-
PDF
- Split View
-
Views
-
Cite
Cite
Gregory Y. H. Lip, Antonio Coca, Thomas Kahan, Giuseppe Boriani, Antonis S. Manolis, Michael Hecht Olsen, Ali Oto, Tatjana S. Potpara, Jan Steffel, Francisco Marín, Márcio Jansen de Oliveira Figueiredo, Giovanni de Simone, Wendy S. Tzou, Chern-En Chiang, Bryan Williams, Reviewers: , Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE), EP Europace, Volume 19, Issue 6, June 2017, Pages 891–911, https://doi.org/10.1093/europace/eux091
Close - Share Icon Share
Abstract
Hypertension is a common cardiovascular risk factor leading to heart failure (HF), coronary artery disease, stroke, peripheral artery disease and chronic renal insufficiency. Hypertensive heart disease can manifest as many cardiac arrhythmias, most commonly being atrial fibrillation (AF). Both supraventricular and ventricular arrhythmias may occur in hypertensive patients, especially in those with left ventricular hypertrophy (LVH) or HF. Also, some of the antihypertensive drugs commonly used to reduce blood pressure, such as thiazide diuretics, may result in electrolyte abnormalities (e.g. hypokalaemia, hypomagnesemia), further contributing to arrhythmias, whereas effective control of blood pressure may prevent the development of the arrhythmias such as AF. In recognizing this close relationship between hypertension and arrhythmias, the European Heart Rhythm Association (EHRA) and the European Society of Cardiology (ESC) Council on Hypertension convened a Task Force, with representation from the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE), with the remit to comprehensively review the available evidence to publish a joint consensus document on hypertension and cardiac arrhythmias, and to provide up-to-date consensus recommendations for use in clinical practice. The ultimate judgment regarding care of a particular patient must be made by the healthcare provider and the patient in light of all of the circumstances presented by that patient.
Introduction
Hypertension is a common cardiovascular risk factor and underlies many cardiovascular conditions, including heart failure (HF), coronary artery disease, and stroke, as well as chronic kidney disease. Hypertensive heart disease can manifest as various cardiac arrhythmias, most commonly being atrial fibrillation (AF). Both AF and hypertension individually contribute to an increased risk of stroke, which is further accentuated when both are present in combination. Both supraventricular arrhythmias and ventricular arrhythmias may occur in the hypertensive patients, especially in the presence of associated left ventricular hypertrophy (LVH) or HF. In addition, some of the antihypertensive drugs commonly used to reduce blood pressure, such as thiazide diuretics, may result in electrolyte abnormalities (e.g. hypokalaemia, hypomagnesemia), further contributing to arrhythmias, whereas effective control of blood pressure may prevent the development of the arrhythmias such as AF.
In recognizing this close relationship between hypertension and arrhythmias, the European Heart Rhythm Association (EHRA) and the European Society of Cardiology (ESC) Council on Hypertension convened a Task Force, with representation from the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE), with the remit to comprehensively review the available evidence to publish a Joint Consensus Document on hypertension and cardiac arrhythmias, and to provide up-to-date consensus recommendations for use in clinical practice. The ultimate judgment regarding care of a particular patient must be made by the healthcare provider and the patient in light of all of the circumstances presented by that patient.
Evidence review
Members of the Task Force were asked to perform a detailed literature review, weigh the strength of evidence for or against a particular treatment or procedure, and include estimates of expected health outcomes where data exist. Patient-specific modifiers, comorbidities, and issues of patient preference that might influence the choice of particular tests or therapies are considered, as are frequency of follow-up and cost effectiveness. In controversial areas, or with regard to issues without evidence other than usual clinical practice, a consensus was achieved by agreement of the expert panel after thorough deliberations.
This document was prepared by the Task Force with representation from EHRA, HRS, APHRS, and SOLAECE. The document was peer-reviewed by official external reviewers representing EHRA, HRS, APHRS, and SOLAECE.
Consensus statements are evidence-based, and derived primarily from published data. In contrast to guidelines, we have opted for an easier and user-friendly system of ranking using ‘coloured hearts’ that should allow physicians to easily assess current status of evidence and consequent guidance (Table 1). This EHRA grading of consensus statements does not have separate definitions of Level of Evidence. This categorization used for consensus statements (used in consensus documents) must not be considered as being directly similar to that used for official society guideline recommendations, which apply a classification (Class I–III) and level of evidence (A, B, and C) to recommendations used in official guidelines.1
| Definitions where related to a treatment or procedure . | Consensus statement instruction . | Symbol . |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk). | ‘Should do this’ |  |
| General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on small number of patients or not widely applicable. | ‘May do this’ |  |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure. | ‘Do not do this’ |  |
| Definitions where related to a treatment or procedure . | Consensus statement instruction . | Symbol . |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk). | ‘Should do this’ |  |
| General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on small number of patients or not widely applicable. | ‘May do this’ |  |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure. | ‘Do not do this’ |  |
This categorization for our consensus document should not be considered as being directly similar to that used for official society guideline recommendations which apply a classification (I–III) and level of evidence (A, B, and C) to recommendations.
| Definitions where related to a treatment or procedure . | Consensus statement instruction . | Symbol . |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk). | ‘Should do this’ |  |
| General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on small number of patients or not widely applicable. | ‘May do this’ |  |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure. | ‘Do not do this’ |  |
| Definitions where related to a treatment or procedure . | Consensus statement instruction . | Symbol . |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk). | ‘Should do this’ |  |
| General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on small number of patients or not widely applicable. | ‘May do this’ |  |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure. | ‘Do not do this’ |  |
This categorization for our consensus document should not be considered as being directly similar to that used for official society guideline recommendations which apply a classification (I–III) and level of evidence (A, B, and C) to recommendations.
Thus, a green heart indicates a ‘should do this’ consensus statement or indicated treatment or procedure, that is based on at least one randomized trial, or is supported by strong observational evidence that it is beneficial and effective. A yellow heart indicates that general agreement and/or scientific evidence favouring a ‘may do this’ statement or the usefulness/efficacy of a treatment or procedure. A ‘yellow heart’ symbol may be supported by randomized trials based on small number of patients or not widely applicable. Treatment strategies for which there has been scientific evidence that they are potentially harmful and should not be used (‘do not do this’) are indicated by a red heart.
Finally, this is a consensus document that includes evidence and expert opinions from several countries. The pharmacologic and non-pharmacologic antiarrhythmic approaches discussed may, therefore, include drugs that do not have the approval of governmental regulatory agencies in all countries.
Relationships with industry and other conflicts
It is EHRA/ESC policy to sponsor position papers and guidelines without commercial support, and all members volunteered their time. Thus, all members of the writing group as well as reviewers have disclosed any potential conflict of interest in detail, at the end of this document.
Pathogenesis of arrhythmias in hypertension
The occurrence of arrhythmias may have important implications on the morbidity and even mortality in hypertensive patients, ranging from supraventricular premature beats to AF, or more serious ventricular arrhythmias and sudden cardiac death (SCD).
Haemodynamic changes, neuroendocrine factors, atrial and ventricular structural remodelling (i.e. myocardial fibrosis) and a proarrhythmogenic electrophysiologic phenotype of a hypertrophied left ventricle all contribute to the complex pathophysiology of arrhythmogenesis in hypertension.2
Atrial fibrillation is the most frequent arrhythmia in hypertensive patients and hypertension is the most prevalent co-morbidity in patients with AF. Poor BP control seems to worsen outcomes in AF via left ventricular diastolic dysfunction [where associated HF is present, this is referred to as ‘heart failure with preserved ejection fraction (HFpEF’)], left atrial overload and remodelling. AF is also related to the circadian rhythm of BP whereby a blunted nocturnal fall increases the occurrence of AF, perhaps due to the sustainability of high BP and the resultant hemodynamic burden on the left atrium.3
Hypertension may induce an atrial cardiomyopathy, and myocardial changes have been described in detail.4 Mechanical overload due to high BP may induce an abnormal expression of ion channels and/or junctional complexes, as connexin 40 and connexin 43 which can enhance myocardium vulnerability by triggering focal ectopic and re-entry activity.5 Activation of the renin-angiotensin-aldosterone system (RAAS) occurs in hypertension and is strongly implicated in the development of AF. AF may also induce microvascular dysfunction in the ventricles.6
Left ventricular hypertrophy is also the major determinant of the development of ventricular arrhythmias and SCD` in hypertensive patients. One of the proarrhythmogenic features in LVH is the presence of early after depolarizations, which may trigger sustained arrhythmias.10
Activation of the sympathetic nervous system and RAAS are important components of the pathophysiology and development of LVH (Figure 1). Sympathetic activation may trigger ventricular arrhythmias.11 Prolongation and dispersion of repolarization is another feature of the pro-arrhythmogenic impact of LVH.12,13 Nocturnal arrhythmias have been reported in up to 50% of sleep apnoea patients, and autonomic changes may be responsible. These arrhythmias including sinus arrest, second-degree atrioventricular (AV) block, ventricular premature beats (VPBs), and non-sustained ventricular tachycardia. Sleep apnoea is also known to predispose to the development of AF. About 50% of sleep apnoea patients are hypertensive,14 and about 30% of hypertensive patients also have sleep apnoea.15,16
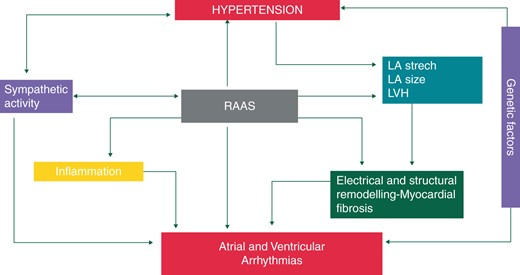
Mechanisms of arrhythmias in hypertension. LA, left atrium; LVH, left ventricular hypertrophy; RAAS, renin-angiotensin-aldosterone system.
