP during VT | 30 | 1 | R amplitude reduction in leads V2-V3 |
| 4 | DP during VT | 44 | 1 | R amplitude reduction in leads V2-V3 |
| 5 | Earliest PP during VT | 40 | 1 | No change |
| 6 | Earliest PP during VT | 32 | 1 | Incomplete LBBB* |
| 7 | Earliest PP during VT | 40 | 1 | LAHB, R amplitude reduction in leads V2-V4* |
| 8 | Earliest PP during VT | 33 | 1 | No change |
| 9 | Earliest PP during VT | 30 | 1 | No change* |
| 10 | No ablation | 34 | 0 | NA |
| 11 | Earliest PP during VT | 38 | 1 | No change* |
A reduction of R amplitude reduction in leads V2-V3 and late precordial R/S transition at lead V4 had already been present before LUS-VT ablation.
DP, diastolic potential; LBBB, incomplete left bundle branch block; LAD, left axis deviation; LAHB, left anterior hemiblock; NA, not associated; PP, Purkinje potential; SR, sinus rhythm; VT, ventricular tachycardia.
When mapping the left ventricle, PPs with a sharp, high-frequency and low amplitude potentials were found at the left septal area in all patients. The distribution of these presystolic sharp potentials was rather diffuse than concentric. Thus, there was no clear landmarks of individual fascicles and anterior or posterior fasciles can only be arbitrarily defined. The activation of presystolic PP was centrifugal during VT with the earliest PP recorded at the left bundle branch (Figure 2). The earliest PP to QRS interval averaged 35.7 ± 4.5 ms (range 30–44 ms).
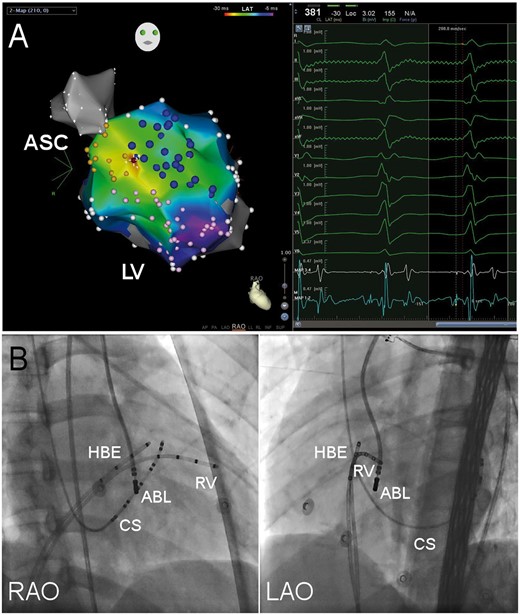
Typical target of LUS-VT. (A) Isochronal map of presystolic PP during VT. Yellow tags stand for His bundle and left bundle brand, pink tags left posterior fascicles and blue tags left anterior fascicles. Early presystolic PP electrograms are depicted in red, late in purple and electrograms in between by shades of color as shown in the reference color bar. White tags are anatomical tags which stand for sites without presystolic PP. The site with the earliest PP recorded was the successful ablation target (red tag) and the local electrogram is shown in the right window. MAP 1-2 and 3-4 stands for the distal and proximal electrodes of ablation catheter. ASC, aortic sinus cusp; LV, left ventricle; RAO, right anterior oblique. (B) Fluoroscopy of left upper septal target. ABL, ablation catheter; CS, coronary sinus catheter; HBE, His bundle electrogram recording catheter; LAO, left anterior oblique projection; RAO, right anterior oblique projection; and RV, right ventricle catheter.
Of note, 5 patients had a second VT morphology induced after left ventricular endocardial mapping at the proximal fascicles, especially at the anterior proximal fascicle (1 in Group 1 vs. 4 in Group 2, P = 0.14). The second VT morphology presented as left deviation of frontal axis, which was consistent with a relatively slower conduction through the anterior fascicle (Figure 3).
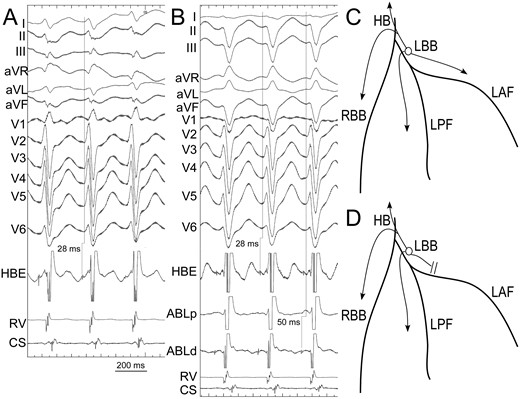
VT ECG change during mapping. In the beginning of the procedure, the VT showed precordial RBBB type and right axis deviation of frontal axis (Panel A). The H-V interval during VT was 28 ms. Due to simultaneous conduction through fascicles (Panel C), the VT QRS width was narrow. After inadvertent block of anterior fascicle during mapping (Panel D), the VT QRS showed left deviation of frontal axis and a precordial morphology similar to sinus QRS but with a deeper S wave in lead V6 (Panel B). The H-V interval was still 28 ms since the retrograde conduction of the earliest PP to His remained unchanged. CS, coronary sinus electrogram; HBE, His bundle electrogram; RV, right ventricle electrogram; ABL, ablation catheter; LAF, left anterior fascicle; LPF, left posterior fascicle; LBB, left bundle branch; HB, His bundle; Right bundle branch.
