-
PDF
- Split View
-
Views
-
Cite
Cite
Uğur Canpolat, Kudret Aytemir, Hikmet Yorgun, Levent Şahiner, Ergün Barış Kaya, Serkan Çay, Serkan Topaloğlu, Dursun Aras, Ali Oto, Usefulness of serum uric acid level to predict atrial fibrillation recurrence after cryoballoon-based catheter ablation, EP Europace, Volume 16, Issue 12, December 2014, Pages 1731–1737, https://doi.org/10.1093/europace/euu198
Close - Share Icon Share
Abstract
Catheter-based atrial fibrillation (AF) ablation has become an important therapeutic option in AF patients. Although there has been significant improvent in procedural success, post-procedural AF recurrences are continuing to be a major clinical problem. To the best of our knowledge, the impact of pre-procedural serum uric acid (SUA) level, as a pro-oxidant and pro-inflammatory marker, on AF recurrence following cryoballoon-based AF ablation has never been studied before. The objective of this study was to establish whether there is a relationship between levels of SUA and recurrence of paroxysmal AF after catheter ablation.
A total of 363 patients (mean age 53.5 ± 11.2 years, 52.6% male) with symptomatic paroxysmal AF underwent initial cryoablation procedure. Patients were categorized into quartiles on the basis of their pre-procedural SUA assays and follow-up, and the Kaplan–Meier estimation with a log-rank test was used for the analysis of the influence of SUA on the recurrence of AF. Post-ablation blanking period was observed for 3 months. At a mean follow-up of 19.2 ± 6.1 months, 68 patients (18.7%) had developed AF recurrence. Atrial fibrillation recurrence rates from the lowest to the highest SUA quartiles were 2.9, 7.4, 11.8, and 77.9%, respectively (P < 0.001). On multivariate Cox regression analysis, pre-ablation SUA level (HR: 1.96, 95% CI: 1.49–2.59, P < 0.001), left atrial diameter (HR: 1.11, 95% CI: 1.04–1.19, P = 0.002) and early AF recurrence (HR: 4.34, 95% CI: 1.9–9.95, P = 0.001) were independent predictors of AF recurrence after cryoablation. Using a cut-off level of 6.37, the pre-ablation SUA level predicted AF recurrence during follow-up with a sensitivity of 85.7% and a specificity of 83.7%.
In this prospective study of patients with paroxysmal AF undergoing cryoablation, increased pre-ablation SUA levels were associated with a higher rate of AF recurrence. Our results support the role of a pre-ablation pro-inflammatory and pro-oxidant environment in the development of AF recurrence after ablation therapy but suggest that other factors are also important.
To the best of our knowledge, the impact of pre-procedural SUA level, as a pro-oxidant and pro-inflammatory marker, on AF recurrence following cryoballoon-based PV isolation has never been studied before.
In this prospective study of patients with paroxysmal AF undergoing cryoablation, increased pre-ablation SUA levels were associated with a higher rate of AF recurrence.
Our results support the role of a pre-ablation pro-inflammatory and pro-oxidant environment in the development of AF recurrence after ablation therapy but suggest that other factors are also important.
Introduction
Catheter-based AF ablation with the primary aim of pulmonary vein (PV) isolation has become an important therapeutic option in symptomatic and drug-refractory AF patients with increased efficacy and safety.1,2 Although there has been significant improvent in procedural success by using recently developed ablation techniques, post-procedural AF recurrences are continuing to be a major clinical problem following catheter ablation, occurring approximately in 25–50% of the patients.3 Various biochemical parameters have been investigated to predict the success of catheter ablation and select appropriate patients for invasive strategy, but results have not been consistent.4 To the best of our knowledge, the impact of pre-procedural SUA level, as an pro-oxidant and pro-inflammatory marker, on AF recurrence following cryoballoon-based PV isolation has never been studied before.
In this study, we aimed to present that the presence of a pre-procedural pro-oxidant and inflammatory state as determined by known clinical parameters and conventional markers including hs-CRP, white blood cell (WBC), and SUA may be associated with AF recurrence following cryoablation.
Methods
Patients
In this prospective and observational study, we enrolled 363 consecutive patients (mean age 53.5 ± 11.2 years, 52.6% males) who underwent initial PV isolation with cryoballoon technique for documented AF. All the patients had symptomatic paroxysmal AF and had failed with at least one antiarrhythmic medication previously. Patients whose episodes of AF have been self-terminated within 7 days were defined as paroxysmal AF.1
Patients who had moderate-to-severe valvular disease, thrombus in left atrium, uncontrolled thyroid dysfunction, heart failure, renal dysfunction (serum creatinine levels ≥1.5 mg/dL), hepatic and haemolytic disorders, concomitant inflammatory diseases, neoplastic diseases or any other systemic disorders, myocardial infarction or cardiac surgery in the previous 3 months, contraindication of anticoagulation, alcohol consumption, vitamin use (including vitamin C, niacin, folate), and patients taking diuretic medications or UA lowering medications like allopurinol, pregnancy and left atrium anteroposterior diameter >55 mm were excluded from the study.
Detailed medical history regarding AF and related cardiovascular and/or systemic conditions was taken from all the patients. Symptomatic severity of the patient was recorded according to the European Heart Rhythm Association (EHRA) score. The CHADS2 and CHA2DS2-VASc scores were calculated for each patient based on a point system.1
Informed consent was taken from each patient before enrollment. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by Institutional Ethics Committee.
Pre-procedural evaluation
All patients underwent standard transthoracic echocardiography to rule structural abnormality, transesophageal echocardiography to rule out thrombus at left atrium and multidetector computerized tomography to evaluate PV configuration and left atrium diameters. Anticoagulation was stopped at least 48–72 h before the procedure and the pre-procedural interval was bridged with enaxoparin 1 mg/kg. Antiarrhythmic drugs were discontinued five half-lives before the procedure.
Blood sample analyses
Samples for the complete blood count analysis were collected in EDTA (ethylene-diamine-tetraacetic acid)-anticoagulated Monovette® tubes (Sarstedt) 24 h before cryoablation. hs-CRP measurement was done using an automatized analyser (Beckman Coulter® IMMAGE) using nephelometric measurement 24 h before cryoablation. Serum UA levels were determined with enzymatic colorimetric method by clinical chemistry auto-analyzer (Aeroset, Abbott Laboratory, Abbott Park).
