-
PDF
- Split View
-
Views
-
Cite
Cite
Fabrizio Drago, Irma Battipaglia, Mario Salvatore Russo, Romolo Remoli, Vincenzo Pazzano, Gino Grifoni, Greta Allegretti, Massimo Stefano Silvetti, Voltage gradient mapping and electrophysiologically guided cryoablation in children with AVNRT, EP Europace, Volume 20, Issue 4, April 2018, Pages 665–672, https://doi.org/10.1093/europace/eux021
Close - Share Icon Share
Abstract
Recently, voltage gradient mapping of Koch’s triangle to find low-voltage connections, or ‘voltage bridges’, corresponding to the anatomic position of the slow pathway, has been introduced as a method to ablate atrioventricular nodal reentry tachycardia (AVNRT) in children. Thus, we aimed to assess the effectiveness of voltage mapping of Koch’s triangle, combined with the search for the slow potential signal in ‘low-voltage bridges’, to guide cryoablation of AVNRT in children.
From June 2015 to May 2016, 35 consecutive paediatric patients (mean age 12.1 ± 4.5 years) underwent 3D-guided cryoablation of AVNRT at our Institution. Fifteen children were enrolled as control group (mean age 14 ± 4 years). A voltage gradient mapping of Koch’s triangle was obtained in all patients, showing low-voltage connections in all children with AVNRT but not in controls. Prior to performing cryoablation, we looked for the typical ‘hump and spike’ electrogram, generally considered to be representative of slow pathway potential within a low-voltage bridge. In all patients the ‘hump and spike’ electrogram was found inside bridges of low voltage. Focal or high-density linear lesions, extended or not, were delivered guided by low-voltage bridge visualization. Acute success rate was 100%, and no recurrence was reported at a mean follow-up of 8 ± 3 months.
Voltage gradient mapping of Koch’s triangle, combined with the search for the slow potential signal in low-voltage bridges, is effective in guiding cryoablation of AVNRT in paediatric patients, with a complete acute success rate and no AVNRT recurrences at mid-term follow-up.
What’s new?
Using an innovative 3D ablation strategy guided by both voltage gradient mapping and the electrophysiological target signal, top results were achieved in cryoablation of AVNRT in children.
Use of a paediatric control group to verify the absence of low-voltage bridges also in patients without AV nodal duality.
The identification of low-voltage bridges within Koch’s triangle allowed to achieve mid-term success also in patients with non-inducible AVNRT.
Introduction
Atrioventricular nodal reentry tachycardia (AVNRT) is one of the most frequent tachyarrhythmias in the paediatric population.1
At present, transcatheter cryoablation is a well-established technique for the successful treatment of AVNRT in children, with no complications reported.2–5 However, conduction in the slow pathway may recur after an acute successful procedure, with a recurrence rate of ∼10% at long-term follow-up.4,6–8
Several protocols for cryoenergy delivery, including bonus cryoapplication,3 linear lesion cryoablation,9 freeze-thaw-freeze cryoablation,10 and 8-mm tip cryoablation,11,12 have been proposed to increase acute and long-term success rates of cryoablation, but AVNRT recurrences still represent a challenging issue.
Follow-up studies on AVNRT cryoablation in paediatric patients have demonstrated that sex, number of cryomappings or ablations, catheter tip size, ablation energy source and ablation endpoints, such as tachycardia elimination with or without persistence of single echo beats, were not significant predictors of recurrences.6,13 Furthermore, a very recent study showed that AVNRT can also recur late after the procedure.8
Recently, our group demonstrated a relation between cryoablation outcome and type of lesions necessary to eliminate the tachycardia.14 In this study, we accurately described the type of atrial electrogram (‘hump and spike’ morphology) used as a target of our cryoablation attempts with an acute success rate of 98.5% and a total recurrence rate of 13.5% (only 5.3% for the focal lesion). Furthermore, in our personal experience, a younger age and a successful cryoablation of the slow pathway obtained with an electrophysiologically guided focal cryoablation seem to be associated with a better outcome.7,14
Recently, the use of 3D voltage gradient mapping has been described as a means to visualize the slow pathway in adults and children with AVNRT.15,16 The results of these studies have introduced a new ablation strategy that can help to overcome the difficulties related to the individual variability in Koch’s triangle size and electrogram distribution.