Clear DPs with high frequency and low amplitude were found in only one patient (Case 4). Different from the findings based on macrorrentry theory,6,8 the area with DP recorded coincided with the site with the earliest PP and both were confined to a small area (Figure 4).
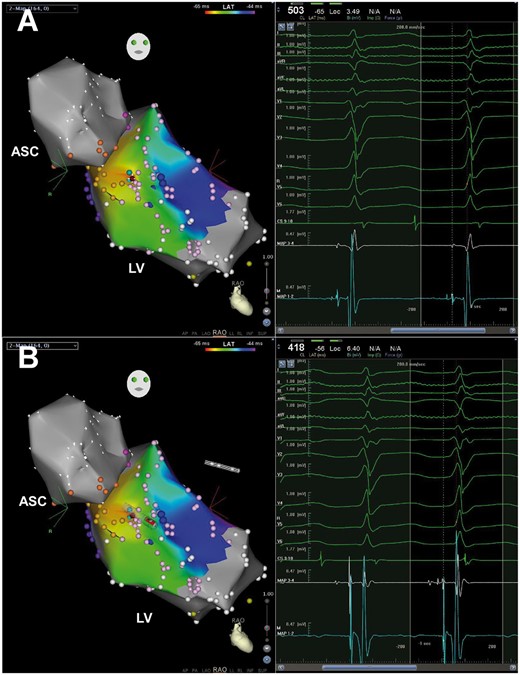
DP in Case 4. Yellow tags stand for His bundle and left bundle brand, pink tags posterior and anterior fascicles. The site with both DP and the earliest PP recorded was the successful ablation target (red tag) and the local electrogram is shown in the right window of Panel A. When advancing the ablation catheter, only the proximal electrodes can record the DP (Panel B). Abbreviations as Figure 2.
Entrainment attempts at the upper septum were not successful, due to unstable capture of local PP or frequent termination or alteration of tachycardia.
RFCA
One patient declined RFCA due to potential risk of atrioventricular block. The other 10 patients were successfully managed by ablation. Successful RFCA target were sites with the earliest PP during VT (9 cases), and sites with DP (1 case) (Table 3). In Cases 3, VT was bumped at the site with the earliest PP during VT and became noninducible.
After ablation, 2 cases (10%) developed new left anterior hemiblock or incomplete LBBB. Minor changes, such as greater right axis deviation and decreased R wave amplitude in leads V2–V4 were resulted in 4 cases, while no changes was found in the remaining 5 patients (Table 3). Of note, the sinus rhythm ECG of Case 3, whose VT was bumped and was no longer inducible, did not change after the bump, but showed reduced R wave amplitude in leads V2 and V3 after a single RF application (Figure 5).

Mild sinus rhythm QRS change after left upper septal ablation. (A) Sinus rhythm ECG before ablation. (B) Sinus rhythm ECG after ablation.
Follow-up
No acute complications occurred in all patients except that one patient developed pseudoaneurysm of the right femoral artery at the site of puncture which resolved with conservative treatment. During a median follow-up period of 3.2 (range 1.0–12.7) years, none of the patients receiving ablation experienced recurrence. Only one patient reported 2 episodes of paroxysmal tachycardia which lasted as short as 3–5 min within the first year after the ablation, but no evidence of VT recurrence could be confirmed either by ECG or by Holter monitoring. The patient who declined catheter ablation had his symptom partially controlled by oral verapamil.
Discussion
Clinical characteristics
In our cohort, the majority of the patients were male and young, and they all had a benign history without syncope or hemodynamic instability during VT. These clinical characteristics also kept consistent throughout prior reports on LUS-VT2–8 and on LPF-VT.11–13 A significant portion (6/11) of LUS-VT patient had prior ablation targeting common type LPF-VT.6,8 Moreover, 3 out of the 6 patients with LPF-VT ablation history had LUS-VT occurrence immediately or shortly after the ablation session targeting LPF-VT, suggesting that the LUS-VT coexisted with or share identical VT substrate with LPF-VT in some patients.
In contrast to LPF-VT, LUS-VT more often manifested as incessancy or persistency and tended to occur spontaneously during the electrophysiological study, which was observed in our cohort and in prior reports.2,4,5,7,8 Higher VT burden could even lead to depressed left ventricular function.4,5 Incomplete ablation lesion formation, leading to slower local fascicular conduction, could partly explained the incessant tendency in patients with prior ablation, but the mechanism of initial presentation as incessant VT in patients without ablation history remained to be studied.