Electrophysiological study and ablation procedure
All procedures were performed under conscious sedation using boluses of midazolam. Invasive arterial blood pressure, oxygen saturation and electrocardiogram (ECG) were continuously monitored throughout the entire procedure. Right femoral vein and left femoral vein/artery punctures were performed with Seldinger technique. A 6Fr steerable decapolar catheter (Dynamic Deca, Bard Electrophysiology) was placed into the coronary sinus. Single transseptal puncture with using a modified Brockenbrough technique (BRK-1, St. Jude Medical) was performed under fluoroscopy and 8Fr transseptal sheath (Biosense Webster) placed into the left atrium. Once left atrial access is obtained, heparin boluses were repeatedly administered to maintain the activated clotting time between 300 and 350 s. The sheath was then exchanged for the 12F steerable transseptal sheath (FlexCath, Medtronic CryoCath) over a guidewire (0.032 inç, 180 cm Super Stiff, St.Jude Medical). Baseline potentials of all PVs were recorded with a Lasso catheter (Biosense Webster, Inc., Diamond Bar). We paced the distal coronary sinus to confirm the presence of left PV potentials. In all patients, the 28 mm cryoballoon catheter (Arctic Front©, Medtronic CryoCath LP) was used for PVI. The cryoballoon was manoeuvered to all PV ostia by use of a steerable 12 Fr sheath and a guidewire inserted through the lumen of the balloon catheter. The balloon is inflated in the left atrium and then directed towards the PV ostia. The assessment of balloon occlusion is performed through the injection of 50% diluted contrast through the cryoballoon catheter's central lumen. The duration of each freezing cycle was 300 s. A minimum of two consecutive freezing cycles for each targeted PV were delivered with excellent or good occlusion. The procedure systematically began with the left superior, then the left inferior, followed by the right superior, and ended with the right inferior PV. The right phrenic nerve was constantly paced from the superior vena cava during freezing at the right-sided PVs. Also direct palpation of the right hemi-diaphragmatic excursion was performed during phrenic nevre stimulation. At the end of the procedure, PV conduction was reevaluated by the Lasso catheter. Successfull PV isolation was defined as the elimination (or dissociation) of all the PV potentials recorded from a Lasso catheter.
Post-procedural follow-up
The patients remained under continuous haemodynamic and ECG monitoring for 24 h. Immediately after the procedure and 24 h following the procedure, transthoracic echocardiography was performed to ascertain the absence of pericardial effusion. Oral anticoagulation with warfarin was initiated after 4–6 h of the procedure and also concomitant enaxoparin 1 mg/kg administered until target INR of 2.0–3.0. For the following 3 months, the patients remained on the antiarrhythmic drug regimen they were prescribed before the ablation procedure.
Follow-up visits were performed at 3, 6, 12 months and for every 6 months thereafter, or earlier if they developed symptoms consistent with recurrent AF. A 24 h Holter ECG was recorded 3 months after the procedure, usually during antiarrhythmic therapy. In the absence of arrhythmia, all antiarrhythmic drugs were discontinued. Another 24 h Holter ECG was recorded 3 months later and for every 6 months thereafter. The need for oral anticoagulation was also evaluated after 3 months, based on the CHA2DS2VASc score.1
Acute procedural success is defined as electrical isolation of all PVs. First 3 months after AF ablation is defined as blanking period. Early recurrence of AF is defined as detection of AF (at least 30 s duration when assessed with ECG monitoring) within 3 months of ablation. Recurrence of AF is defined as detection of AF (at least 30 s duration when assessed with ECG monitoring) >3 months following AF ablation.3
Statistical analysis
Continuous variables are presented as mean values ± SD, whereas categorical ones are presented as percentages. The Shapiro–Wilk criterion was used for the assessment of normality. The study population was assigned into quartiles (Q) based on pre-procedural SUA levels (Q1: <4.79; Q2: 4.79–5.62; Q3: 5.63–6.54; Q4: >6.54). Comparisons of multiple mean values were carried out by Kruskal–Wallis tests or analysis of variance as appropriate. Comparisons of continuous data between two groups (reccurrence/no recurrence) were made by unpaired t-test, whereas categorical data were compared in both groups using the χ2 test. Correlations were assessed using Spearman's rank test. Receiver operating characteristic curve analysis was used to determine the optimum cut-off levels of pre-procedural SUA level, LA diameter, and duration of AF history to predict the recurrence of AF after cryoablation. Also ROC curves for each parameter were compared with each other by using MedCalc 11.4.2 (MedCalc Software).5 Time to recurrence of AF was plotted using Kaplan–Meier curves for patients with AF due to SUA level quartiles separately (with a blanking period of 3 months following cryoablation applied). Cox proportional hazard regression was used in order to test the effect of the explanatory variables on AF recurrence, adjusted for other variables. A P value of <0.05 was considered statistically significant. All analyses were performed, using the SPSS software, version 20.0 (SPSS, Inc.).
Results
Baseline characteristics and demographical features of the study population were given in Table 1. After a mean follow-up period of 19.2 ± 6.1 months (range 6–32), early recurrence was developed in 33 (9.1%) patients and recurrence after blanking period was observed in 68 (18.7%) patients. As shown in Table 1, patients with AF recurrence exhibited older age, higher rate of male gender, CAD, alcohol intake and smoking, higher duration of AF history, early recurrence, and also increased pre-procedural SUA level compared with those who remained in sinus rhythm (P < 0.05). Also, Table 2 presents the baseline demographic and clinical data of the patients by quartile of pre-procedural SUA levels. Patients in Q4 showed an increased duration of AF history, increased hsCRP, WBC count and SUA levels, higher rate of early recurrence, and recurrence rates compared with those in Q1, Q2, and Q3 (P < 0.05).