In this study, we aimed to assess the acute and long-term effectiveness of cryoablation of the slow pathway in paediatric patients affected by AVNRT, combining voltage gradient mapping and the search for the typical atrial electrogram at the site of slow pathway potential.
Methods
Patient population
From June 2015 to May 2016, 35 consecutive paediatric patients [13 males (37%), mean age 12.1 ± 4.5 years] underwent 3D-guided transcatheter cryoablation of AVNRT at our Institution.
Thirty-two patients had structurally normal hearts, whereas congenital heart disease was present in three patients (two patients with a device implanted for ostium secundum atrial septal defect and one patient treated for aortic coarctation).
Thirty-one children had previously undergone a transoesophageal electrophysiological study confirming the diagnosis of AVNRT. Four patients had already been unsuccessfully treated, three with cryoenergy, and one with radiofrequency.
Fifteen patients (6 males; mean age 14 ± 4 years) with palpitations underwent an endocavitary electrophysiological study, resulted negative for AVNRT induction or atrioventricular (AV) nodal duality, and were enrolled as control group to verify the absence of low-voltage bridges.
Electrophysiological study
All patients underwent an electrophysiological study before cryoablation to identify dual physiology of the AV node and/or inducibility of AVNRT.
Written informed consent was obtained from parents of all patients (or patients themselves for those aged ≥ 18 years) prior to the procedure.
The procedure was performed under general anaesthesia, induced with sevoflurane or propofol and maintained with sevoflurane. A thermal mattress was used to maintain normal body temperature. All antiarrhythmic drugs were discontinued for at least five half-lives before the procedure to ensure complete pharmacological washout.
Surface electrocardiogram (ECG) leads and endocardial potentials (EGM) were recorded and stored on a multichannel recorder (Bard Electrophysiology, Billerica, MA, USA). The bipolar bandwidth filter was set at a range of 30–300 Hz.
Atrial or ventricular single, double, and triple premature extrastimuli, as well as incremental pacing, were used to induce sustained ventricular tachycardia (SVT). The same stimulation protocol was repeated under isoproterenol infusion (0.01–0.04 mcg/kg/min in incremental doses) if SVT was not inducible at baseline.
3D electroanatomical mapping
The EnSite Velocity was used as electroanatomical mapping system.
Details on catheter positioning into the heart and on geometric reconstruction of the right atrium have already been described elsewhere.14,17 Briefly, a quadripolar catheter (Inquiry or Livewire, St Jude Medical, St Paul, MN, USA) was inserted into a femoral vein and advanced through the inferior vena cava toward the right atrium. Then, the catheter was gently moved to acquire geometric reconstruction of this heart chamber and to create voltage gradient maps. A second quadripolar catheter was positioned in the His bundle region via a femoral vein. The catheter position was shadowed on the EnSite Velocity System map to provide a constant marker for the His bundle.
The creation of voltage maps has been previously validated.18 The peak-to-peak amplitude of bipolar atrial potentials was recorded, and voltage at each site was used to generate a 3D colour coded endocardial voltage map using a graded colour scale. Very high-density voltage maps were created for the atrial septum within Koch’s triangle. Interpolation was set at 10 mm, and the interior/exterior projection was set at 7 mm. The voltage colour bar was individually adjusted for each patient in order to highlight a low-voltage bridge, if present, starting from a voltage range of 0.1–2 mV. Voltage data below the lowest value were shown as grey, while those above the upper limit as purple.15 The values between the two thresholds were displayed as green, yellow and red. Both high- and low-voltage values were modified until a voltage bridge was evident. This adjustment was necessary because of voltage variability in each patient.
As already described in the literature,15,16 bridges were classified as follows:
Type I low-voltage bridge was represented by a clear, rather large area of low voltage within Koch’s triangle between the coronary sinus ostium and the AV node (Figure 1A);
Type II low-voltage bridge consisted in a narrow corridor made of low-voltage points between adjacent normal-voltage regions (Figure 1B).