ECG features
In our study, all patients shared a common sinus rhythm ECG feature, i.e. S wave in lead I and aVL and small Q wave and tall R wave in inferior leads, which point to rightward shift of frontal axis and slower conduction through left posterior fascicle and/or midseptal fascicle. This ECG feature was newly developed in patient with prior LPF-VT ablation history, supporting iatrogenic conduction disturbance may be proarrhythmic. However, the presence of this feature, albeit in milder form (i.e. narrower sinus rhythm QRS width and absence of left posterior hemiblock), in ablation-naïve patient could only be explained by preexisting diseased fascicle, which might serve as the substrate of VT.
Because of penetration into the proximal left His-Purkinje system at upper septum, quicker retrograde conduction to His, and similar activation sequence through the His-Purkinje system, the QRS morphology of LUS-VT was similar to that of sinus rhythm or mimicked incomplete RBBB aberrancy. Notably, unlike the normal frontal axis in patients without LPF-VT history, the frontal axes were all right deviation in patients with prior LPF-VT ablation, which was consistent with slower conduction through the left posterior fascicle during VT due to prior ablation.
Multiform left anterior and posterior fascicular VTs was initially reported by Sung et al.14 and by Suzuki et al.15 LUS-VT could also presented as multiple VT morphologies.6 In our case series, 7 patients (50%) presented as multiple VT morphologies in the electrophysiological study. However, the observations that H-VVT of different VT morphologies in the same patient were identical and a single target for ablation rendered all VTs non-inducible, which strongly suggested that the upper turn-around point of the reentrant circuit or the tachycardia foci was anatomically located in the same region with different exit due to the mechanical/previous ablation lesions and functional conduction block in some part of left Purkinje conduction system. This finding also stressed importance of targeting the critical site of the VT, i.e. the earliest PP during VT.
Mapping and ablation
The mechanism of LUS-VT was still controversial. Furtherly, the disease might be heterogeneous involving different mechansims in different populations, since targeting DP during VT based on macro-reentry theory6,8 and targeting the earliest PP during VT based on focal theory2,5,7 both yielded favorable result. In the present study using three dimensional mapping instead of multipolar catheter mapping, clear DP could not be universally recorded. In the only patient with clear DP recorded, the DP was confined to a small area and coincided with the recording of the earliest PP. Therefore, no macro-reentrant circuit could be determined in any case. These findings argued for a mechasism of microreentry or reentry in a very small area within the fascicles where reentrant circuit was unmappable. We chose the earliest PP during VT as the ablation target in most cases, which had a favorable acute success and a long-term follow-up result, mainly based on the following reasons: (1) The earliest PP during VT was a critical site of the VT, which was deemed as an essential part of the reentry (i.e. the upper turnaround site) in the macroreentry theory8 or the site of origin in the focal theory.7 It clearly identify the site where activation propagated from the diseased fascile to the normal fascicle, regardless of the mechanism involved. (2)Both distal DP recorded using mutipolar catheter8 and PP recorded along the His-Purkinje system other than the earliest one16 could be the bystander of the reentrant circuit. Targeting these sites might fail to abolish the circuit.
Injury of left His-Purkinje system
In this retrospective study, out of 10 patients whose VT were ablated in 11 sessions in our center, 1 patient had newly developed left anterior hemiblock and another 1 patient developed incomplete LBBB. While in the remaining patients, no change of sinus rhythm ECG was found in 5 and R amplitude reduction in anteroseptal precordial leads (V2–V3) was observed in 3. This result pointed to the facts that (1) directly targeting proximal fascicles carried a higher risk of transecting the main trunk of fascicles, which was well acknowledged by other researchers,2,7 and even discouraged some from ablation attempt4; (2) ablation destructing a diseased portion of the fascicle, would suffice for successful elimination of LUS-VT, while a considerable portion of the fascicles remained intact. Therefore, normal antegrade conduction through His-Purkinje system can be preserved in most patients and only mild ECG change would be observed. Thus, we assumed that an effective and safe ablation would ideally be a ‘modification’ of the fascicles, weighing destruction of the diseased fascicle against total abolishment of local fascicular conduction.
Limitations
Our study also had some limitations as follows: (1) A multipolar electrode catheter was not placed in the left ventricle. However, three dimensional electroanatomical mapping was used instead to reveal the activation sequence. (2) Verapamil responsiveness was not available in all cases, which may help differentiate the mechanism of the VT.
Conclusions
LUS-VT presented as narrow QRS complex tachycardia. Some LUS-VTs occurred after ablation targeting left posterior fascicular VT. The VTs can be managed successfully by focal ablation at the left upper septum with a mild risk of fascicular injury.
Conflict of interest: none declared.
Funding
This work was supported by a grant from National Natural Science Foundation of China (81270242).
References
Chen M, Yang B, Zou J, Shan Q, Chen C, Xu D et al . Non-contact mapping and linear ablation of the left posterior fascicle during sinus rhythm in the treatment of idiopathic left ventricular tachycardia. Europace 2005;7:138–44.
Ouyang F, Cappato R, Ernst S, Goya M, Volkmer M, Hebe J et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macroreentry within the purkinje network. Circulation 2002;105:462–9.
Author notes
Xiao-Gang Guo and Xu Liu contributed equally to the study.