Baseline characteristics and demographical features of the study population (n = 363)
| Parameters . | Total n = 363 . | Recurrence (−) n = 295 . | Recurrence (+) n = 68 . | P . |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years (mean ± SD) | 53.5 ± 11.2 | 52.6 ± 11.1 | 57.2 ± 10.9 | 0.002 |
| Gender, male; n (%) | 191 (52.6%) | 146 (49.5%) | 45 (66.2%) | 0.015 |
| BMI, kg/m2 | 24.3 ± 3.7 | 24.1 ± 3.8 | 24.7 ± 2.8 | 0.277 |
| CAD, n (%) | 38 (10.5%) | 24 (8.1%) | 14 (20.6%) | 0.007 |
| Diabetes mellitus, n (%) | 46 (12.7%) | 35 (11.9%) | 11 (16.2%) | 0.319 |
| Dyslipidaemia, n (%) | 77 (21.2%) | 57 (19.3%) | 20 (29.4%) | 0.072 |
| Hypertension, n (%) | 132 (36.4%) | 104 (35.3%) | 28 (41.2%) | 0.402 |
| Alcohol, n (%) | 31 (8.5%) | 19 (6.4%) | 12 (17.6%) | 0.006 |
| Smoking, n (%) | 111 (30.6%) | 79 (26.8%) | 32 (47.1%) | 0.002 |
| Duration of AF history, years | 5.9 ± 4.4 | 5.2 ± 3.3 | 8.8 ± 6.8 | <0.001 |
| LA diameter (AP), mm | 37.7 ± 4.7 | 37.3 ± 4.4 | 39.6 ± 5.6 | 0.033 |
| LVEF (%) | 65.1 ± 4.2 | 65.2 ± 4.0 | 64.9 ± 4.9 | 0.075 |
| Failed antiarrhythmics, mean ± SD | 1.82 ± 0.75 | 1.79 ± 0.58 | 1.85 ± 0.65 | 0.072 |
| EHRA score, mean | 2.80 ± 0.59 | 2.83 ± 0.56 | 3.0 ± 0.57 | 0.096 |
| CHADS2 score, mean ± SD | 1.52 ± 0.81 | 1.49 ± 0.82 | 1.60 ± 0.79 | 0.323 |
| CHA2DS2-vasc score, mean ± SD | 2.11 ± 1.05 | 2.10 ± 1.03 | 2.15 ± 1.10 | 0.729 |
| Statins, n (%) | 68 (18.7%) | 55 (18.6%) | 13 (19.1%) | 0.224 |
| ACE inhibitor/ARB, n (%) | 128 (35.2%) | 103 (34.9%) | 25 (36.7%) | 0.064 |
| Laboratory parameters | ||||
| Haemoglobin, g/dL | 13.6 ± 1.9 | 13.6 ± 1.9 | 13.9 ± 2.0 | 0.202 |
| WBC, ×106/L | 7786 ± 2301 | 7644 ± 2279 | 7837 ± 2104 | 0.452 |
| SUA, mg/dL | 5.74 ± 1.31 | 5.38 ± 1.0 | 7.25 ± 1.3 | <0.001 |
| SUA quartiles, n (%) | ||||
| Q1 (<4.79 mg/dL) | 87 (24.0%) | 85 (28.8%) | 2 (2.9%) | <0.001 |
| Q2 (4.79–5.62 mg/dL) | 94 (25.9%) | 89 (30.2%) | 5 (7.4%) | |
| Q3 (5.63–6.54 mg/dL) | 90 (24.8%) | 82 (27.8%) | 8 (11.8%) | |
| Q4 (>6.54 mg/dL) | 92 (25.3%) | 39 (13.2%) | 53 (77.9%) | |
| hsCRP, mg/L | 1.77 ± 0.36 | 1.79 ± 0.36 | 1.69 ± 0.38 | 0.457 |
| Serum creatinine, mg/dL | 0.89 ± 0.21 | 0.88 ± 0.15 | 0.92 ± 0.21 | 0.645 |
| Follow-up parameters | ||||
| Early recurrence, n (%) | 33 (9.1%) | 7 (2.4%) | 26 (38.2%) | <0.001 |
| Follow-up time, months | 19.2 ± 6.1 | 18.7 ± 6.4 | 20.1 ± 6.2 | 0.065 |
| Parameters . | Total n = 363 . | Recurrence (−) n = 295 . | Recurrence (+) n = 68 . | P . |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years (mean ± SD) | 53.5 ± 11.2 | 52.6 ± 11.1 | 57.2 ± 10.9 | 0.002 |
| Gender, male; n (%) | 191 (52.6%) | 146 (49.5%) | 45 (66.2%) | 0.015 |
| BMI, kg/m2 | 24.3 ± 3.7 | 24.1 ± 3.8 | 24.7 ± 2.8 | 0.277 |
| CAD, n (%) | 38 (10.5%) | 24 (8.1%) | 14 (20.6%) | 0.007 |
| Diabetes mellitus, n (%) | 46 (12.7%) | 35 (11.9%) | 11 (16.2%) | 0.319 |
| Dyslipidaemia, n (%) | 77 (21.2%) | 57 (19.3%) | 20 (29.4%) | 0.072 |
| Hypertension, n (%) | 132 (36.4%) | 104 (35.3%) | 28 (41.2%) | 0.402 |
| Alcohol, n (%) | 31 (8.5%) | 19 (6.4%) | 12 (17.6%) | 0.006 |
| Smoking, n (%) | 111 (30.6%) | 79 (26.8%) | 32 (47.1%) | 0.002 |
| Duration of AF history, years | 5.9 ± 4.4 | 5.2 ± 3.3 | 8.8 ± 6.8 | <0.001 |
| LA diameter (AP), mm | 37.7 ± 4.7 | 37.3 ± 4.4 | 39.6 ± 5.6 | 0.033 |
| LVEF (%) | 65.1 ± 4.2 | 65.2 ± 4.0 | 64.9 ± 4.9 | 0.075 |
| Failed antiarrhythmics, mean ± SD | 1.82 ± 0.75 | 1.79 ± 0.58 | 1.85 ± 0.65 | 0.072 |
| EHRA score, mean | 2.80 ± 0.59 | 2.83 ± 0.56 | 3.0 ± 0.57 | 0.096 |
| CHADS2 score, mean ± SD | 1.52 ± 0.81 | 1.49 ± 0.82 | 1.60 ± 0.79 | 0.323 |
| CHA2DS2-vasc score, mean ± SD | 2.11 ± 1.05 | 2.10 ± 1.03 | 2.15 ± 1.10 | 0.729 |
| Statins, n (%) | 68 (18.7%) | 55 (18.6%) | 13 (19.1%) | 0.224 |
| ACE inhibitor/ARB, n (%) | 128 (35.2%) | 103 (34.9%) | 25 (36.7%) | 0.064 |
| Laboratory parameters | ||||
| Haemoglobin, g/dL | 13.6 ± 1.9 | 13.6 ± 1.9 | 13.9 ± 2.0 | 0.202 |
| WBC, ×106/L | 7786 ± 2301 | 7644 ± 2279 | 7837 ± 2104 | 0.452 |
| SUA, mg/dL | 5.74 ± 1.31 | 5.38 ± 1.0 | 7.25 ± 1.3 | <0.001 |
| SUA quartiles, n (%) | ||||
| Q1 (<4.79 mg/dL) | 87 (24.0%) | 85 (28.8%) | 2 (2.9%) | <0.001 |
| Q2 (4.79–5.62 mg/dL) | 94 (25.9%) | 89 (30.2%) | 5 (7.4%) | |
| Q3 (5.63–6.54 mg/dL) | 90 (24.8%) | 82 (27.8%) | 8 (11.8%) | |
| Q4 (>6.54 mg/dL) | 92 (25.3%) | 39 (13.2%) | 53 (77.9%) | |
| hsCRP, mg/L | 1.77 ± 0.36 | 1.79 ± 0.36 | 1.69 ± 0.38 | 0.457 |
| Serum creatinine, mg/dL | 0.89 ± 0.21 | 0.88 ± 0.15 | 0.92 ± 0.21 | 0.645 |
| Follow-up parameters | ||||
| Early recurrence, n (%) | 33 (9.1%) | 7 (2.4%) | 26 (38.2%) | <0.001 |
| Follow-up time, months | 19.2 ± 6.1 | 18.7 ± 6.4 | 20.1 ± 6.2 | 0.065 |
AF, atrial fibrillation; AP, anteroposterior; BMI, body mass index; CAD, coronary artery disease; EHRA, European Heart Rhythm Association; hsCRP, high sensitive C-reactive protein; LA, left atrium; LVEF, left ventricular ejection fraction; Q, quartile; SD, standard deviation; SUA, serum uric acid; WBC, white blood cell count.