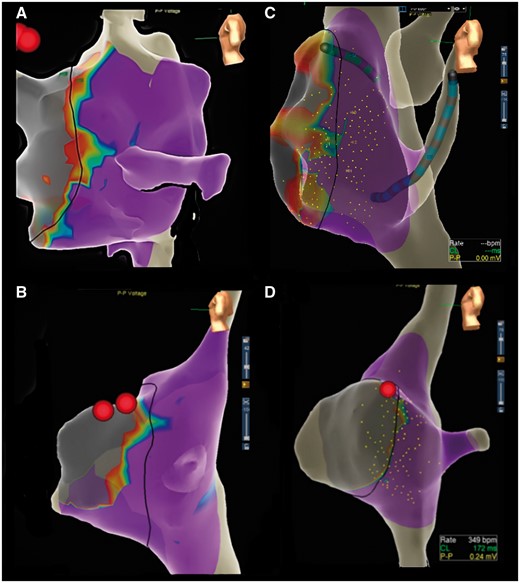
(A) Type I low-voltage bridges at voltage gradient mapping of Koch’s triangle. (B) Type II low-voltage bridges at voltage gradient mapping of Koch’s triangle. (C) A ‘double bridge’ case, with a low-voltage bridge extension in a mid-septal location and another appendage in the lower part of Koch’s triangle, at the coronary sinus ostium level. (D) Voltage gradient map of Koch’s triangle in a control patient.
During mapping of the atrial septum, the peculiar ‘hump and spike’ electrogram pattern (the slow and low atrial electrogram immediately followed by a sharp and high-voltage component) was searched and its position was analyzed in relation to the area of the low-voltage bridge. Although this signal is not yet universally accepted as slow pathway potential, it was used as a target for cryoablation as previously described in detail.14
Cryoablation
Our protocol of cryoablation has been described previously in.6,7,14,17 Briefly, cryoablation was performed in patients with: (i) inducible AVNRT; (ii) an atrial-His (AH) jump clearly diagnostic of dual AV nodal physiology (defined as a sudden prolongation of the AH interval of >50 ms when shortening the cycle length of atrial pacing or the coupling interval of the atrial extra stimulus by 10 ms), and (iii) a negative electrophysiological study for SVT or AH jump inducibility, if a reciprocating AVNRT with a ventriculo-atrial interval <70 ms was clearly documented on a previous transoesophageal electrophysiological study. A 7Fr cryoablation catheter (Freezor, Medtronic Cryocath LP, Montreal, Quebec, Canada, 4–8 mm tip size catheters) was used for cryomapping and ablation.
A steerable 7Fr catheter (Freezor, Medtronic Cryocath LP, Montreal, Quebec, Canada) with a 4- or 6–8-mm tip electrode was preferably used in patients <30 or >30 kg, respectively.
‘Fixed cryomapping’, performed by reducing the tip temperature to −30 °C for a maximum of 60 s, or ‘step-by-step cryomapping’, performed by progressively decreasing the tip temperature by 10 °C every 5 s from −30 to −70 °C, was attempted at the discretion of the operator on the specific site of the low-voltage bridge, carefully identified through the technique described earlier. During cryomapping, repeat extrastimulus testing, or ramps were performed, using the diagnostic catheter placed in the high right atrium, to assess tachycardia inducibility or absence of AV nodal slow pathway conduction. If cryomapping was effective (i.e. no AH jump, no reentry beats, no inducibility of tachyarrhythmia), the tip temperature was further reduced to create a permanent lesion.
Our protocol of cryoablation has already been described in.7,14 Briefly, focal cryoablation was performed using the single lesion technique followed by one or more cryobonus in the same spot to consolidate and slightly enlarge the cryolesion. After each delivered cryoablation, repeat extrastimulus testing or ramps were conducted to re-assess tachycardia inducibility or absence of slow pathway conduction. When it appeared to be effective, repeat extrastimulus testing or ramps were conducted for 30 min to confirm the acute success.
In case of recurrent tachycardia or slow pathway conduction after an initially successful cryoablation, a 3D-guided ‘high-density linear lesion’ (HDLL), recently described in (13), was carried out delivering multiple overlapping cryolesions from the ventricular to the atrial side of the tricuspid annulus.
Cryoablation was performed at the site of the low-voltage bridge, taking into account the extension of the bridge and the presence of the ‘hump and spike’ electrogram (Figure 2).