Myocardial fibrosis in the left ventricle is part of the structural remodelling process associated with LVH, and may lead to distortion of myocardial structure and increased myocardial stiffness, as part of the hypertensive diastolic dysfunction. At the cellular level, structural remodelling induced by hypertension is associated with impaired cell-to-cell communication at gap junctions, and these changes are the basis of non-homogenous impulse propagation and re-entrant ventricular arrhythmias.8,17 Finally, LVH is also a source of myocardial ischaemia due to the mismatch of oxygen supply and demand. Microvascular dysfunction with myocardial ischaemia has also been reported in the early stages of hypertension, even in the absence of LVH, particularly in patients treated with thiazide diuretics.2,18 Such myocardial ischemia may be a trigger of ventricular arrhythmias and SCD in some cases.2,13,19
Supraventricular arrhythmias
Supraventricular ectopics
Previous studies demonstrated that supraventricular (SVPBs) and VPBs occur frequently in hypertensive patients with LVH.20
Different variables could influence these underlying structural changes. Non-dipping profile (nocturnal BP reduction < 10% vs. diurnal BP) and increased nocturnal BP are markers of more advanced target organ damage; thus, non-dipping is commonly associated with arrhythmias, rather than dipping pattern being causal or directly related to arrhythmia.21
Recovery from exercise may be another triggering factor for SVPBs and the subsequent occurrence of AF. For example, in a study of 258 patients with LVH undergoing an exercise test, SVPBs and supraventricular tachycardia (SVT) occurring during the recovery phase, but not during exercise, predicted the occurrence of subsequent AF.22 The number of SVPBs during recovery was correlated to the development of AF, and the incidence of AF increased from 12% if no SVPBs occurred to 15% with 0–1 SVPB/min, and further to 37% if ≥1 SVPB/min or if an SVT was seen.
Thus, patients with excessive SVPBs and LVH have a greater risk of developing AF, which is associated with increased age, systolic blood pressure and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.23 Interestingly, stroke was commonly the first clinical presentation, beyond manifest AF, in these study subjects. Even short runs of 20–50 SVPBs are associated with AF or some cryptogenic stroke events. Similarly, SVPBs were previously associated with an increased risk of ischaemic stroke.24 In the EMBRACE (30-day cardiac event monitor belt for recording AF after a cerebral ischaemic event) trial,25 among older cryptogenic stroke or transient ischaemic attack (TIA) patients (median CHADS2 score 3), the number of SVPBs on a routine 24-h Holter was a strong independent predictor of subclinical AF.
| Consensus statements . | . | References . |
|---|---|---|
| Patients with frequent SVPBs and LVH have a higher probability of AF and prolonged electrocardiographic (ECG) monitoring for AF detection may be used |  | 23 |
| Lifestyle changes may be used when managing majority of patients with SVPBs, including addressing precipitants relevant to some patients (e.g. alcohol, caffeine) and optimizing BP control especially where LVH is present |  | 20 |
| Consensus statements . | . | References . |
|---|---|---|
| Patients with frequent SVPBs and LVH have a higher probability of AF and prolonged electrocardiographic (ECG) monitoring for AF detection may be used |  | 23 |
| Lifestyle changes may be used when managing majority of patients with SVPBs, including addressing precipitants relevant to some patients (e.g. alcohol, caffeine) and optimizing BP control especially where LVH is present |  | 20 |
| Consensus statements . | . | References . |
|---|---|---|
| Patients with frequent SVPBs and LVH have a higher probability of AF and prolonged electrocardiographic (ECG) monitoring for AF detection may be used |  | 23 |
| Lifestyle changes may be used when managing majority of patients with SVPBs, including addressing precipitants relevant to some patients (e.g. alcohol, caffeine) and optimizing BP control especially where LVH is present |  | 20 |
| Consensus statements . | . | References . |
|---|---|---|
| Patients with frequent SVPBs and LVH have a higher probability of AF and prolonged electrocardiographic (ECG) monitoring for AF detection may be used |  | 23 |
| Lifestyle changes may be used when managing majority of patients with SVPBs, including addressing precipitants relevant to some patients (e.g. alcohol, caffeine) and optimizing BP control especially where LVH is present |  | 20 |
Atrial fibrillation
Due to its high prevalence in the general population, hypertension is the most significant population-attributable risk for AF and has been estimated to be responsible for 14% of all AF cases.26 Hypertension was present in >70% of AF patients in epidemiological studies27,28 and recent AF real-world registries,29–31 and in 49–90% of patients in randomized AF trials.32,33
A pooled risk estimate revealed a 73% greater likelihood of AF in patients with hypertension or taking antihypertensive medication (Odds Ratio [OR] 1.73%; 95% Confidence Interval [CI], 1.31–2.28), especially in the presence of LVH.34 Increased AF risk was also reported in individuals with upper normal blood pressure.35,36
One population-based, case-control study of patients treated for hypertension showed a ‘J’ shaped relationship between blood pressure and incident AF over a 12-year follow-up, with the lowest rates of incident AF at systolic blood pressure of 120–130 mmHg and diastolic blood pressure of 60–69 mmHg,37 thus suggesting that optimal blood pressure control might decrease AF burden in hypertensive patients.38 Atrial fibrillation could represent a manifestation of hypertensive heart disease or hypertensive target organ damage.39
In a study of patients with essential hypertension free of other cardiovascular conditions (n ≈ 2500), the incidence of AF was 0.46% per year, and age or increased left ventricular mass were the only independent predictors of incident AF during a 5-year follow-up.40 Of note, the annual incidence of stroke in that study was significantly higher in hypertensive patients with intermittent or chronic AF (2.7 and 4.6%, respectively) than in those without AF (0.81%, P = 0.0005).
Hypertension has been identified as an independent risk factor for incident AF41–43 or AF progression,44–46 AF-related stroke and mortality47–49 and bleeding complications of oral anticoagulant therapy in AF patients,50–52 and a contributor to increased risk of poor quality of treatment with vitamin K antagonists, as predicted by the SAMe-TT2R2 score53 (see Supplementary material online, Table S1).
Importantly, AF may be asymptomatic in up to 35% of patients (including those who also have symptomatic AF episodes),54 particularly in patients with less co-morbidity (e.g. with hypertension only).55
| Consensus statements . | . | References . |
|---|---|---|
| AF should be considered as a manifestation of hypertensive heart disease, and optimization of hypertension management should be made. |  | 26,35,36 |
| Given that stroke prevention is central to the management of AF patients, detection of hypertension and good blood pressure control should be done to minimize the risk of stroke and thromboembolism, as well as the bleeding risk whilst on antithrombotic therapy. |  | 47–49 |
| Consensus statements . | . | References . |
|---|---|---|
| AF should be considered as a manifestation of hypertensive heart disease, and optimization of hypertension management should be made. |  | 26,35,36 |
| Given that stroke prevention is central to the management of AF patients, detection of hypertension and good blood pressure control should be done to minimize the risk of stroke and thromboembolism, as well as the bleeding risk whilst on antithrombotic therapy. |  | 47–49 |
| Consensus statements . | . | References . |
|---|---|---|
| AF should be considered as a manifestation of hypertensive heart disease, and optimization of hypertension management should be made. |  | 26,35,36 |
| Given that stroke prevention is central to the management of AF patients, detection of hypertension and good blood pressure control should be done to minimize the risk of stroke and thromboembolism, as well as the bleeding risk whilst on antithrombotic therapy. |  | 47–49 |
| Consensus statements . | . | References . |
|---|---|---|
| AF should be considered as a manifestation of hypertensive heart disease, and optimization of hypertension management should be made. |  | 26,35,36 |
| Given that stroke prevention is central to the management of AF patients, detection of hypertension and good blood pressure control should be done to minimize the risk of stroke and thromboembolism, as well as the bleeding risk whilst on antithrombotic therapy. |  | 47–49 |
Supraventricular tachycardia
Left ventricular hypertrophy is the most important predictor for developing supraventricular arrhythmias. In a recent meta-analysis of 10 studies with 27 141 patients, the incidence of SVT (especially atrial tachycardias, AF or flutter) in patients with LVH was 11.1% compared with 1.1% among patients without LVH (P < 0.001).56
Patients with LVH have a 3.4-fold greater odds of developing SVT (OR 3.39; 95% CI, 1.57–7.31) than those without LVH, although significant heterogeneity was present (I2 = 98%) due to differences in the baseline covariates such as age, male gender, hypertension, and diabetes in the individual studies.56
Other arrhythmias
Drug-related bradyarrhythmias
While dihydropyridine calcium channel blockers (CCBs) combine well with β-blockers in the management of hypertension, caution should be exercised when combining non-dihydropyridine CCBs with beta-blockers.57 There is a risk of bradycardia and AV block with non-dihydropyridine CCBs particularly with the use of verapamil, but also with diltiazem at higher doses. A disproportionality analysis of side-effects caused by drug interactions reported to the US FDA between 1968 and 2001 indicated that the rate of conduction-related interactions between beta-blocker and verapamil leading to bradyarrhythmias was approximately 10%, a two-fold increase in the rate reported for either agent taken alone.58 In the Diltiazem Reinfarction study,59 second-degree AV block was noted in 3.4% of patients in the treatment group receiving high-dose (360 mg) diltiazem compared with 0.5% of patients in the placebo group (∼60% concomitant use of a beta-blocker in both groups).
In patients with chronic kidney disease, the accumulation of beta-blockers or active metabolites could exacerbate concentration-dependent side effects, such as bradyarrhythmias.60 Such accumulation occurs with atenolol, nadolol, and bisoprolol, but less so with carvedilol, propranolol, or metoprolol.
Sick sinus syndrome and atrioventricular conduction disturbances
The association of LVH with bradyarrhythmias, including complete atrioventricular block (AVB) and symptomatic sick sinus syndrome (SSS) requiring permanent pacemaker implantation, was examined in a study comprising 260 patients with complete heart block (n = 130) or symptomatic SSS (n = 130) receiving a permanent pacemaker, who were compared with 45 patients without cardiac conduction disturbances.61 A significant association with LVH was observed in the AVB group, but not in the SSS group, as the majority of patients with complete AVB were hypertensive with LVH. This association was stronger in patients with infra-Hisian block.
In a post hoc analysis of the RACE trial62 studying differences in outcome between hypertensive and normotensive persistent AF patients, it was demonstrated that morbidity and mortality and in particular SSS and AV conduction disturbances occurred more commonly in persistent AF patients with hypertension as compared to normotensive patients.