Baseline characteristics and demographical features of the study population (n = 363)
| Parameters . | Total n = 363 . | Recurrence (−) n = 295 . | Recurrence (+) n = 68 . | P . |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years (mean ± SD) | 53.5 ± 11.2 | 52.6 ± 11.1 | 57.2 ± 10.9 | 0.002 |
| Gender, male; n (%) | 191 (52.6%) | 146 (49.5%) | 45 (66.2%) | 0.015 |
| BMI, kg/m2 | 24.3 ± 3.7 | 24.1 ± 3.8 | 24.7 ± 2.8 | 0.277 |
| CAD, n (%) | 38 (10.5%) | 24 (8.1%) | 14 (20.6%) | 0.007 |
| Diabetes mellitus, n (%) | 46 (12.7%) | 35 (11.9%) | 11 (16.2%) | 0.319 |
| Dyslipidaemia, n (%) | 77 (21.2%) | 57 (19.3%) | 20 (29.4%) | 0.072 |
| Hypertension, n (%) | 132 (36.4%) | 104 (35.3%) | 28 (41.2%) | 0.402 |
| Alcohol, n (%) | 31 (8.5%) | 19 (6.4%) | 12 (17.6%) | 0.006 |
| Smoking, n (%) | 111 (30.6%) | 79 (26.8%) | 32 (47.1%) | 0.002 |
| Duration of AF history, years | 5.9 ± 4.4 | 5.2 ± 3.3 | 8.8 ± 6.8 | <0.001 |
| LA diameter (AP), mm | 37.7 ± 4.7 | 37.3 ± 4.4 | 39.6 ± 5.6 | 0.033 |
| LVEF (%) | 65.1 ± 4.2 | 65.2 ± 4.0 | 64.9 ± 4.9 | 0.075 |
| Failed antiarrhythmics, mean ± SD | 1.82 ± 0.75 | 1.79 ± 0.58 | 1.85 ± 0.65 | 0.072 |
| EHRA score, mean | 2.80 ± 0.59 | 2.83 ± 0.56 | 3.0 ± 0.57 | 0.096 |
| CHADS2 score, mean ± SD | 1.52 ± 0.81 | 1.49 ± 0.82 | 1.60 ± 0.79 | 0.323 |
| CHA2DS2-vasc score, mean ± SD | 2.11 ± 1.05 | 2.10 ± 1.03 | 2.15 ± 1.10 | 0.729 |
| Statins, n (%) | 68 (18.7%) | 55 (18.6%) | 13 (19.1%) | 0.224 |
| ACE inhibitor/ARB, n (%) | 128 (35.2%) | 103 (34.9%) | 25 (36.7%) | 0.064 |
| Laboratory parameters | ||||
| Haemoglobin, g/dL | 13.6 ± 1.9 | 13.6 ± 1.9 | 13.9 ± 2.0 | 0.202 |
| WBC, ×106/L | 7786 ± 2301 | 7644 ± 2279 | 7837 ± 2104 | 0.452 |
| SUA, mg/dL | 5.74 ± 1.31 | 5.38 ± 1.0 | 7.25 ± 1.3 | <0.001 |
| SUA quartiles, n (%) | ||||
| Q1 (<4.79 mg/dL) | 87 (24.0%) | 85 (28.8%) | 2 (2.9%) | <0.001 |
| Q2 (4.79–5.62 mg/dL) | 94 (25.9%) | 89 (30.2%) | 5 (7.4%) | |
| Q3 (5.63–6.54 mg/dL) | 90 (24.8%) | 82 (27.8%) | 8 (11.8%) | |
| Q4 (>6.54 mg/dL) | 92 (25.3%) | 39 (13.2%) | 53 (77.9%) | |
| hsCRP, mg/L | 1.77 ± 0.36 | 1.79 ± 0.36 | 1.69 ± 0.38 | 0.457 |
| Serum creatinine, mg/dL | 0.89 ± 0.21 | 0.88 ± 0.15 | 0.92 ± 0.21 | 0.645 |
| Follow-up parameters | ||||
| Early recurrence, n (%) | 33 (9.1%) | 7 (2.4%) | 26 (38.2%) | <0.001 |
| Follow-up time, months | 19.2 ± 6.1 | 18.7 ± 6.4 | 20.1 ± 6.2 | 0.065 |
| Parameters . | Total n = 363 . | Recurrence (−) n = 295 . | Recurrence (+) n = 68 . | P . |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years (mean ± SD) | 53.5 ± 11.2 | 52.6 ± 11.1 | 57.2 ± 10.9 | 0.002 |
| Gender, male; n (%) | 191 (52.6%) | 146 (49.5%) | 45 (66.2%) | 0.015 |
| BMI, kg/m2 | 24.3 ± 3.7 | 24.1 ± 3.8 | 24.7 ± 2.8 | 0.277 |
| CAD, n (%) | 38 (10.5%) | 24 (8.1%) | 14 (20.6%) | 0.007 |
| Diabetes mellitus, n (%) | 46 (12.7%) | 35 (11.9%) | 11 (16.2%) | 0.319 |
| Dyslipidaemia, n (%) | 77 (21.2%) | 57 (19.3%) | 20 (29.4%) | 0.072 |
| Hypertension, n (%) | 132 (36.4%) | 104 (35.3%) | 28 (41.2%) | 0.402 |
| Alcohol, n (%) | 31 (8.5%) | 19 (6.4%) | 12 (17.6%) | 0.006 |
| Smoking, n (%) | 111 (30.6%) | 79 (26.8%) | 32 (47.1%) | 0.002 |
| Duration of AF history, years | 5.9 ± 4.4 | 5.2 ± 3.3 | 8.8 ± 6.8 | <0.001 |
| LA diameter (AP), mm | 37.7 ± 4.7 | 37.3 ± 4.4 | 39.6 ± 5.6 | 0.033 |
| LVEF (%) | 65.1 ± 4.2 | 65.2 ± 4.0 | 64.9 ± 4.9 | 0.075 |
| Failed antiarrhythmics, mean ± SD | 1.82 ± 0.75 | 1.79 ± 0.58 | 1.85 ± 0.65 | 0.072 |
| EHRA score, mean | 2.80 ± 0.59 | 2.83 ± 0.56 | 3.0 ± 0.57 | 0.096 |
| CHADS2 score, mean ± SD | 1.52 ± 0.81 | 1.49 ± 0.82 | 1.60 ± 0.79 | 0.323 |
| CHA2DS2-vasc score, mean ± SD | 2.11 ± 1.05 | 2.10 ± 1.03 | 2.15 ± 1.10 | 0.729 |
| Statins, n (%) | 68 (18.7%) | 55 (18.6%) | 13 (19.1%) | 0.224 |
| ACE inhibitor/ARB, n (%) | 128 (35.2%) | 103 (34.9%) | 25 (36.7%) | 0.064 |
| Laboratory parameters | ||||
| Haemoglobin, g/dL | 13.6 ± 1.9 | 13.6 ± 1.9 | 13.9 ± 2.0 | 0.202 |
| WBC, ×106/L | 7786 ± 2301 | 7644 ± 2279 | 7837 ± 2104 | 0.452 |
| SUA, mg/dL | 5.74 ± 1.31 | 5.38 ± 1.0 | 7.25 ± 1.3 | <0.001 |
| SUA quartiles, n (%) | ||||
| Q1 (<4.79 mg/dL) | 87 (24.0%) | 85 (28.8%) | 2 (2.9%) | <0.001 |
| Q2 (4.79–5.62 mg/dL) | 94 (25.9%) | 89 (30.2%) | 5 (7.4%) | |
| Q3 (5.63–6.54 mg/dL) | 90 (24.8%) | 82 (27.8%) | 8 (11.8%) | |
| Q4 (>6.54 mg/dL) | 92 (25.3%) | 39 (13.2%) | 53 (77.9%) | |
| hsCRP, mg/L | 1.77 ± 0.36 | 1.79 ± 0.36 | 1.69 ± 0.38 | 0.457 |
| Serum creatinine, mg/dL | 0.89 ± 0.21 | 0.88 ± 0.15 | 0.92 ± 0.21 | 0.645 |
| Follow-up parameters | ||||
| Early recurrence, n (%) | 33 (9.1%) | 7 (2.4%) | 26 (38.2%) | <0.001 |
| Follow-up time, months | 19.2 ± 6.1 | 18.7 ± 6.4 | 20.1 ± 6.2 | 0.065 |
AF, atrial fibrillation; AP, anteroposterior; BMI, body mass index; CAD, coronary artery disease; EHRA, European Heart Rhythm Association; hsCRP, high sensitive C-reactive protein; LA, left atrium; LVEF, left ventricular ejection fraction; Q, quartile; SD, standard deviation; SUA, serum uric acid; WBC, white blood cell count.