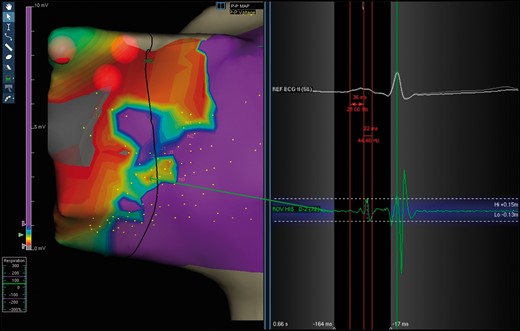
Typical ‘hump-and-spike’ electrogram (length and amplitude) recorded inside a type I bridge.
The types of cryolesions were defined as follows (Figure 3):
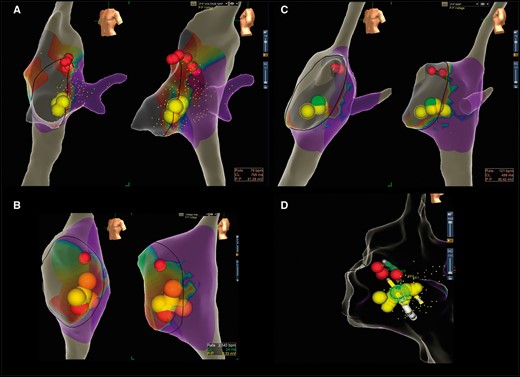
The different types of cryolesions performed depending on the size of bridges: (A) focal lesion; (B) extended focal lesion; (C) HDLL; (D) extended HDLL.
Focal lesion: single lesion followed by one or more cryoenergy applications (cryobonus) in the same spot to consolidate and slightly enlarge the successful cryolesion.
Extended focal lesion: single lesion followed by one or more cryoenergy applications delivered slightly anteriorly and posteriorly to the first successful or apparently successful lesion.
HDLL: multiple overlapping cryolesions from the ventricular to the atrial side of the tricuspid annulus (with a final cryolesion at least 10 mm long, obtaining an overlapping of 3D spheres/lesions of at least half of their diameter or at least 2 mm).
Extended HDLL: produced when extending the lesion anteriorly and posteriorly to the original HDLL to achieve successful cryoablation of the arrhythmia.
Cryoablation was performed also in patients without inducible AVNRT or dual AV nodal conduction during the procedure but with previously documented AVNRT. In this case, cryoablation was guided only by the voltage mapping bridge and the typical ‘hump and spike’ electrogram.
Endpoints and follow-up
AVNRT recurrence was defined as ECG-documented tachycardia, or resumption of pre-procedural clinical symptoms with evidence of arrhythmia inducibility by transoesophageal atrial pacing. Acute and long-term success rates, defined as absence of recurrences, were efficacy primary endpoints. Clinical examination and standard ECG were performed in all patients 24 h and 1 month after the procedure. Twenty-four-hour ECG Holter monitoring and exercise stress test were also performed after 6 months.
Statistical analysis
The unpaired t-test was used for group comparisons of procedural characteristics and individual parameters. The significance level was set at P ≤ 0.05. GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all descriptive and inferential statistical analyses.
Results
Demographic and procedural characteristics of the study population are reported in Table 1.