According with the results of an autopsy study comprising 35 hearts obtained from patients who died suddenly, and compared with 27 both age- and disease-matched and 30 age-matched control hearts from individuals who had not died suddenly, sclerosis of the sinus node artery was seen in the cases of sudden death with hypertensive heart disease.63
Thus, AV conduction disturbances may occur in hypertensive patients with LVH, and sinus node dysfunction may occur. Both clinical conditions may be encountered in the subgroup of hypertensive patients suffering from sleep-disordered breathing.64
In these situations, the electrophysiological properties of the sinus node and AV conduction system in obstructive sleep apnoea (OSA) patients with nocturnal bradyarrhythmias are usually normal while awake, and thus primary therapy of bradyarrhythmias in the setting of sleep apnoeas and normal AV conduction would consist of treatment of OSA with continuous positive airway pressure (CPAP) which can reverse these bradyarrhythmias, suggesting that OSA most likely induced the arrhythmias.64 Finally, beneficial effects, albeit moderate and variable, of CPAP on blood pressure have been reported in patients with OSA; patients with more severe OSA, resistant hypertension, and better CPAP compliance may have a greater blood pressure reduction with CPAP.
Intra- and inter-atrial/inter- and intra-ventricular conduction delays
Interatrial and intra-atrial conduction delays have been reported to be longer in patients with hypertension compared to controls.65 Among 9131 patients with hypertension and LVH, 564 (0.6%) had left bundle branch block (LBBB).66 Left bundle branch block was independently associated with 1.6-fold more cardiovascular death (P < 0.05), 1.7-fold more hospitalization for HF (P < 0.01), 3.5-fold more SCD (P < 0.001), and 3.4-fold more cardiovascular death within 24 h (P < 0.001). The authors concluded that in hypertension with LVH on ECG, LBBB identifies patients at increased risk of cardiovascular mortality, SCD, and HF.
In an analysis of the losartan intervention for endpoint reduction in hypertension (LIFE) study population, the duration of the QRS complex predicted all-cause and cardiovascular mortality in hypertensive patients with ECG-LVH in the setting of aggressive hypertensive therapy.67 An ancillary analysis from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) also found that lisinopril significantly reduced these conduction disturbances.68 In a subsequent analysis of the LIFE study, among 9193 hypertensive patients with LVH treated with atenolol or losartan, QRS duration was independently predictive of SCD over an approximate mean 5-year follow-up69 and remained a significant predictor of SCD even after controlling for the presence or absence of LBBB and for changes in ECG LVH severity over the course of the study.
Elevated resting heart rate in sinus rhythm
A high resting heart rate has been related to an adverse prognosis in patients with coronary artery disease (CAD) and HF.70–74 In hypertensive patients free from other overt cardiac disease, this is less clear, and an elevated resting heart rate in hypertensive subjects free from overt cardiac disease seems to be more a risk marker than a risk factor.74,75 Studies assessing ambulatory heart rate tend to demonstrate that night-time heart rate may be a better predictor of cardiovascular events than daytime heart rate. Heart rate during sleep represents persistent sympathetic overactivity and appears to be a better reflection of the mechanical stress on the arterial wall than daytime heart rate. Nevertheless, intervention trials have not demonstrated that lowering the heart rate with use of beta-blockers is beneficial in hypertensive subjects, although some benefit may be evident with ivabradine where concomitant reduced ejection fraction is evident.76 Paradoxically, the use of ivabradine in HF is associated with more AF. Inappropriate sinus tachycardia can sometimes occur and in symptomatic patients, heart rate control with, e.g. beta blockers may be indicated.77
A resting heart rate > 80—85 b.p.m. may be used as a guide to further investigate for occult HF symptoms by clinical examination or determining biomarkers (such as BNP) or performing an echocardiogram, or looking for associated comorbidities, such as arrhythmias (e.g. AF and atrial flutter), anaemia, hyperthyroidism and sepsis.74 A high resting heart rate may also be associated with obesity.
In addition, a high sleeping heart rate obtained via ambulatory blood pressure measurement may reflect episodes of resistant hypertension with a non-dipping heart rate and blood pressure profile or explore the possibility for sleep apnoea associated with sympathetic overactivity by clinical history and/or by performing a polysomnography study.74,78
In AF, note that rate control should initially aim for a HR < 110 b.p.m., with stricter rate control if symptomatic or LV function deteriorates.79 The beneficial effects of beta-blockers on outcomes may be less apparent in AF and reduced LV systolic function.80
| Consensus Statements . | . | References . |
|---|---|---|
| Both sinus node and AV conduction disturbances (particularly in patients with LVH) can occur in hypertensive patients as a consequence of sleep apnoea, and sleep disordered breathing is more common in hypertensive patient. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 61,64 |
| Conduction delays occur both at the atrial and ventricular level in hypertensive patients, particularly in those with LVH, leading to AF or SCD, respectively. The presence of LBBB in hypertension, especially with LVH identifies patients at increased cardiovascular risk. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 65 |
| An increased resting heart rate (>80–85 b.p.m.), portends an adverse prognosis not only in patients with CAD and HF, but in hypertensive patients as well. Routine lowering of the heart rate with use of beta-blockers or other agents may be considered in hypertensive subjects uncomplicated by other comorbidities (e.g. impaired LV function). |  | 70–74 |
| Consensus Statements . | . | References . |
|---|---|---|
| Both sinus node and AV conduction disturbances (particularly in patients with LVH) can occur in hypertensive patients as a consequence of sleep apnoea, and sleep disordered breathing is more common in hypertensive patient. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 61,64 |
| Conduction delays occur both at the atrial and ventricular level in hypertensive patients, particularly in those with LVH, leading to AF or SCD, respectively. The presence of LBBB in hypertension, especially with LVH identifies patients at increased cardiovascular risk. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 65 |
| An increased resting heart rate (>80–85 b.p.m.), portends an adverse prognosis not only in patients with CAD and HF, but in hypertensive patients as well. Routine lowering of the heart rate with use of beta-blockers or other agents may be considered in hypertensive subjects uncomplicated by other comorbidities (e.g. impaired LV function). |  | 70–74 |
| Consensus Statements . | . | References . |
|---|---|---|
| Both sinus node and AV conduction disturbances (particularly in patients with LVH) can occur in hypertensive patients as a consequence of sleep apnoea, and sleep disordered breathing is more common in hypertensive patient. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 61,64 |
| Conduction delays occur both at the atrial and ventricular level in hypertensive patients, particularly in those with LVH, leading to AF or SCD, respectively. The presence of LBBB in hypertension, especially with LVH identifies patients at increased cardiovascular risk. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 65 |
| An increased resting heart rate (>80–85 b.p.m.), portends an adverse prognosis not only in patients with CAD and HF, but in hypertensive patients as well. Routine lowering of the heart rate with use of beta-blockers or other agents may be considered in hypertensive subjects uncomplicated by other comorbidities (e.g. impaired LV function). |  | 70–74 |
| Consensus Statements . | . | References . |
|---|---|---|
| Both sinus node and AV conduction disturbances (particularly in patients with LVH) can occur in hypertensive patients as a consequence of sleep apnoea, and sleep disordered breathing is more common in hypertensive patient. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 61,64 |
| Conduction delays occur both at the atrial and ventricular level in hypertensive patients, particularly in those with LVH, leading to AF or SCD, respectively. The presence of LBBB in hypertension, especially with LVH identifies patients at increased cardiovascular risk. Thus, assessment for these conditions may be performed in hypertensive patients. |  | 65 |
| An increased resting heart rate (>80–85 b.p.m.), portends an adverse prognosis not only in patients with CAD and HF, but in hypertensive patients as well. Routine lowering of the heart rate with use of beta-blockers or other agents may be considered in hypertensive subjects uncomplicated by other comorbidities (e.g. impaired LV function). |  | 70–74 |
Proposal for a standardized ‘workup’
In most patients with hypertension and suspected arrhythmias, all efforts should be made to obtain a diagnosis by documenting the arrhythmia.
First, because regular SVTs including atrioventricular nodal re-entrant tachycardia (AVNTR), atrioventricular re-entrant tachycardia (AVRT), atrial flutter and focal atrial tachycardia may lead to severe symptoms in patients with hypertension (particularly in those with advanced diastolic dysfunction). In these patients, curative treatment with catheter ablation is available (as well as medical therapy) and can be associated with a high success and low complications rates.82
Second, AF can rarely be ruled out as the underlying problem on clinical grounds alone, and the diagnosis of AF usually carries important implications at least regarding anticoagulation.83,84 The increasing evidence that silent AF is associated with a higher risk for stroke54,85 has led to the recommendation of ‘opportunistic screening’ for AF using pulse taking or ECG in the most recent guidelines86. This recommendation is clearly also valid in hypertensive patients because they are at greater risk of stroke. However, further research is needed to define best practice for younger patients diagnosed, e.g. with hypertension (especially those with asymptomatic organ damage),87–89 as well as new technologies such as automated BP monitors with algorithms for AF detection, or other new technologies.
Third, a number of studies suggest that lower blood pressure goals reduce the frequency of episodes with paroxysmal SVT.5,6 Lifestyle changes reducing blood pressure and AF burden may also contribute.90
The order and type of workup of patients with arrhythmias and hypertension depends on various factors including duration and severity of symptoms, frequency of episodes and potential therapeutic implications. A proposal for a standardized initial work up is shown in Figure 2. A personal ECG device (e.g., mobile phone applications) may be another option. Implantable loop recorder (ILR) may be indicated earlier in ‘high risk’ patients with cryptogenic stroke (embolic stroke of uncertain source, ESUS) or presyncope/syncope where bradyarrhythmias are suspected as opposed to patients with palpitations, at least in daily practice. Note that a highly symptomatic patient even with low CHA2DS2-VASc score may still warrant a further work up with 30d event monitor or ILR.
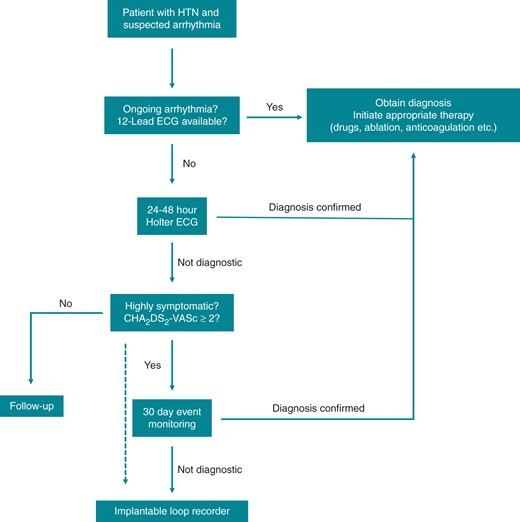
Proposed ‘workup’ standard for patients with hypertension and suspected cardiac arrhythmias. HTN, Hypertension; ECG, Electrocardiogram.