Clinical and laboratory characteristics according to pre-ablation serum uric acid quartiles
| Parameters . | Quartile 1 (<4.79; n = 87) . | Quartile 2 (4.79–5.62; n = 94) . | Quartile 3 (5.63–6.54; n = 90) . | Quartile 4 (>6.54; n = 92) . | P . |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 53.0 ± 11.3 | 52.6 ± 9.9 | 54.6 ± 11.2 | 54.1 ± 10.6 | 0.082 |
| Gender, male, n (%) | 52 (59.8%) | 49 (52.1%) | 38 (42.2%) | 52 (56.5%) | 0.101 |
| BMI, kg/m2 | 24.1 ± 3.4 | 24.4 ± 3.2 | 24.8 ± 3.6 | 25.2 ± 3.7 | 0.132 |
| Smoking, n (%) | 29 (33.3%) | 21 (22.3%) | 27 (27.8%) | 36 (39.1%) | 0.078 |
| Diabetes mellitus, n (%) | 6 (6.9%) | 14 (14.9%) | 17 (18.9%) | 9 (9.8%) | 0.076 |
| Hypertension, n (%) | 30 (34.5%) | 35 (37.2%) | 32 (35.5%) | 35 (38.0%) | 0.065 |
| CAD, n (%) | 12 (13.8%) | 7 (7.4%) | 8 (8.9%) | 11 (12.0%) | 0.494 |
| Dyslipidaemia, n (%) | 18 (20.6%) | 20 (21.2%) | 19 (21.1%) | 20 (21.7%) | 0.814 |
| Alcohol, n (%) | 9 (10.3%) | 8 (8.5%) | 6 (6.6%) | 8 (8.6%) | 0.152 |
| Duration of AF history, years | 5.0 ± 3.1 | 6.4 ± 5.3 | 4.9 ± 3.2 | 7.3 ± 5.2 | <0.001 |
| EHRA score | 2.83 ± 0.60 | 2.89 ± 0.58 | 2.82 ± 0.57 | 2.87 ± 0.57 | 0.488 |
| CHADS2 score, mean ± SD | 1.40 ± 0.77 | 1.46 ± 0.73 | 1.71 ± 0.93 | 1.49 ± 0.79 | 0.057 |
| CHA2DS2-vasc score, mean ± SD | 1.94 ± 0.92 | 2.02 ± 1.04 | 2.42 ± 1.15 | 2.04 ± 1.0 | 0.010 |
| LA diameter, mm | 37.3 ± 4.9 | 38.1 ± 4.4 | 37.6 ± 4.2 | 38.1 ± 5.6 | 0.626 |
| LVEF, % | 65.3 ± 4.2 | 65.6 ± 3.2 | 64.2 ± 4.3 | 65.5 ± 4.9 | 0.087 |
| Statins, n (%) | 15 (17.2%) | 19 (20.2%) | 16 (17.7%) | 18 (19.5%) | 0.312 |
| ACE inhibitor/ARB, n (%) | 30 (34.4%) | 34 (36.1%) | 31 (34.4%) | 33 (35.8%) | 0.182 |
| Pre-procedural laboratory parameters | |||||
| Haemoglobin, g/dL | 13.2 ± 1.8 | 13.6 ± 1.9 | 13.7 ± 1.9 | 13.5 ± 2.2 | 0.258 |
| WBC, ×106/L | 7256 ± 2155 | 7492 ± 2471 | 7997 ± 2189 | 8382 ± 2232 | 0.004 |
| SUA, mg/dL | 4.15 ± 0.48 | 5.19 ± 0.26 | 6.0 ± 0.28 | 7.5 ± 0.77 | <0.001 |
| Serum creatinine, mg/dL | 0.88 ± 0.11 | 0.92 ± 0.13 | 0.89 ± 0.15 | 0.90 ± 0.21 | 0.794 |
| hsCRP, mg/L | 1.76 ± 0.40 | 1.80 ± 0.36 | 1.83 ± 0.34 | 1.85 ± 0.38 | 0.011 |
| Follow-up parameters | |||||
| Follow-up time, months | 19.1 ± 6.5 | 18.7 ± 6.1 | 19.4 ± 5.8 | 19.3 ± 6.4 | 0.572 |
| Early recurrence, n (%) | 5 (5.7%) | 7 (7.4%) | 3 (3.3%) | 18 (19.6%) | 0.001 |
| Recurrence, n (%) | 2 (2.3%) | 5 (5.3%) | 8 (8.9%) | 53 (57.9%) | <0.001 |
| Parameters . | Quartile 1 (<4.79; n = 87) . | Quartile 2 (4.79–5.62; n = 94) . | Quartile 3 (5.63–6.54; n = 90) . | Quartile 4 (>6.54; n = 92) . | P . |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 53.0 ± 11.3 | 52.6 ± 9.9 | 54.6 ± 11.2 | 54.1 ± 10.6 | 0.082 |
| Gender, male, n (%) | 52 (59.8%) | 49 (52.1%) | 38 (42.2%) | 52 (56.5%) | 0.101 |
| BMI, kg/m2 | 24.1 ± 3.4 | 24.4 ± 3.2 | 24.8 ± 3.6 | 25.2 ± 3.7 | 0.132 |
| Smoking, n (%) | 29 (33.3%) | 21 (22.3%) | 27 (27.8%) | 36 (39.1%) | 0.078 |
| Diabetes mellitus, n (%) | 6 (6.9%) | 14 (14.9%) | 17 (18.9%) | 9 (9.8%) | 0.076 |
| Hypertension, n (%) | 30 (34.5%) | 35 (37.2%) | 32 (35.5%) | 35 (38.0%) | 0.065 |
| CAD, n (%) | 12 (13.8%) | 7 (7.4%) | 8 (8.9%) | 11 (12.0%) | 0.494 |
| Dyslipidaemia, n (%) | 18 (20.6%) | 20 (21.2%) | 19 (21.1%) | 20 (21.7%) | 0.814 |
| Alcohol, n (%) | 9 (10.3%) | 8 (8.5%) | 6 (6.6%) | 8 (8.6%) | 0.152 |
| Duration of AF history, years | 5.0 ± 3.1 | 6.4 ± 5.3 | 4.9 ± 3.2 | 7.3 ± 5.2 | <0.001 |
| EHRA score | 2.83 ± 0.60 | 2.89 ± 0.58 | 2.82 ± 0.57 | 2.87 ± 0.57 | 0.488 |