Demographic and procedural characteristics of patients and controls who underwent electrophysiological study and voltage gradient mapping ± cryoablation of AVNRT and Voltage gradient mapping characteristics obtained using the 3D mapping system EnSite Velocity
| . | Patients . | Controls . | P . |
|---|---|---|---|
| Number of patients | 35 | 15 | – |
| Age (years) | 12.1 ± 4.5 (range 5–31) | 14 ±4 (range 7–8) | 0.13 |
| Sex | M 13 (37%) F 22 (63%) | M 6 (40%) F 9 (60%) | 0.85 |
| Weight (kg) | 46.5 ± 15.7 (range 16–86) | 59.8±24.3 (range 21–135) | 0.02 |
| Tip size (mm) | 4 mm (5 pts) 6 mm (29 pts) 8 mm (1 pt) | – | – |
| Number of cryo-applications | 4.5 ± 1.2 (range 2–7) | – | – |
| Type of lesion | Focal 19 Extended focal 7 HDLL 6 Extended HDLL 4 | – | – |
| Acute success | 35 pts (100%) | – | – |
| Mean follow- up (months) | 8.0 ± 3.0 (range 3–13) | – | – |
| Recurrence rate | 0% | – | – |
| No. points voltage map/No. points collected | 115.0 ± 49.5/281.4 ± 138.4 | 105±19/240±56 | 0.2 |
| High voltage value (mV) | 1.1 ± 0.45 (range 0.37–2.0) | 1.35±0.4 (range 0.56-2) | 0.04 |
| Low voltage value (mV) | 0.29 ± 0.19 (range 0–0.76) | 0.33±0.3 (range 0-0.9) | 0.09 |
| Type of bridge | Type I (25 pts) Type II (10 pts) | – | – |
| . | Patients . | Controls . | P . |
|---|---|---|---|
| Number of patients | 35 | 15 | – |
| Age (years) | 12.1 ± 4.5 (range 5–31) | 14 ±4 (range 7–8) | 0.13 |
| Sex | M 13 (37%) F 22 (63%) | M 6 (40%) F 9 (60%) | 0.85 |
| Weight (kg) | 46.5 ± 15.7 (range 16–86) | 59.8±24.3 (range 21–135) | 0.02 |
| Tip size (mm) | 4 mm (5 pts) 6 mm (29 pts) 8 mm (1 pt) | – | – |
| Number of cryo-applications | 4.5 ± 1.2 (range 2–7) | – | – |
| Type of lesion | Focal 19 Extended focal 7 HDLL 6 Extended HDLL 4 | – | – |
| Acute success | 35 pts (100%) | – | – |
| Mean follow- up (months) | 8.0 ± 3.0 (range 3–13) | – | – |
| Recurrence rate | 0% | – | – |
| No. points voltage map/No. points collected | 115.0 ± 49.5/281.4 ± 138.4 | 105±19/240±56 | 0.2 |
| High voltage value (mV) | 1.1 ± 0.45 (range 0.37–2.0) | 1.35±0.4 (range 0.56-2) | 0.04 |
| Low voltage value (mV) | 0.29 ± 0.19 (range 0–0.76) | 0.33±0.3 (range 0-0.9) | 0.09 |
| Type of bridge | Type I (25 pts) Type II (10 pts) | – | – |
HDLL, high-density linear lesion; AVNRT, atrioventricular nodal reentry tachycardia.
Demographic and procedural characteristics of patients and controls who underwent electrophysiological study and voltage gradient mapping ± cryoablation of AVNRT and Voltage gradient mapping characteristics obtained using the 3D mapping system EnSite Velocity
| . | Patients . | Controls . | P . |
|---|---|---|---|
| Number of patients | 35 | 15 | – |
| Age (years) | 12.1 ± 4.5 (range 5–31) | 14 ±4 (range 7–8) | 0.13 |
| Sex | M 13 (37%) F 22 (63%) | M 6 (40%) F 9 (60%) | 0.85 |
| Weight (kg) | 46.5 ± 15.7 (range 16–86) | 59.8±24.3 (range 21–135) | 0.02 |
| Tip size (mm) | 4 mm (5 pts) 6 mm (29 pts) 8 mm (1 pt) | – | – |
| Number of cryo-applications | 4.5 ± 1.2 (range 2–7) | – | – |
| Type of lesion | Focal 19 Extended focal 7 HDLL 6 Extended HDLL 4 | – | – |
| Acute success | 35 pts (100%) | – | – |
| Mean follow- up (months) | 8.0 ± 3.0 (range 3–13) | – | – |
| Recurrence rate | 0% | – | – |
| No. points voltage map/No. points collected | 115.0 ± 49.5/281.4 ± 138.4 | 105±19/240±56 | 0.2 |
| High voltage value (mV) | 1.1 ± 0.45 (range 0.37–2.0) | 1.35±0.4 (range 0.56-2) | 0.04 |
| Low voltage value (mV) | 0.29 ± 0.19 (range 0–0.76) | 0.33±0.3 (range 0-0.9) | 0.09 |
| Type of bridge | Type I (25 pts) Type II (10 pts) | – | – |
| . | Patients . | Controls . | P . |