With a CHA2DS2-VASc score ≥2 (i.e. two or more stroke risk factors) there is sufficient risk to either consider or recommend stroke prevention in patients with AF or suspected AF on basis of (prolonged) atrial high rate episodes (AHRE).
As a final step, 30 day event monitoring or an implantable cardiac monitor (ICM) may be used to detect rare arrhythmias. In the EMBRACE (30-day cardiac event monitor belt for recording AF after a cerebral ischaemic event) trial and CRYSTAL-AF (CRYptogenic STroke and underlying AtriaL Fibrillation) study respectively, these strategies were superior to conventional follow-up (mostly 24 h Holter and symptom-based diagnostics) for the detection of AF in patients post-cryptogenic stroke.7,8
The optimal duration cut-off for the definition of device detected AF, however, currently remains elusive; a 6 min cut-off is mostly widely used, based on findings of the ASSERT trial (ASymptomatic AF and Stroke Evaluation in Pacemaker Patients and the AF Reduction Atrial Pacing).54 Closely connected to this is the question of the necessary AF burden to initiate anticoagulation, but >5–6 min burden is generally considered as ‘significant’. Finally, use of new technology that can be incorporated into a smartphone may be another option to record an infrequent arrhythmic event or detect silent AF.89 However, there is a disconnect between AHRE and thromboembolic events, raising the possibility that AF is marker of increased risk for stroke rather than a cause of stroke.91
| Consensus Statements . | . | References . |
|---|---|---|
| Silent AF is common, and opportunistic screening for underlying AF amongst hypertensive patients should be performed. |  | 87–89 |
| In hypertensive patients with symptoms suggestive of a cardiac rhythm disorder, documentation of the presence and type of arrhythmia should be done, for adequate management of the arrhythmia. |
| Consensus Statements . | . | References . |
|---|---|---|
| Silent AF is common, and opportunistic screening for underlying AF amongst hypertensive patients should be performed. |  | 87–89 |
| In hypertensive patients with symptoms suggestive of a cardiac rhythm disorder, documentation of the presence and type of arrhythmia should be done, for adequate management of the arrhythmia. |
| Consensus Statements . | . | References . |
|---|---|---|
| Silent AF is common, and opportunistic screening for underlying AF amongst hypertensive patients should be performed. |  | 87–89 |
| In hypertensive patients with symptoms suggestive of a cardiac rhythm disorder, documentation of the presence and type of arrhythmia should be done, for adequate management of the arrhythmia. |
| Consensus Statements . | . | References . |
|---|---|---|
| Silent AF is common, and opportunistic screening for underlying AF amongst hypertensive patients should be performed. |  | 87–89 |
| In hypertensive patients with symptoms suggestive of a cardiac rhythm disorder, documentation of the presence and type of arrhythmia should be done, for adequate management of the arrhythmia. |
Management approaches
The management of patients with hypertension and SVT is primarily driven by the type of arrhythmia (see Figure 3). In addition, hypertension should be proactively managed, with the type of treatment determined by associated compelling indications and/or comorbidities.18 In general, RAAS blockade with angiotensin converting enzyme (ACE) inhibitors or ARB should be considered where LVH is present. Rate control in the presence of AF may be facilitated with a beta-blocker or non-dihydropyridine calcium blocker (verapamil, diltiazem). Supraventricular tachycardia may respond to a beta-blocker or non-dihydropyridine calcium blocker.

Management of atrial fibrillation in patients with hypertension. *Assess indication for oral anticoagulation in typical isthmus-dependent flutter (the same as in atrial fibrillation); Until further evidence is available, oral anticoagulation needs to be continued post AF and/or AFl ablation depending on the patient's CHA2DS2-VASc score, independent of ablation “success” or “failure. HTN, Hypertension; AVNRT, Atrio-ventricular nodal re-entrant tachycardia; AVRT, Atrio-ventricular re-entrant tachycardia; TX, Therapy.
Supraventricular tachycardia
For acute management of SVT, these patients are treated as other patients with no hypertension according to published guidelines.92 Vagal manoeuvres or intravenous adenosine are recommended as the initial therapy.93,94 In haemodynamically unstable patients, synchronized cardioversion is recommended.94 Intravenous diltiazem, verapamil, or beta-blockers are recommended for haemodynamically stable patients.93,94 Intravenous esmolol is especially useful for short-term control of SVT and hypertension.95
For more chronic management of SVT catheter ablation is the first choice therapy.94,96 Similarly, focal ectopic atrial tachycardia can usually be treated by ablation. Unlike re-entrant tachycardias, focal atrial tachycardia (AT) is likely reflective of a diseased atrium, particularly in patients with long-standing hypertension and more severe diastolic dysfunction and atrial remodelling. For patients who refuse catheter ablation, possible options in symptomatic patients who do not have ventricular pre-excitation during sinus rhythm include oral beta-blockers, diltiazem, or verapamil. Flecainide, propafenone, or sotalol are reasonable choices in patients without structural heart disease (e.g. severe LVH) who have symptomatic SVT and are not candidates for, or prefer not to undergo, catheter ablation.94
Atrial fibrillation
The priority in the treatment of patients with AF is stroke prevention.83,86 The default is to offer oral anticoagulation (OAC) to all AF patients unless they are at low risk (defined as a CHA2DS2-VASc Score 0 in males, 1 in females).97 Thus, the initial step is to identify ‘low risk’ patients where no antithrombotic therapy is recommended, following which OAC can be considered for those with ≥1 additional stroke risk factors.97 Even a single stroke risk factor confers excess risk of stroke and mortality, and the net clinical benefit is positive for treating such patients.98–100
Patients with hypertension have a CHA2DS2-VASc score of at least 1, and hence at least a Class IIa/Level of Evidence A indication for anticoagulation (which becomes a class I indication with any additional risk factor). Bleeding risk should be assessed using well-validated simple bleeding risk scores that draw attention to the potentially reversible bleeding risk factors,86 such as the HAS-BLED score, but should not by itself be used to deny anticoagulation to a patient.83,101 Indeed, patients with a class I indication for anticoagulation and a HAS-BLED score ≥ 3 may profit as much if not more from anticoagulation based on net clinical benefit than those with a lower HAS-BLED score.102 Uncontrolled hypertension (e.g. SBP > 160 mmHg) and other modifiable risk factors (e.g. concomitant aspirin or non-steroidal anti-inflammatory drug treatment, excessive alcohol use) should be addressed, to minimize the risk of bleeding.103
The non-vitamin K antagonist oral anticoagulants (NOACs) are recommended as the preferred treatment modality over vitamin K antagonists for anticoagulation,83 based on the results of four independent large-scale clinical trials.104–107 Subgroup analyses in patients with hypertension have mostly been consistent with the main outcome of the trials.105,107–109 The use of aspirin for stroke prevention in AF is associated with minimal efficacy, but comes with a substantial bleeding risk; thus, aspirin is therefore no longer recommended (Class III).83
Persistent as well as permanent AF is common in hypertensive patients, particularly in the elderly, where rhythm control may not be an option. Elderly hypertensives often have associated HFpEF. A beta-blocker or non-dihydropyridine calcium blocker may be considered for rate control in such patients, although RAAS blockade may help LVH regression. In the elderly, digoxin may be a 2nd line option. Note that during rhythm control therapy, persistent AF patients with hypertension have more morbidity and mortality as compared to rate control, albeit in an era before ablation therapy.62
Atrial fibrillation ablation has emerged as an effective method for treatment of AF. In paroxysmal AF and normal sized atria, long-term freedom from symptoms can be achieved in up to 80%, but may require multiple procedures.110,111 In patients with persistent AF and diseased atria, which is frequently found in patients with long-standing hypertension, long term success rates are substantially less below 70%.110,111
| Consensus statements . | . | References . |
|---|---|---|
| Oral amiodarone should be used for ongoing management in patients witd symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, sotalol, or verapamil are ineffective or contraindicated. |  | 94,92,114 |
| The priority in the treatment of patients with AF is stroke prevention, and AF patients with hypertension have a CHA2DS2-VASc score of at least 1; thus, effective stroke prevention may be considered with OAC in addition to good BP control. |  | 98–100 |
| With additional stroke risk factors, CHA2DS2-VASc score ≥2, OAC should be used, as well controlled VKA (TTR >70%) or a non-vitamin K antagonist oral anticoagulants (NOAC), with a preference for the latter. |  | |
Bleeding risk should be assessed with focus on the modifiable bleeding risk factors, most of which can be identified using the HAS-BLED score.
|  | 103,83,101,115 |
| Atrial fibrillation ablation should be used in hypertensive patients with symptomatic recurrences of AF on antiarrhythmic drug therapy who prefer further rhythm control therapy, and is first therapy in selected individuals as an alternative to antiarrhythmic drug therapy depending on patient choice, benefit, and risk. |  | 92,110,111 |
| In patients with re-entrant SVT and isthmus dependent flutter, catheter ablation should be used and is associated with a high success and low complication rate. | ||
| In patients with severe structural heart diseases, such as severe LVH, history of myocardial infarction and HF, a haemodynamically significant valvular disease, do not use flecainide or propafenone. Do not use Sotalol in LVH patients, or Diltiazem and verapamil in HFrEF. |  | 112,113 |
| Consensus statements . | . | References . |
|---|---|---|
| Oral amiodarone should be used for ongoing management in patients witd symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, sotalol, or verapamil are ineffective or contraindicated. |  | 94,92,114 |
| The priority in the treatment of patients with AF is stroke prevention, and AF patients with hypertension have a CHA2DS2-VASc score of at least 1; thus, effective stroke prevention may be considered with OAC in addition to good BP control. |  | 98–100 |
| With additional stroke risk factors, CHA2DS2-VASc score ≥2, OAC should be used, as well controlled VKA (TTR >70%) or a non-vitamin K antagonist oral anticoagulants (NOAC), with a preference for the latter. |  | |
Bleeding risk should be assessed with focus on the modifiable bleeding risk factors, most of which can be identified using the HAS-BLED score.