| CHADS2 score, mean ± SD | 1.40 ± 0.77 | 1.46 ± 0.73 | 1.71 ± 0.93 | 1.49 ± 0.79 | 0.057 |
| CHA2DS2-vasc score, mean ± SD | 1.94 ± 0.92 | 2.02 ± 1.04 | 2.42 ± 1.15 | 2.04 ± 1.0 | 0.010 |
| LA diameter, mm | 37.3 ± 4.9 | 38.1 ± 4.4 | 37.6 ± 4.2 | 38.1 ± 5.6 | 0.626 |
| LVEF, % | 65.3 ± 4.2 | 65.6 ± 3.2 | 64.2 ± 4.3 | 65.5 ± 4.9 | 0.087 |
| Statins, n (%) | 15 (17.2%) | 19 (20.2%) | 16 (17.7%) | 18 (19.5%) | 0.312 |
| ACE inhibitor/ARB, n (%) | 30 (34.4%) | 34 (36.1%) | 31 (34.4%) | 33 (35.8%) | 0.182 |
| Pre-procedural laboratory parameters | |||||
| Haemoglobin, g/dL | 13.2 ± 1.8 | 13.6 ± 1.9 | 13.7 ± 1.9 | 13.5 ± 2.2 | 0.258 |
| WBC, ×106/L | 7256 ± 2155 | 7492 ± 2471 | 7997 ± 2189 | 8382 ± 2232 | 0.004 |
| SUA, mg/dL | 4.15 ± 0.48 | 5.19 ± 0.26 | 6.0 ± 0.28 | 7.5 ± 0.77 | <0.001 |
| Serum creatinine, mg/dL | 0.88 ± 0.11 | 0.92 ± 0.13 | 0.89 ± 0.15 | 0.90 ± 0.21 | 0.794 |
| hsCRP, mg/L | 1.76 ± 0.40 | 1.80 ± 0.36 | 1.83 ± 0.34 | 1.85 ± 0.38 | 0.011 |
| Follow-up parameters | |||||
| Follow-up time, months | 19.1 ± 6.5 | 18.7 ± 6.1 | 19.4 ± 5.8 | 19.3 ± 6.4 | 0.572 |
| Early recurrence, n (%) | 5 (5.7%) | 7 (7.4%) | 3 (3.3%) | 18 (19.6%) | 0.001 |
| Recurrence, n (%) | 2 (2.3%) | 5 (5.3%) | 8 (8.9%) | 53 (57.9%) | <0.001 |
Data are means ± SD or n (%). AF, atrial fibrillation; CAD, coronary artery disease; EHRA, European Heart Rhythm Association; hsCRP, high sensitive C-reactive protein; LA, left atrium; LVEF, left ventricular ejection fraction; SUA, serum uric acid; WBC, white blood cell count.
Clinical and laboratory characteristics according to pre-ablation serum uric acid quartiles
| Parameters . | Quartile 1 (<4.79; n = 87) . | Quartile 2 (4.79–5.62; n = 94) . | Quartile 3 (5.63–6.54; n = 90) . | Quartile 4 (>6.54; n = 92) . | P . |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 53.0 ± 11.3 | 52.6 ± 9.9 | 54.6 ± 11.2 | 54.1 ± 10.6 | 0.082 |
| Gender, male, n (%) | 52 (59.8%) | 49 (52.1%) | 38 (42.2%) | 52 (56.5%) | 0.101 |
| BMI, kg/m2 | 24.1 ± 3.4 | 24.4 ± 3.2 | 24.8 ± 3.6 | 25.2 ± 3.7 | 0.132 |
| Smoking, n (%) | 29 (33.3%) | 21 (22.3%) | 27 (27.8%) | 36 (39.1%) | 0.078 |
| Diabetes mellitus, n (%) | 6 (6.9%) | 14 (14.9%) | 17 (18.9%) | 9 (9.8%) | 0.076 |
| Hypertension, n (%) | 30 (34.5%) | 35 (37.2%) | 32 (35.5%) | 35 (38.0%) | 0.065 |
| CAD, n (%) | 12 (13.8%) | 7 (7.4%) | 8 (8.9%) | 11 (12.0%) | 0.494 |
| Dyslipidaemia, n (%) | 18 (20.6%) | 20 (21.2%) | 19 (21.1%) | 20 (21.7%) | 0.814 |
| Alcohol, n (%) | 9 (10.3%) | 8 (8.5%) | 6 (6.6%) | 8 (8.6%) | 0.152 |
| Duration of AF history, years | 5.0 ± 3.1 | 6.4 ± 5.3 | 4.9 ± 3.2 | 7.3 ± 5.2 | <0.001 |
| EHRA score | 2.83 ± 0.60 | 2.89 ± 0.58 | 2.82 ± 0.57 | 2.87 ± 0.57 | 0.488 |
| CHADS2 score, mean ± SD | 1.40 ± 0.77 | 1.46 ± 0.73 | 1.71 ± 0.93 | 1.49 ± 0.79 | 0.057 |
| CHA2DS2-vasc score, mean ± SD | 1.94 ± 0.92 | 2.02 ± 1.04 | 2.42 ± 1.15 | 2.04 ± 1.0 | 0.010 |
| LA diameter, mm | 37.3 ± 4.9 | 38.1 ± 4.4 | 37.6 ± 4.2 | 38.1 ± 5.6 | 0.626 |
| LVEF, % | 65.3 ± 4.2 | 65.6 ± 3.2 | 64.2 ± 4.3 | 65.5 ± 4.9 | 0.087 |
| Statins, n (%) | 15 (17.2%) | 19 (20.2%) | 16 (17.7%) | 18 (19.5%) | 0.312 |
| ACE inhibitor/ARB, n (%) | 30 (34.4%) | 34 (36.1%) | 31 (34.4%) | 33 (35.8%) | 0.182 |
| Pre-procedural laboratory parameters | |||||
| Haemoglobin, g/dL | 13.2 ± 1.8 | 13.6 ± 1.9 | 13.7 ± 1.9 | 13.5 ± 2.2 | 0.258 |
| WBC, ×106/L | 7256 ± 2155 | 7492 ± 2471 | 7997 ± 2189 | 8382 ± 2232 | 0.004 |
| SUA, mg/dL | 4.15 ± 0.48 | 5.19 ± 0.26 | 6.0 ± 0.28 | 7.5 ± 0.77 | <0.001 |