|---|---|---|---|
| Number of patients | 35 | 15 | – |
| Age (years) | 12.1 ± 4.5 (range 5–31) | 14 ±4 (range 7–8) | 0.13 |
| Sex | M 13 (37%) F 22 (63%) | M 6 (40%) F 9 (60%) | 0.85 |
| Weight (kg) | 46.5 ± 15.7 (range 16–86) | 59.8±24.3 (range 21–135) | 0.02 |
| Tip size (mm) | 4 mm (5 pts) 6 mm (29 pts) 8 mm (1 pt) | – | – |
| Number of cryo-applications | 4.5 ± 1.2 (range 2–7) | – | – |
| Type of lesion | Focal 19 Extended focal 7 HDLL 6 Extended HDLL 4 | – | – |
| Acute success | 35 pts (100%) | – | – |
| Mean follow- up (months) | 8.0 ± 3.0 (range 3–13) | – | – |
| Recurrence rate | 0% | – | – |
| No. points voltage map/No. points collected | 115.0 ± 49.5/281.4 ± 138.4 | 105±19/240±56 | 0.2 |
| High voltage value (mV) | 1.1 ± 0.45 (range 0.37–2.0) | 1.35±0.4 (range 0.56-2) | 0.04 |
| Low voltage value (mV) | 0.29 ± 0.19 (range 0–0.76) | 0.33±0.3 (range 0-0.9) | 0.09 |
| Type of bridge | Type I (25 pts) Type II (10 pts) | – | – |
HDLL, high-density linear lesion; AVNRT, atrioventricular nodal reentry tachycardia.
Thirty-one patients were diagnosed with typical AVNRT by transoesophageal or endocavitary electrophysiological study. Two patients had both typical and atypical AVNRT inducible during the diagnostic test. The day of the procedure two patients had only evidence of AH jump and eight patients had AH jump and echo beats.
Low-voltage bridge
In all patients with inducible AVNRT, the voltage map showed a low-voltage bridge in the region of Koch’s triangle (voltage range 0, 3–1, 0 mV).
A type I bridge was found in 25 patients (71.4%), whereas a type II bridge was found in 10 patients (28.6%) (Figure 1). The characteristics of the voltage gradient maps obtained are described in Table 1.
Interestingly, in one of the two patients with both typical and atypical AVNRT, two low-voltage bridges were found: one in the mid-septal position and the other one in the lower part of Koch’s triangle.
The ‘hump and spike’ signal
The ‘hump and spike’ signal was found in all patients with AVNRT inducibility. The characteristics of this target electrogram are described in Table 2.
| . | 35 patients with AVNRT . |
|---|---|
| Length of local atrial activation (ms) | 31.7 ± 7 (range 19–56) |
| Length of slow pathway potential (ms) | 21.9 ± 5.6 (range 12–33) |
| Amplitude of local atrial activation (mV) | 0.09 ± 0.05 (range 0.05–0.23) |
| Amplitude of slow pathway potential (mV) | 0.45 ± 0.3 (range 0.1–1.52) |
| Atrial/ventricular ratio | 2/3 |
| . | 35 patients with AVNRT . |
|---|---|
| Length of local atrial activation (ms) | 31.7 ± 7 (range 19–56) |
| Length of slow pathway potential (ms) | 21.9 ± 5.6 (range 12–33) |
| Amplitude of local atrial activation (mV) | 0.09 ± 0.05 (range 0.05–0.23) |
| Amplitude of slow pathway potential (mV) | 0.45 ± 0.3 (range 0.1–1.52) |
| Atrial/ventricular ratio | 2/3 |
EGM, electrogram.
| . | 35 patients with AVNRT . |
|---|---|
| Length of local atrial activation (ms) | 31.7 ± 7 (range 19–56) |
| Length of slow pathway potential (ms) | 21.9 ± 5.6 (range 12–33) |
| Amplitude of local atrial activation (mV) | 0.09 ± 0.05 (range 0.05–0.23) |
| Amplitude of slow pathway potential (mV) | 0.45 ± 0.3 (range 0.1–1.52) |
| Atrial/ventricular ratio | 2/3 |
| . | 35 patients with AVNRT . |
|---|---|
| Length of local atrial activation (ms) | 31.7 ± 7 (range 19–56) |
| Length of slow pathway potential (ms) | 21.9 ± 5.6 (range 12–33) |
| Amplitude of local atrial activation (mV) | 0.09 ± 0.05 (range 0.05–0.23) |
| Amplitude of slow pathway potential (mV) | 0.45 ± 0.3 (range 0.1–1.52) |
| Atrial/ventricular ratio | 2/3 |
EGM, electrogram.