|  | 103,83,101,115 |
| Atrial fibrillation ablation should be used in hypertensive patients with symptomatic recurrences of AF on antiarrhythmic drug therapy who prefer further rhythm control therapy, and is first therapy in selected individuals as an alternative to antiarrhythmic drug therapy depending on patient choice, benefit, and risk. |  | 92,110,111 |
| In patients with re-entrant SVT and isthmus dependent flutter, catheter ablation should be used and is associated with a high success and low complication rate. | ||
| In patients with severe structural heart diseases, such as severe LVH, history of myocardial infarction and HF, a haemodynamically significant valvular disease, do not use flecainide or propafenone. Do not use Sotalol in LVH patients, or Diltiazem and verapamil in HFrEF. |  | 112,113 |
LVH, left ventricular hypertrophy; HFrEF, Heart failure with reduced ejection fraction; SVT, Supraventricular tachycardia; AF, atrial fibrilation; CHA2DS2-VASc score, Congestive Heart failure, hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Sex (female); OAC, oral anticoagulation/oral anticoagulant; VKA, vitamin K anticoagulants; TTR, time in therapeutic range; NOAC, no oral anticoagulation; HAS-BLED score, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (65 years), drugs/alcohol concomitantly (1 point each); AVNRT, Atrioventricular nodal re-entrant tachycardia; AVRT, Atrioventricular re-entrant tachycardia.
| Consensus statements . | . | References . |
|---|---|---|
| Oral amiodarone should be used for ongoing management in patients witd symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, sotalol, or verapamil are ineffective or contraindicated. |  | 94,92,114 |
| The priority in the treatment of patients with AF is stroke prevention, and AF patients with hypertension have a CHA2DS2-VASc score of at least 1; thus, effective stroke prevention may be considered with OAC in addition to good BP control. |  | 98–100 |
| With additional stroke risk factors, CHA2DS2-VASc score ≥2, OAC should be used, as well controlled VKA (TTR >70%) or a non-vitamin K antagonist oral anticoagulants (NOAC), with a preference for the latter. |  | |
Bleeding risk should be assessed with focus on the modifiable bleeding risk factors, most of which can be identified using the HAS-BLED score.
|  | 103,83,101,115 |
| Atrial fibrillation ablation should be used in hypertensive patients with symptomatic recurrences of AF on antiarrhythmic drug therapy who prefer further rhythm control therapy, and is first therapy in selected individuals as an alternative to antiarrhythmic drug therapy depending on patient choice, benefit, and risk. |  | 92,110,111 |
| In patients with re-entrant SVT and isthmus dependent flutter, catheter ablation should be used and is associated with a high success and low complication rate. | ||
| In patients with severe structural heart diseases, such as severe LVH, history of myocardial infarction and HF, a haemodynamically significant valvular disease, do not use flecainide or propafenone. Do not use Sotalol in LVH patients, or Diltiazem and verapamil in HFrEF. |  | 112,113 |
| Consensus statements . | . | References . |
|---|---|---|
| Oral amiodarone should be used for ongoing management in patients witd symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, sotalol, or verapamil are ineffective or contraindicated. |  | 94,92,114 |
| The priority in the treatment of patients with AF is stroke prevention, and AF patients with hypertension have a CHA2DS2-VASc score of at least 1; thus, effective stroke prevention may be considered with OAC in addition to good BP control. |  | 98–100 |
| With additional stroke risk factors, CHA2DS2-VASc score ≥2, OAC should be used, as well controlled VKA (TTR >70%) or a non-vitamin K antagonist oral anticoagulants (NOAC), with a preference for the latter. |  | |
Bleeding risk should be assessed with focus on the modifiable bleeding risk factors, most of which can be identified using the HAS-BLED score.
|  | 103,83,101,115 |
| Atrial fibrillation ablation should be used in hypertensive patients with symptomatic recurrences of AF on antiarrhythmic drug therapy who prefer further rhythm control therapy, and is first therapy in selected individuals as an alternative to antiarrhythmic drug therapy depending on patient choice, benefit, and risk. |  | 92,110,111 |
| In patients with re-entrant SVT and isthmus dependent flutter, catheter ablation should be used and is associated with a high success and low complication rate. | ||
| In patients with severe structural heart diseases, such as severe LVH, history of myocardial infarction and HF, a haemodynamically significant valvular disease, do not use flecainide or propafenone. Do not use Sotalol in LVH patients, or Diltiazem and verapamil in HFrEF. |  | 112,113 |
LVH, left ventricular hypertrophy; HFrEF, Heart failure with reduced ejection fraction; SVT, Supraventricular tachycardia; AF, atrial fibrilation; CHA2DS2-VASc score, Congestive Heart failure, hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Sex (female); OAC, oral anticoagulation/oral anticoagulant; VKA, vitamin K anticoagulants; TTR, time in therapeutic range; NOAC, no oral anticoagulation; HAS-BLED score, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (65 years), drugs/alcohol concomitantly (1 point each); AVNRT, Atrioventricular nodal re-entrant tachycardia; AVRT, Atrioventricular re-entrant tachycardia.
Ventricular arrhythmias
Ventricular ectopics
Ventricular arrhythmias are common among hypertensive patients, and this association may have important clinical implications.116–118 Data from the Atherosclerosis Risk in Communities (ARIC) study of more than 15 000 African American and white men and women showed that frequent or complex ventricular ectopic beats are associated with high blood pressure.119 Epidemiological data have shown that hypertension induced LVH is associated with sustained ventricular arrhythmias.56,120
High blood pressure is not arrhythmogenic per se but the induced ventricular overload. Ventricular arrhythmias are commonly observed in aortic stenosis, even when peripheral blood pressure is low; the frequency of these arrhythmias have been demonstrated to be reduced after transcatheter aortic valve implantation.121 Changes in electrophysiological properties can occur during volume overload,122 which may be even more important under pathological conditions, such as ischaemic scars.
Ventricular tachycardia (VT), Ventricular fibrillation (VF), and sudden death
Hypertension is a risk factor for SCD, particularly in the context of increased LV mass.123 Left ventricular hypertrophy is associated with long-term risk of SCD independent of blood pressure, and the risk of SCD increases progressively with LV mass.120,124,125
As discussed in Section II, several mechanisms have been proposed to explain the relationship between the presence of LVH and SCD in hypertension (Figure 4). The prolongation of repolarization as measured by the QT interval or transmural dispersion of repolarization, present in hypertensive patients can be related to the degree of LVH and may be associated with an increased risk of ventricular arrhythmias.13,126 Other mechanisms, such as mismatch between oxygen supply and demand, particularly with stress, reduced coronary flow reserve and subsequent myocardial ischaemia may play a role.127
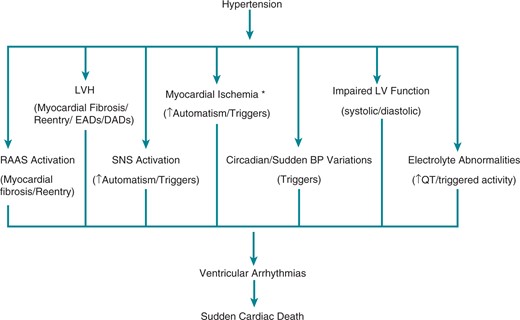
Potential mechanisms of ventricular arrhythmias and sudden cardiac death in hypertensive patients. BP, blood pressure; CAD, coronary artery disease; DADs, delayed afterdepolarizations; EADs, early afterdepolarizations; LV, left ventricular; LVH, left ventricular hypertrophy; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system. * CAD/LVH causing myocardial blood supply and oxygen consumption mismatch/decreased diastolic coronary blood flow causing subendocardial ischemia/microvascular dysfunction.
There is evidence that optimal control of blood pressure and regression of LVH by antihypertensive treatment can help prevent cardiac arrhythmias.128,129 Although an effect on the burden of ventricular ectopy has not been consistently observed even in the context of reversal of LVH,130–132 a reduced incidence of SCD has been demonstrated with effective BP control and regression of LVH. For example, in one study regression of electrocardiographic LVH during antihypertensive therapy was associated with a 30% lower risk of SCD independently of blood pressure lowering and other known predictors of SCD.133
However, it is also important to consider the potential influence of antihypertensive drugs on the risk of SCD. The use of thiazide diuretics has been associated with an increased risk of cardiac arrhythmias, with a dose-dependent increase in SCD.134 Although the exact mechanism is unknown, hypokalaemia may be involved, with increased risk for QT prolongation, QT dispersion, and propensity for arrhythmogenic early and delayed after-depolarizations.135 There was no difference in SCD among high-risk Japanese patients treated with the ARB candesartan or the calcium antagonist amlodipine, suggesting that blood pressure lowering itself may be important in affecting SCD risk.136 However, in a high risk group for SCD consisting of hypertensive patients with LVH and diabetes, ARB based treatment with losartan was superior to treatment based on the beta blocker atenolol to prevent SCD.137 Also, treatment with ACEi in high-risk cardiovascular patients was associated with a 26% reduction in cardiac mortality and a 37% decrease in cardiac arrest compared to placebo.138 This provides some supportive evidence for blocking the RAAS in hypertensive patients at high risk of SCD.
Proposal for a standardized ‘workup’
Frequent non-sustained ventricular tachycardia (NSVT) or single VPBs among patients with hypertension are treated similarly to those found among patients without hypertension. A 12-lead ECG and a 24-h Holter recording may help to potentially localize site(s) of origin and to quantify VPBs. Transthoracic echocardiography may be useful to assess for other signs of hypertensive or structural heart disease, as well as to assess left ventricular systolic function. The latter is particularly important to identify, especially when a high VPB burden, defined as >20% of all beats in a 24-h recording, is documented.139–141 If underlying coronary disease is suspected, frequent VPBs, associated symptoms, or LV systolic dysfunction, exercise testing may be useful for assessing effect on VPBs and for evaluating the presence of myocardial ischaemia (Figure 5).
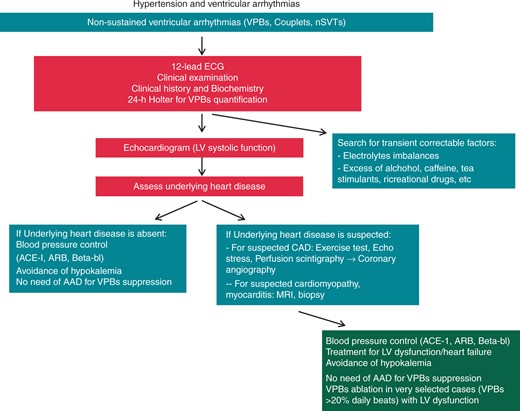
Proposal for a standardized ‘workup’. NB. Only in rare cases does myocardial biopsy change management, and the benefit: risk of this is low. Consider ICD implantation if LVEF ≤35% despite goal-directed medical therapy and sustained hypertension control. ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CAD, coronary artery disease; LV, left ventricular; MRI, magnetic resonance imaging; nSVTs, no supraventricular tachycardia; VPBs, ventricular premature beats.