| Serum creatinine, mg/dL | 0.88 ± 0.11 | 0.92 ± 0.13 | 0.89 ± 0.15 | 0.90 ± 0.21 | 0.794 |
| hsCRP, mg/L | 1.76 ± 0.40 | 1.80 ± 0.36 | 1.83 ± 0.34 | 1.85 ± 0.38 | 0.011 |
| Follow-up parameters | |||||
| Follow-up time, months | 19.1 ± 6.5 | 18.7 ± 6.1 | 19.4 ± 5.8 | 19.3 ± 6.4 | 0.572 |
| Early recurrence, n (%) | 5 (5.7%) | 7 (7.4%) | 3 (3.3%) | 18 (19.6%) | 0.001 |
| Recurrence, n (%) | 2 (2.3%) | 5 (5.3%) | 8 (8.9%) | 53 (57.9%) | <0.001 |
| Parameters . | Quartile 1 (<4.79; n = 87) . | Quartile 2 (4.79–5.62; n = 94) . | Quartile 3 (5.63–6.54; n = 90) . | Quartile 4 (>6.54; n = 92) . | P . |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 53.0 ± 11.3 | 52.6 ± 9.9 | 54.6 ± 11.2 | 54.1 ± 10.6 | 0.082 |
| Gender, male, n (%) | 52 (59.8%) | 49 (52.1%) | 38 (42.2%) | 52 (56.5%) | 0.101 |
| BMI, kg/m2 | 24.1 ± 3.4 | 24.4 ± 3.2 | 24.8 ± 3.6 | 25.2 ± 3.7 | 0.132 |
| Smoking, n (%) | 29 (33.3%) | 21 (22.3%) | 27 (27.8%) | 36 (39.1%) | 0.078 |
| Diabetes mellitus, n (%) | 6 (6.9%) | 14 (14.9%) | 17 (18.9%) | 9 (9.8%) | 0.076 |
| Hypertension, n (%) | 30 (34.5%) | 35 (37.2%) | 32 (35.5%) | 35 (38.0%) | 0.065 |
| CAD, n (%) | 12 (13.8%) | 7 (7.4%) | 8 (8.9%) | 11 (12.0%) | 0.494 |
| Dyslipidaemia, n (%) | 18 (20.6%) | 20 (21.2%) | 19 (21.1%) | 20 (21.7%) | 0.814 |
| Alcohol, n (%) | 9 (10.3%) | 8 (8.5%) | 6 (6.6%) | 8 (8.6%) | 0.152 |
| Duration of AF history, years | 5.0 ± 3.1 | 6.4 ± 5.3 | 4.9 ± 3.2 | 7.3 ± 5.2 | <0.001 |
| EHRA score | 2.83 ± 0.60 | 2.89 ± 0.58 | 2.82 ± 0.57 | 2.87 ± 0.57 | 0.488 |
| CHADS2 score, mean ± SD | 1.40 ± 0.77 | 1.46 ± 0.73 | 1.71 ± 0.93 | 1.49 ± 0.79 | 0.057 |
| CHA2DS2-vasc score, mean ± SD | 1.94 ± 0.92 | 2.02 ± 1.04 | 2.42 ± 1.15 | 2.04 ± 1.0 | 0.010 |
| LA diameter, mm | 37.3 ± 4.9 | 38.1 ± 4.4 | 37.6 ± 4.2 | 38.1 ± 5.6 | 0.626 |
| LVEF, % | 65.3 ± 4.2 | 65.6 ± 3.2 | 64.2 ± 4.3 | 65.5 ± 4.9 | 0.087 |
| Statins, n (%) | 15 (17.2%) | 19 (20.2%) | 16 (17.7%) | 18 (19.5%) | 0.312 |
| ACE inhibitor/ARB, n (%) | 30 (34.4%) | 34 (36.1%) | 31 (34.4%) | 33 (35.8%) | 0.182 |
| Pre-procedural laboratory parameters | |||||
| Haemoglobin, g/dL | 13.2 ± 1.8 | 13.6 ± 1.9 | 13.7 ± 1.9 | 13.5 ± 2.2 | 0.258 |
| WBC, ×106/L | 7256 ± 2155 | 7492 ± 2471 | 7997 ± 2189 | 8382 ± 2232 | 0.004 |
| SUA, mg/dL | 4.15 ± 0.48 | 5.19 ± 0.26 | 6.0 ± 0.28 | 7.5 ± 0.77 | <0.001 |
| Serum creatinine, mg/dL | 0.88 ± 0.11 | 0.92 ± 0.13 | 0.89 ± 0.15 | 0.90 ± 0.21 | 0.794 |
| hsCRP, mg/L | 1.76 ± 0.40 | 1.80 ± 0.36 | 1.83 ± 0.34 | 1.85 ± 0.38 | 0.011 |
| Follow-up parameters | |||||
| Follow-up time, months | 19.1 ± 6.5 | 18.7 ± 6.1 | 19.4 ± 5.8 | 19.3 ± 6.4 | 0.572 |
| Early recurrence, n (%) | 5 (5.7%) | 7 (7.4%) | 3 (3.3%) | 18 (19.6%) | 0.001 |
| Recurrence, n (%) | 2 (2.3%) | 5 (5.3%) | 8 (8.9%) | 53 (57.9%) | <0.001 |
Data are means ± SD or n (%). AF, atrial fibrillation; CAD, coronary artery disease; EHRA, European Heart Rhythm Association; hsCRP, high sensitive C-reactive protein; LA, left atrium; LVEF, left ventricular ejection fraction; SUA, serum uric acid; WBC, white blood cell count.
According to multivariate Cox proportional hazard regression analysis, pre-ablation SUA level (HR: 1.96; 95% CI: 1.49–2.59, P < 0.0001), LA diameter (HR: 1.11; 95% CI: 1.04–1.19, P = 0.002), duration of AF history (HR: 1.08; 95% CI: 1.02–1.14, P = 0.013), and early AF recurrence (HR: 4.34; 95% CI: 1.90–9.95, P = 0.001) were independent predictors of AF recurrence following cryoballon-based PV isolation, after adjustment of all other variables (Table 3).