In all cases, the low-voltage bridge corresponded to the region of slow pathway and the required electrophysiological signal was found exactly inside this region. In particular, in 12 cases, the ‘hump and spike’ pattern was identified in the lower border of the bridge, in 17 patients the signal was scattered in the low-voltage bridge area, in 5 it was localized in the middle part of the bridge, and only in 1 case the slow pathway signal was identified in the higher portion of the low-voltage area.
Procedural success and recurrences
The procedural acute success rate was 100%. There were no major complications during and after the procedures. In one patient, a minor complication occurred (a femoral artero-venous fistula that resolved spontaneously).
No recurrences occurred during a median follow-up of 8 ± 3 months (range 3–13 months).
Ablation details
In 29 patients, a 6 mm tip cryocatheter was used, whereas a 4 mm tip catheter was used in five cases and an 8 mm tip cryocatheter in one patient only.
The average number of cryoablations delivered was 4.5 ± 1.2 (either in patients with type I or type II low-voltage bridges).
Focal and extended focal cryolesions were used in 19 and 7 patients, respectively, whereas HDLLs and extended HDLLs were applied in 6 and four patients, respectively.
Regarding the two patients with both typical and atypical AVNRT, in one two low-voltage bridges were detected and a focal lesion was created on the first bridge eliminating the typical form, and a HDLL was created on the second bridge eliminating the atypical form. In the other patient, a focal lesion was performed on a type I low-voltage bridge eliminating both tachycardias.
Control group
In seven patients of the control group, a concealed accessory pathway was identified (four anteroseptal, one mid-septal, one posteroseptal, one left lateral).
None of the control patients had evidence of low-voltage bridges (Figure 2).
Discussion
In the last decade, the efficacy of cryoablation treatment of AVNRT in children has continuously improved, reaching an acute success rate of about 97%, with a recurrence rate of 13.7% (range 2.8–23%).4,6–8,11,19,20
Although several cryoablation strategies have been attempted and proposed in the literature to improve long-term outcomes, AVNRT recurrence remains a challenging issue. Furthermore, predictors of long-term efficacy are still under debate and, recently, analysing data from 241 patients with a history of AVNRT ablation (median follow-up 5.9 years), Backhoff et al.8 surprisingly documented that a substantial portion of AVNRT recurrences can also occur late, sometimes even 5 years after ablation.
Recently, we demonstrated that a younger age and a successful cryoablation of the slow pathway obtained with a less aggressive protocol are associated with a better outcome.6,7 Indeed, in our latter study,7 the 3D electrophysiologically guided cryoablation protocol used to eliminate the slow pathway, showed an acute success rate of 98.5% with an AVNRT recurrence rate at long-term follow-up of only 5.2% in patients treated with a focal lesion, compared with 23.3% in patients treated with HDLL cryoablation (P = 0.036). In the same study, the characteristics of the ‘hump and spike’ electrogram used as a target of cryoablation were accurately described.
In the literature, the complexity of the electrograms in the region of Koch’s triangle has been acknowledged and differently interpreted by various authors in the attempt to use them as a target for AVNRT ablation.21 In addition, extension and localization of the slow pathway may vary considerably and, therefore, the search for appropriate electrogram recordings can be very challenging due to the variable anatomy and size of Koch’s triangle.
Recently, Bailin et al.15 described the use of 3D voltage gradient mapping of the right atrium as a means to visualize the slow pathway in the adult population. Voltage values were adjusted until low-voltage bridges were observed within the triangle of Koch. Ablation at the site of low-voltage bridges resulted in loss of slow pathway function and significant changes in the post-ablation voltage map. The slow pathway was identified and successfully ablated in all patients. The majority of patients had a type I low-voltage bridge, with only 8% showing a type II low-voltage bridge.