Since the presence and number of VPBs may be modulated by many factors, a blood biochemistry profile, including electrolytes (potassium, magnesium, calcium), renal function, thyroid function, and glucose should be assessed. Moreover, it is necessary to review prescriptions and over-the-counter agents that may lengthen the QT interval or may induce sympathetic stimulation, particularly if LVH is evident on ECG or echocardiography.131,142,143 Excessive intake of alcohol or caffeine or other non-pharmacologic stimulants, as well as use of recreational drugs, should be investigated and appropriately corrected. Identification of chronic exposure to high-stress conditions is important in order to counsel avoidance or ways to mitigate the stress, in view of the facilitating effect of adrenergic stimulation on arrhythmogenesis (Figure 5).
Additionally, cardiac magnetic resonance imaging (MRI) may be useful for providing a detailed analysis of myocardial substrate and to quantify the extent of myocardial fibrosis and possibly prognosis.144–146 Coronary angiography may also be indicated to rule out myocardial ischemia and to assess the need for revascularization, particularly if abnormal findings are elicited on exercise stress testing or echocardiography.144,145 Revascularization can suppress polymorphic ventricular tachycardias and VF secondary to acute myocardial ischaemia; however, revascularization has no effect on sustained ventricular tachycardias related to re-entry utilizing scar tissue related to a previous myocardial infarction and usually has no effect on isolated monomorphic VPBs. With regard to treatments for sustained ventricular arrhythmias and prevention of SCD, hypertension does not modify the indications for ICDs147 or ablation, as recommended by current guidelines.145,135
| Consensus statements . | . | References . |
|---|---|---|
| Frequent VPBs, couplets, or non-sustained ventricular arrhytdmias should need a careful clinical history and examination, blood chemistry, a 12-lead ECG, and a 24-h Holter recording. |  | 116–118 |
| Transthoracic echocardiography should be used when assessing hypertensive patients with arrhythmias to assess for signs of hypertensive or structural heart disease. |  | 56 |
| Finding of frequent VPBs and/or NSVT should lead to investigation for structural heart disease, including with transthoracic echocardiography or cardiac MRI. |  | 131,142,143 |
| Exercise testing or other functional testing for ischaemia may be used for patients with suspected coronary disease and frequent VPBs or associated symptoms, both for assessing suppression or worsening of VPBs and for evaluating the presence of myocardial ischaemia. Further non-invasive testing or coronary angiography may be used if necessary/needed. |  | 139–141 |
| Serological studies including electrolyte levels, glucose, and thyroid studies may be checked to assess for reversible, secondary causes of increased ventricular ectopy. |  | 131,142,143 |
| Identification of non-prescription or non-pharmacologic sources of increased adrenergic stimulation, including intake of alcohol, caffeine, other stimulants including recreational drugs, may be documented through history taking in order to provide appropriate counselling and/or assistance as needed. |  | 131,142,143 |
| Consensus statements . | . | References . |
|---|---|---|
| Frequent VPBs, couplets, or non-sustained ventricular arrhytdmias should need a careful clinical history and examination, blood chemistry, a 12-lead ECG, and a 24-h Holter recording. |  | 116–118 |
| Transthoracic echocardiography should be used when assessing hypertensive patients with arrhythmias to assess for signs of hypertensive or structural heart disease. |  | 56 |
| Finding of frequent VPBs and/or NSVT should lead to investigation for structural heart disease, including with transthoracic echocardiography or cardiac MRI. |  | 131,142,143 |
| Exercise testing or other functional testing for ischaemia may be used for patients with suspected coronary disease and frequent VPBs or associated symptoms, both for assessing suppression or worsening of VPBs and for evaluating the presence of myocardial ischaemia. Further non-invasive testing or coronary angiography may be used if necessary/needed. |  | 139–141 |
| Serological studies including electrolyte levels, glucose, and thyroid studies may be checked to assess for reversible, secondary causes of increased ventricular ectopy. |  | 131,142,143 |
| Identification of non-prescription or non-pharmacologic sources of increased adrenergic stimulation, including intake of alcohol, caffeine, other stimulants including recreational drugs, may be documented through history taking in order to provide appropriate counselling and/or assistance as needed. |  | 131,142,143 |
| Consensus statements . | . | References . |
|---|---|---|
| Frequent VPBs, couplets, or non-sustained ventricular arrhytdmias should need a careful clinical history and examination, blood chemistry, a 12-lead ECG, and a 24-h Holter recording. |  | 116–118 |
| Transthoracic echocardiography should be used when assessing hypertensive patients with arrhythmias to assess for signs of hypertensive or structural heart disease. |  | 56 |
| Finding of frequent VPBs and/or NSVT should lead to investigation for structural heart disease, including with transthoracic echocardiography or cardiac MRI. |  | 131,142,143 |
| Exercise testing or other functional testing for ischaemia may be used for patients with suspected coronary disease and frequent VPBs or associated symptoms, both for assessing suppression or worsening of VPBs and for evaluating the presence of myocardial ischaemia. Further non-invasive testing or coronary angiography may be used if necessary/needed. |  | 139–141 |
| Serological studies including electrolyte levels, glucose, and thyroid studies may be checked to assess for reversible, secondary causes of increased ventricular ectopy. |  | 131,142,143 |
| Identification of non-prescription or non-pharmacologic sources of increased adrenergic stimulation, including intake of alcohol, caffeine, other stimulants including recreational drugs, may be documented through history taking in order to provide appropriate counselling and/or assistance as needed. |  | 131,142,143 |
| Consensus statements . | . | References . |
|---|---|---|
| Frequent VPBs, couplets, or non-sustained ventricular arrhytdmias should need a careful clinical history and examination, blood chemistry, a 12-lead ECG, and a 24-h Holter recording. |  | 116–118 |
| Transthoracic echocardiography should be used when assessing hypertensive patients with arrhythmias to assess for signs of hypertensive or structural heart disease. |  | 56 |
| Finding of frequent VPBs and/or NSVT should lead to investigation for structural heart disease, including with transthoracic echocardiography or cardiac MRI. |  | 131,142,143 |
| Exercise testing or other functional testing for ischaemia may be used for patients with suspected coronary disease and frequent VPBs or associated symptoms, both for assessing suppression or worsening of VPBs and for evaluating the presence of myocardial ischaemia. Further non-invasive testing or coronary angiography may be used if necessary/needed. |  | 139–141 |
| Serological studies including electrolyte levels, glucose, and thyroid studies may be checked to assess for reversible, secondary causes of increased ventricular ectopy. |  | 131,142,143 |
| Identification of non-prescription or non-pharmacologic sources of increased adrenergic stimulation, including intake of alcohol, caffeine, other stimulants including recreational drugs, may be documented through history taking in order to provide appropriate counselling and/or assistance as needed. |  | 131,142,143 |
Management approaches
Management approaches for ventricular arrhythmias in hypertensive patients can vary widely based on primary presenting problem. The most common ventricular arrhythmias associated with hypertension are VPBs, although NSVT also has been observed and can affect prognosis, particularly in the context of LVH (Figure 5).117,130,143,148,149
Although a direct relation between VPB reduction and antihypertensive treatment has not been clearly shown, reduced fatal ventricular arrhythmia event risk has been demonstrated, and efforts to control blood pressure remain important. Some results suggest that beta blockers are inferior to other major antihypertensive drug classes in reducing LV mass and major cardiovascular event risk.150,151 However, other studies have indicated overall benefit in SCD reduction with BP lowering regardless of drug classes and demonstrated additional benefit with the use of beta blockers in patients with concomitant coronary heart disease.152 There is also agent-specific evidence of SCD reduction using ACEI or ARB, which also appears to be independent of blood pressure reduction.139,152 Thus, ACEI and ARB are also recommended in hypertensive patients at high risk for SCD.
Patients with hypertension-induced LVH may have greater QTc dispersion, particularly in the context of hypokalemia.131,142,143 A relationship between QT and RR intervals has also been observed in hypertensive patients with LVH, which is similar to other conditions associated with proarrhythmic potential, including subsets of long QT syndrome.13 Thus, avoiding marked hypokalaemia, or anything that prolongs repolarization time, may be important.
In asymptomatic hypertensive patients with normal LV systolic function and non-sustained ventricular arrhythmias, there is no role for prophylactic use of antiarrhythmic drugs. Sustained monomorphic VT is unusual in the absence of other structural heart disease.131,142,149 However, the subgroup of hypertensive patients in whom this may present are those who also have CAD with or without preserved LV function, although the hypertensive heart may also have advanced patchy fibrosis even in the absence of coronary heart disease. In the normal heart e.g. outflow tract VT (often sustained) and hypertension can occur together, and may need CMR for work up.