Following this very innovative experience in adults, Malloy et al.16 evaluated the same technique in 28 paediatric patients with inducible AVNRT. The voltage map gradients were adjusted to identify a ‘bridge’ of low-voltage signals. This bridge was used as a surrogate of the slow pathway to guide cryoablation. Long-term procedural success was achieved in all but two patients (7%), who experienced AVNRT recurrence during follow-up.
The new strategy for cryoablation of AVNRT in paediatric patients adopted in our study consisted in the identification of low-voltage bridges and the search for typical ‘hump and spike’ electrograms. The combination of these two methods allowed us to perform the procedure in a more precise and effective way than with only electrophysiologically guided cryoablation of the slow pathway. In fact, using this new technique, a complete and mid-term success was obtained in all patients at a mean follow-up of 8 ± 3 months.
Interestingly, the ‘hump and spike’ signal was found in all low-voltage bridges, in most cases scattered in the low-voltage bridge area or localized in its inferior border. This finding allowed us to confirm the target of our cryoablation, especially in borderline and unclear cases such as type II low-voltage bridges. Moreover, in larger type I bridges, we started cryoablation at the site of the ‘hump and spike’ potential. Afterwards, in order to consolidate the lesion or in case of only transiently effective ablation, the cryolesion was extended so as to cover the entire low-voltage bridge, under direct visual 3D guide. With this method, extended focal lesions, HDLLs, or extended HDLLs were performed to destroy a well-defined myocardial substrate.
The excellent long-term result of this new approach clarifies the reason why our electrophysiological approach, previously described in,7 had resulted in more recurrences when a HDLL was necessary to obtain the acute success. Probably, in some of these cases, the entire extension of the largest low-voltage bridges was not completely covered by our linear cryolesions,14 making AVNRT easier to recur.
Notably, the efficacy of our combined approach is also confirmed by the absence of recurrences in patients with previously documented AVNRT but no inducible tachycardia during the procedure.
Another important contribution of our study is the absence of low-voltage bridges in control patients without AVNRT inducibility. This strengthens the efficacy of the 3D voltage gradient approach, suggesting that low-voltage bridges can actually identify the exact critical area of the arrhythmic substrate, thus enabling successful cryoablation.
In our study population, there were no differences in the type of bridge between patients with typical or atypical AVNRT, nor between patients at the first ablation procedure and those who were redo cases. However, some differences in the type of lesions applied to achieve a successful ablation were noted in patients with typical or atypical AVNRT, who needed lesions in a lower position of Koch’s triangle and in the low-voltage bridges.
Summarizing our strategy for cryoablation of AVNRT, we suggest to follow these three steps: (i) to create a very high-density voltage gradient map, paying special attention to the Koch’s triangle region with the aim at identifying a low-voltage bridge; (ii) to carefully search for the ‘hump and spike’ signal within Koch’s triangle, mapping the posterior, medial, and anterior parts of this region and exploring in particular the low-voltage bridge; (iii) to perform repeat electrophysiological studies after an apparently successful focal cryolesion to prove its real effectiveness and, in case of transient success or presence of a bridge larger than the lesion, to create a more extended focal lesion or HDLL or extended HDLL to cover the entire extension of the voltage bridge.
Conclusions
On the basis of the results of this study, we can conclude that:
Voltage gradient mapping of Koch’s triangle, combined with the search for the typical ‘hump and spike’ electrogram, is completely effective in guiding cryoablation of AVNRT in paediatric patients. Indeed, the combination of both strategies can identify the exact substrate responsible for the tachycardia and even guide cryoablation when no target tachycardia is inducible the day of the procedure.
The excellent efficacy of this innovative approach clearly demonstrates that the exact identification and complete destruction of the arrhythmic substrate, rather than the type of energy used (radiofrequency vs. cryoenergy), determine the acute and long-term success rate of the procedure.
Acknowledgements
The authors would like to thank Dr Michele Ciani (F.T.E., St Jude Medical) for his technical contribution and Dr Elisa Del Vecchio for her valuable collaboration in the editorial revision.
Conflict of interest: The authors have no conflict of interest.