Antiarrhythmic drugs e.g. Class IC agents such as flecainide, is not recommended, especially where structural heart disease such as severe LVH or left ventricular systolic dysfunction is evident. In addition to beta blockers and ACE inhibitors or angiotensin receptor blockers (ARB), or catheter ablation should be considered in these patients, as well as implantable cardioverter-defibrillator (ICD).153 Similarly, among patients with low ejection fraction and persistently high frequency of ventricular ectopic beats (>15–20% of total beats in a day, or >10 000 PVCs/24 h) and/or associated symptoms, antiarrhythmic drugs or catheter ablation should be considered to potentially reverse tachycardia-induced cardiomyopathy.140,141 In the recent DANISH trial, prophylactic ICD implantation in patients with symptomatic systolic HF not caused by CAD was not associated with a significantly lower long-term rate of death from any cause than was usual clinical care.154
Finally, achieving adequate blood pressure control and promoting regression of LVH is a central goal in management, and any combination of antihypertensive drug classes should be considered as needed in order to achieve this goal, with the considerations as discussed above. Importantly, as LV systolic and diastolic dysfunction can result from poorly controlled hypertension alone, efforts to control BP and reduce associated risk of ventricular arrhythmias and SCD by improving LV ejection fraction are important. In the context of persistently severe LV systolic dysfunction (EF < 35%) despite adequate medical management, including BP control, implantation of an ICD should be considered, although in the absence of coronary disease the prognostic benefit is not evident.154,155
| Consensus statements . | . | References . |
|---|---|---|
| Achieving and maintaining adequate BP control should be an important priority when managing patients witd hypertension and ventricular arrhytdmias, especially if severe LV systolic dysfunction (EF < 35%) is present |  | 131,132,137,152 |
| Beta blockers should be used for management of hypertension in the setting of CAD and HF |  | 152 |
| Angiotensin converting enzyme inhibitors and ARB a should be used for hypertension management in patients at high risk for SCD |  | 137,152 |
| Avoiding hypokalaemia or QT prolonging drugs should be done in the context of HTN and LVH |  | 13,131,142,143 |
| In patients with sustained ventricular arrhythmias or frequent non-sustained ventricular arrhythmias with LV systolic dysfunction, treatment with beta-blocker, MRA and sacubitril/valsartan reduces the risk of sudden death and should be used for patients with HFrEF and ventricular arrhythmias, and, catheter ablation, and/or ICD implantation should be used in addition to antihypertensive therapy. |  | 155 |
An ICD should be used to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status.
|  | 155 |
| Routine use of antiarrhythmic drugs is not to be used in patients with HF and asymptomatic ventricular arrhythmias because of safety concerns (worsening HF, proarrhythmia, and death). |  | 154 |
| Consensus statements . | . | References . |
|---|---|---|
| Achieving and maintaining adequate BP control should be an important priority when managing patients witd hypertension and ventricular arrhytdmias, especially if severe LV systolic dysfunction (EF < 35%) is present |  | 131,132,137,152 |
| Beta blockers should be used for management of hypertension in the setting of CAD and HF |  | 152 |
| Angiotensin converting enzyme inhibitors and ARB a should be used for hypertension management in patients at high risk for SCD |  | 137,152 |
| Avoiding hypokalaemia or QT prolonging drugs should be done in the context of HTN and LVH |  | 13,131,142,143 |
| In patients with sustained ventricular arrhythmias or frequent non-sustained ventricular arrhythmias with LV systolic dysfunction, treatment with beta-blocker, MRA and sacubitril/valsartan reduces the risk of sudden death and should be used for patients with HFrEF and ventricular arrhythmias, and, catheter ablation, and/or ICD implantation should be used in addition to antihypertensive therapy. |  | 155 |
An ICD should be used to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status.
|  | 155 |
| Routine use of antiarrhythmic drugs is not to be used in patients with HF and asymptomatic ventricular arrhythmias because of safety concerns (worsening HF, proarrhythmia, and death). |  | 154 |
| Consensus statements . | . | References . |
|---|---|---|
| Achieving and maintaining adequate BP control should be an important priority when managing patients witd hypertension and ventricular arrhytdmias, especially if severe LV systolic dysfunction (EF < 35%) is present |  | 131,132,137,152 |
| Beta blockers should be used for management of hypertension in the setting of CAD and HF |  | 152 |
| Angiotensin converting enzyme inhibitors and ARB a should be used for hypertension management in patients at high risk for SCD |  | 137,152 |
| Avoiding hypokalaemia or QT prolonging drugs should be done in the context of HTN and LVH |  | 13,131,142,143 |
| In patients with sustained ventricular arrhythmias or frequent non-sustained ventricular arrhythmias with LV systolic dysfunction, treatment with beta-blocker, MRA and sacubitril/valsartan reduces the risk of sudden death and should be used for patients with HFrEF and ventricular arrhythmias, and, catheter ablation, and/or ICD implantation should be used in addition to antihypertensive therapy. |  | 155 |
An ICD should be used to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status.
|  | 155 |
| Routine use of antiarrhythmic drugs is not to be used in patients with HF and asymptomatic ventricular arrhythmias because of safety concerns (worsening HF, proarrhythmia, and death). |  | 154 |
| Consensus statements . | . | References . |
|---|---|---|
| Achieving and maintaining adequate BP control should be an important priority when managing patients witd hypertension and ventricular arrhytdmias, especially if severe LV systolic dysfunction (EF < 35%) is present |  | 131,132,137,152 |
| Beta blockers should be used for management of hypertension in the setting of CAD and HF |  | 152 |
| Angiotensin converting enzyme inhibitors and ARB a should be used for hypertension management in patients at high risk for SCD |  | 137,152 |
| Avoiding hypokalaemia or QT prolonging drugs should be done in the context of HTN and LVH |  | 13,131,142,143 |
| In patients with sustained ventricular arrhythmias or frequent non-sustained ventricular arrhythmias with LV systolic dysfunction, treatment with beta-blocker, MRA and sacubitril/valsartan reduces the risk of sudden death and should be used for patients with HFrEF and ventricular arrhythmias, and, catheter ablation, and/or ICD implantation should be used in addition to antihypertensive therapy. |  | 155 |
An ICD should be used to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status.
|  | 155 |
| Routine use of antiarrhythmic drugs is not to be used in patients with HF and asymptomatic ventricular arrhythmias because of safety concerns (worsening HF, proarrhythmia, and death). |  | 154 |
Complications related to arrhythmias and hypertension
Heart failure
Hypertension is one of the most common causes of HF. Antihypertensive therapy markedly reduces the incidence of HF. About half of all HF patients exhibit reduced ejection fraction.
Coronary artery disease is the cause of approximately two-thirds of cases of systolic HF, although hypertension and diabetes remain contributing factors.156 Incident HF with preserved ejection fraction (HFpEF) is not always directly a consequence of CAD, and increases with advancing age, hypertension, diabetes, obesity, and chronic renal dysfunction. Important triggers for HFpEF are uncontrolled hypertension and AF, two conditions that require aggressive management.157 Rhythm control in HFpEF is particularly relevant especially in those patients with substantial atrial contribution to LV filling.
In general, AF is predicted by the same markers of risk predicting HF, including target organ damage.158 In the context of hypertension, the association with HFpEF is particularly important because the LV filling pattern is always abnormal, requiring a greater atrial contribution.159 Although uncontrolled hypertension is certainly a trigger for AF, consolidated organ damage is the hallmark of risk.158 Thus, attention should be paid to the global management of risk (including metabolic factors and obesity) in addition to the aggressive antihypertensive therapy that is always required.
A rate control strategy is mandatory in persistent/permanent AF, to facilitate LV filling, and is obtained more frequently using cardiospecific beta-blockers.79 Uncontrolled heart rate may lead to a tachycardia-induced cardiomyopathy, with LV dilatation and impairment. In patients with systolic HF, the combination of digoxin and a beta-blocker could be effective. In patients with HFpEF, non-dihydropyridine calcium-channel blockers could be an alternative to a beta-blocker. In patients with chronic HF, a rhythm-control strategy has not been demonstrated to be superior to a rate-control strategy in reducing mortality or morbidity. In acute HF, emergency cardioversion may be required due to hemodynamic instability.
Postural hypotension
Postural (orthostatic) hypotension is usually defined as drop of 20 mmHg in systolic BP or 10 mmHg in diastolic BP within 2–5 min of standing up, and symptoms lasting a few seconds to several minutes of lightheadedness.161,162
Orthostatic hypotension (OH) is common in the elderly hypertensive population with a reported prevalence ranging from 6–30%. Due to its association with increased risk of falling, the presence of OH in elderly patients with HTN and AF may inappropriately prevent the use of OAC for stroke prevention. Also, the symptoms of lightheadedness or presyncope may raise suspicion of a cardiac arrhythmia in hypertensive patient, thus unnecessarily re-directing the diagnostic work-up.
Hypertension itself and commonly used antihypertensive drugs increase the incidence of OH. Orthostatic hypotension appears to be associated with immediate, and not prolonged, standing balance impairment, which is about three-fold higher in elderly hypertensive patients with OH or uncontrolled hypertension.160 The risk for OH increases with ageing and diabetes due to slower baroreceptors function, impaired cardiac performance, and stiffer arteries.163,164 Some antihypertensive and cardiodepressant medications (e.g. diuretics, alpha and beta blockers, calcium channel blockers, RAAS blockers, and nitrates), and drugs for Parkinson's disease and certain antidepressants and antipsychotics may increase the risk of orthostatic hypotension.165–167
Thromboembolism and bleeding risk, including safe use of antithrombotic therapy in hypertension
Increased blood pressure (BP, systolic BP of >130 mmHg or a diagnosis/history of hypertension) doubles the risk of stroke in patients with AF.168,169 Offer oral anticoagulation with VKAs or NOACs reduces stroke risk and mortality in AF170,171 but their benefit must be balanced against the risk of OAC-related major bleeding (especially ICH, due to its high fatality172,173) because uncontrolled hypertension (but not a diagnosis/history of hypertension) increases bleeding risk.174
Optimal BP control is crucial for both stroke and bleeding risk reduction in AF patients taking OAC. However, more data are needed to better define the target blood pressure values securing the most favourable risk/benefit ratio of OAC therapy in AF patients. Available evidence from randomized trials clearly shows a substantial increase in stroke risk (including both ischaemic and haemorrhagic stroke) at systolic BP values above 140 mmHg in AF patients taking warfarin.175 In a posthoc analysis of the ARISTOTLE trial Apixaban for Reduction In STroke and Other ThromboemboLic Events in AF), elevated BP (a systolic BP of ≥140 mmHg and/or a diastolic BP of ≥90 mmHg) at any point during the trial was associated with increased risk of stroke or systemic embolism (HR 1.53; 95%CI, 1.26–1.86), haemorrhagic stroke (HR 1.85; 95%CI, 1.26–2.72) and a composite of major and clinically relevant non-major bleeding (HR 1.14; 95%CI, 1.011.28) in both treatment arms (i.e. apixaban or warfarin), whilst history of hypertension was significantly associated with increased stroke, but not major bleeding.
Patients with uncontrolled hypertension defined as a systolic BP of ≥ 170–180 mmHg and/or diastolic BP of ≥ 100 mmHg were excluded in all four NOAC trials, whilst hypertension defined as the use of antihypertensive medications104,106,107 (or persistent systolic BP > 140 mmHg or diastolic BP > 90 mmHg107) was present in 78.8104–93.7%107 of participants. Most AF guidelines now favour the use of NOACs over VKAs (see Supplementary material online, Table S2). In patients with AF, all emphasize the importance of well-controlled anticoagulation with VKAs (i.e. the international normalized ratio time in therapeutic range of ≥70%). The SAMe-TT2R2 score of >2 (see Supplementary material online, Table S1, Section III-2) can be used to identify patients who are unlikely to do well on VKAs and hence should be prescribed NOACs (Figure 6).53
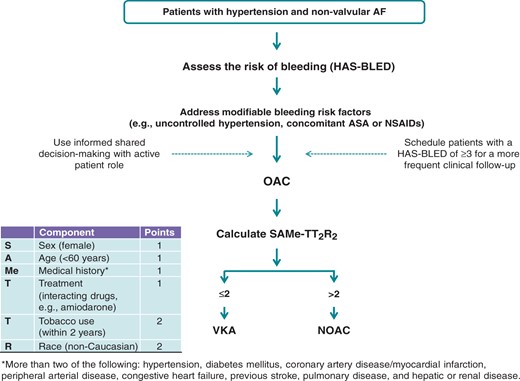
Proposed algorithm for antithrombotic management of patients with hypertension and non-valvular atrial fibrillation. ASA, Acetylsalicylic acid; NSAID, Non-steroidal anti-inflammatory drug; OAC, Oral anticoagulant; VKA, Vitamin K antagonist; NOAC, Non-vitamin K oral anticoagulant. SAMe-TT2R2, sex female, age, 60 years, medical history (more than two comorbidities), treatment (interacting drugs, e.g. amiodarone for rhythm control), tobacco use (doubled), race (doubled); TTR, time in therapeutic range.
Given its high prevalence among AF patients, hypertension may often be the single risk factor requiring a decision on OAC use, and data from contemporary real-world AF registries shows that physicians often underestimate the significance of hypertension as a stroke risk factor176,177 despite clearly positive net clinical benefit (the balance of stroke reduction against serious bleeding) of OAC in patients with ≥1 stroke risk factors in contemporary large AF cohorts.102,178
A recent analysis showed that the threshold for OAC use at ≥1.7%/year annual stroke risk considering VKAs should be decreased to ≥0.9%/year annual stroke risk with the safer NOACs.179 Two recent analyses of large AF cohorts of untreated patients with 1 stroke risk factor reported the annual stroke rates well above the NOACs threshold (1.55%180 and 2.55–2.75%181), and hypertension was associated with significant increase in stroke risk (HR 1.71; 95% CI, 1.48–1.98 [females], and 1.95; 95%CI, 1.73–2.19 [males]).181 The presence of one stroke risk factor in untreated AF patients was associated with increased rates of stroke, bleeding and death,180 and warfarin use was associated with a positive net clinical benefit compared to no therapy or aspirin.99 In clinical practice, shared informed decision-making is useful, as AF patients commonly attribute stronger value to the avoidance of stroke than the risk of bleeding.182
| Consensus statements . | . | References . |
|---|---|---|
| Offer oral anticoagulation should be used to reduce tde risk of stroke in most AF patients with hypertension, including tdose with AF in whom hypertension is the single additional stroke risk factor. |  | 97,168,169 |
| Shared, informed decision-making regarding the risks and benefits of OAC therapy should be used, especially where hypertension is the single additional risk factor for stroke. |  | |
| Well-controlled anticoagulation intensity (i.e. a TTR of ≥ 65–70%) should be used for achieving the optimal risk/benefit ratio with VKA therapy. |  | 53,97 |
| Compared to VKAs, NOACs offer additional safety benefit provided that there is good adherence to treatment. Optimal blood pressure control should be used for minimizing the risks of AF-related stroke and OAC-related bleeding. Until more data are available, target BP values in AF patients taking OAC should be below 140 mmHg for systolic BP and below 90 mmHg for diastolic BP. Oral anticoagulation should be used with caution in patients with persistent uncontrolled hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 100 mmHg) but strenuous efforts to control BP should be made. |  | 97,175 |
| Consensus statements . | . | References . |
|---|---|---|
| Offer oral anticoagulation should be used to reduce tde risk of stroke in most AF patients with hypertension, including tdose with AF in whom hypertension is the single additional stroke risk factor. |  | 97,168,169 |
| Shared, informed decision-making regarding the risks and benefits of OAC therapy should be used, especially where hypertension is the single additional risk factor for stroke. |  | |
| Well-controlled anticoagulation intensity (i.e. a TTR of ≥ 65–70%) should be used for achieving the optimal risk/benefit ratio with VKA therapy. |  | 53,97 |
| Compared to VKAs, NOACs offer additional safety benefit provided that there is good adherence to treatment. Optimal blood pressure control should be used for minimizing the risks of AF-related stroke and OAC-related bleeding. Until more data are available, target BP values in AF patients taking OAC should be below 140 mmHg for systolic BP and below 90 mmHg for diastolic BP. Oral anticoagulation should be used with caution in patients with persistent uncontrolled hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 100 mmHg) but strenuous efforts to control BP should be made. |  | 97,175 |
| Consensus statements . | . | References . |
|---|---|---|
| Offer oral anticoagulation should be used to reduce tde risk of stroke in most AF patients with hypertension, including tdose with AF in whom hypertension is the single additional stroke risk factor. |  | 97,168,169 |
| Shared, informed decision-making regarding the risks and benefits of OAC therapy should be used, especially where hypertension is the single additional risk factor for stroke. |  | |
| Well-controlled anticoagulation intensity (i.e. a TTR of ≥ 65–70%) should be used for achieving the optimal risk/benefit ratio with VKA therapy. |  | 53,97 |
| Compared to VKAs, NOACs offer additional safety benefit provided that there is good adherence to treatment. Optimal blood pressure control should be used for minimizing the risks of AF-related stroke and OAC-related bleeding. Until more data are available, target BP values in AF patients taking OAC should be below 140 mmHg for systolic BP and below 90 mmHg for diastolic BP. Oral anticoagulation should be used with caution in patients with persistent uncontrolled hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 100 mmHg) but strenuous efforts to control BP should be made. |  | 97,175 |
| Consensus statements . | . | References . |
|---|---|---|
| Offer oral anticoagulation should be used to reduce tde risk of stroke in most AF patients with hypertension, including tdose with AF in whom hypertension is the single additional stroke risk factor. |  | 97,168,169 |
| Shared, informed decision-making regarding the risks and benefits of OAC therapy should be used, especially where hypertension is the single additional risk factor for stroke. |  | |
| Well-controlled anticoagulation intensity (i.e. a TTR of ≥ 65–70%) should be used for achieving the optimal risk/benefit ratio with VKA therapy. |  | 53,97 |
| Compared to VKAs, NOACs offer additional safety benefit provided that there is good adherence to treatment. Optimal blood pressure control should be used for minimizing the risks of AF-related stroke and OAC-related bleeding. Until more data are available, target BP values in AF patients taking OAC should be below 140 mmHg for systolic BP and below 90 mmHg for diastolic BP. Oral anticoagulation should be used with caution in patients with persistent uncontrolled hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 100 mmHg) but strenuous efforts to control BP should be made. |  | 97,175 |
Health economic considerations
Taking into account the risk of cardiovascular events linked to high blood pressure, the costs of untreated or inadequately controlled hypertension are of great relevance for any health care system.183 According to a meta-regression analyses, every 10 mmHg reduction in systolic blood pressure the risk of major cardiovascular disease events is reduced by 20%, the risk of coronary heart disease by 17%, the risk of stroke by 27%, the risk of HF by 28% and the risk of all-cause mortality by 13%.150
The high prevalence of both hypertension and AF and the increasing costs for their treatment constitute an important financial burden, and therefore many economic analyses have been done with the aim to assess the cost-effectiveness of treating these diseases.184 In these analyses the focus is mainly on the risk of stroke and the savings that can be obtained by preventing stroke occurrence and the consequent health care resources for hospitalization, rehabilitation, and assistance resulting from disability, which are associated with high direct and indirect costs.185
For stroke associated with AF, the direct costs per patient are approximately 33% greater than the costs for stroke unrelated to AF186 and are in the range of Euro 30 000 over a 2-year period for a severe ischaemic stroke.197 Within this scenario it is noteworthy that use of NOACs in patients with non-valvular AF has been found to be cost-effective.188,189 In a series of analysis focused both on cost effectiveness and cost-benefit, the higher initial cost of NOACs compared to warfarin is offset by the reduction in intracranial bleeding and stroke prevention, making these agents cost-effective in the long-term.188,189
Finally, from an economic perspective, it is noteworthy to stress that since AF is frequently asymptomatic, opportunistic screening for asymptomatic AF is indicated in order to institute antithromboembolic prophylaxis in patients at risk, and proved to be cost-effective.89
Areas for further research
Many areas linking hypertension and cardiac arrhythmias deserve additional clarification and merit future study. Whilst perhaps rather selective, some areas of uncertainty are summarized as follows:
How different circadian BP profiles, particularly blunted nocturnal blood pressure, influence the presence of different arrhythmias
Detection and management of hypertensive patients with silent AF to prevent stroke risk, and whether OAC instituted in population based screen detected AF results in a meaningful stroke reduction.
Antihypertensive drugs and regression of myocardial fibrosis in patients with hypertension and LVH
Primary prevention of arrhythmias in patients with uncomplicated hypertension: is there a preferred antihypertensive drug or combination?
Optimal antihypertensive treatment in patients with HF and preserved ejection fraction.
Optimal BP targets in patients with hypertension and OAC therapy
Atrial fibrillation management in asymptomatic cases detected by remote monitoring of implantable cardiac devices
Supplementary material
Supplementary material is available at Europace online.
Acknowledgement
European Heart Rhythm Association Scientific Committee: Prof. Gregory Lip (chair), Prof. Bulent Gorenek (co-chair), Prof. Christian Sticherling, Prof. Laurent Fauchier, Prof. A. Goette, Prof. Werner Jung, Prof. Marc A Vos, Dr Michele Brignole, Dr. Christian Elsner, Prof. Gheorghe-Andrei Dan, Dr Francisco Marin, Prof. Giuseppe Boriani, Dr Deirdre Lane, Prof. Carina Blomstrom Lundqvist, Dr Irina Savelieva.
Conflict of interest: Conflict of Interest information is available in the supplementary data online.