Univariate and multivariate Cox proportional hazard modelling results of the AF recurrence after cryoballoon-based catheter ablation
| Variables . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Clinical parameters | ||||||
| Age, years | 1.04 | 1.01–1.07 | 0.012 | 1.03 | 0.98–1.07 | 0.245 |
| Male gender | 0.52 | 0.26–1.04 | 0.065 | 0.89 | 0.54–1.12 | 0.434 |
| Hypertension | 1.38 | 0.71–2.69 | 0.347 | |||
| Diabetes mellitus | 1.55 | 0.65–3.75 | 0.326 | |||
| Dyslipidaemia | 1.61 | 0.77–3.35 | 0.206 | |||
| BMI | 1.44 | 0.83–2.13 | 0.342 | |||
| CAD | 2.10 | 0.92–4.82 | 0.079 | 1.56 | 0.87–2.43 | 0.548 |
| Smoking | 2.23 | 1.15–4.34 | 0.018 | 1.23 | 0.54–2.86 | 0.622 |
| Alcohol | 2.61 | 1.08–3.31 | 0.032 | 1.65 | 0.84–2.12 | 0.532 |
| Duration of AF, years | 1.12 | 1.07–1.19 | <0.001 | 1.08 | 1.02–1.14 | 0.013 |
| EHRA score | 1.87 | 1.03–3.39 | 0.039 | 0.98 | 0.78–1.57 | 0.850 |
| CHADS2 score | 1.17 | 0.89–1.55 | 0.257 | |||
| CHA2DS2-vasc score | 1.03 | 0.82–1.29 | 0.779 | |||
| Echocardiographic parameters | ||||||
| LVEF, % | 0.98 | 0.90–1.06 | 0.608 | |||
| LAD, mm | 1.09 | 1.03–1.16 | 0.004 | 1.11 | 1.04–1.19 | 0.002 |
| Laboratory parameters | ||||||
| WBC, ×106/L | 1.45 | 0.94–2.14 | 0.565 | |||
| SUA, mg/dL | 2.24 | 1.80–2.79 | <0.001 | 1.96 | 1.49–2.59 | <0.001 |
| hsCRP | 1.44 | 0.97–1.85 | 0.087 | 1.05 | 0.88–2.11 | 0.137 |
| eGFR | 0.76 | 0.58–1.34 | 0.342 | |||
| Follow-up parameters | ||||||
| Early recurrence | 13.5 | 6.7–27.4 | <0.001 | 4.34 | 1.90–9.95 | 0.001 |
| Variables . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Clinical parameters | ||||||
| Age, years | 1.04 | 1.01–1.07 | 0.012 | 1.03 | 0.98–1.07 | 0.245 |
| Male gender | 0.52 | 0.26–1.04 | 0.065 | 0.89 | 0.54–1.12 | 0.434 |
| Hypertension | 1.38 | 0.71–2.69 | 0.347 | |||
| Diabetes mellitus | 1.55 | 0.65–3.75 | 0.326 | |||
| Dyslipidaemia | 1.61 | 0.77–3.35 | 0.206 | |||
| BMI | 1.44 | 0.83–2.13 | 0.342 | |||
| CAD | 2.10 | 0.92–4.82 | 0.079 | 1.56 | 0.87–2.43 | 0.548 |
| Smoking | 2.23 | 1.15–4.34 | 0.018 | 1.23 | 0.54–2.86 | 0.622 |
| Alcohol | 2.61 | 1.08–3.31 | 0.032 | 1.65 | 0.84–2.12 | 0.532 |
| Duration of AF, years | 1.12 | 1.07–1.19 | <0.001 | 1.08 | 1.02–1.14 | 0.013 |
| EHRA score | 1.87 | 1.03–3.39 | 0.039 | 0.98 | 0.78–1.57 | 0.850 |
| CHADS2 score | 1.17 | 0.89–1.55 | 0.257 | |||
| CHA2DS2-vasc score | 1.03 | 0.82–1.29 | 0.779 | |||
| Echocardiographic parameters | ||||||
| LVEF, % | 0.98 | 0.90–1.06 | 0.608 | |||
| LAD, mm | 1.09 | 1.03–1.16 | 0.004 | 1.11 | 1.04–1.19 | 0.002 |
| Laboratory parameters | ||||||
| WBC, ×106/L | 1.45 | 0.94–2.14 | 0.565 | |||
| SUA, mg/dL | 2.24 | 1.80–2.79 | <0.001 | 1.96 | 1.49–2.59 | <0.001 |
| hsCRP | 1.44 | 0.97–1.85 | 0.087 | 1.05 | 0.88–2.11 | 0.137 |
| eGFR | 0.76 | 0.58–1.34 | 0.342 | |||
| Follow-up parameters | ||||||
| Early recurrence | 13.5 | 6.7–27.4 | <0.001 | 4.34 | 1.90–9.95 | 0.001 |
AF recurrence is the dependent variable. AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; EHRA, European Heart Rhythm Association; HR, hazard ratio; LA, left atrium; LAD, left atrial diameter; LAVmax, left atrial maximum volume; LVEF, left ventricular ejection fraction; SUA, serum uric acid.
Univariate and multivariate Cox proportional hazard modelling results of the AF recurrence after cryoballoon-based catheter ablation
| Variables . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Clinical parameters | ||||||
| Age, years | 1.04 | 1.01–1.07 | 0.012 | 1.03 | 0.98–1.07 | 0.245 |
| Male gender | 0.52 | 0.26–1.04 | 0.065 | 0.89 | 0.54–1.12 | 0.434 |
| Hypertension | 1.38 | 0.71–2.69 | 0.347 | |||
| Diabetes mellitus | 1.55 | 0.65–3.75 | 0.326 | |||
| Dyslipidaemia | 1.61 | 0.77–3.35 | 0.206 | |||
| BMI | 1.44 | 0.83–2.13 | 0.342 | |||
| CAD | 2.10 | 0.92–4.82 | 0.079 | 1.56 | 0.87–2.43 | 0.548 |
| Smoking | 2.23 | 1.15–4.34 | 0.018 | 1.23 | 0.54–2.86 | 0.622 |
| Alcohol | 2.61 | 1.08–3.31 | 0.032 | 1.65 | 0.84–2.12 | 0.532 |
| Duration of AF, years | 1.12 | 1.07–1.19 | <0.001 | 1.08 | 1.02–1.14 | 0.013 |
| EHRA score | 1.87 | 1.03–3.39 | 0.039 | 0.98 | 0.78–1.57 | 0.850 |
| CHADS2 score | 1.17 | 0.89–1.55 | 0.257 | |||
| CHA2DS2-vasc score | 1.03 | 0.82–1.29 | 0.779 | |||
| Echocardiographic parameters | ||||||
| LVEF, % | 0.98 | 0.90–1.06 | 0.608 | |||
| LAD, mm | 1.09 | 1.03–1.16 | 0.004 | 1.11 | 1.04–1.19 | 0.002 |
| Laboratory parameters | ||||||
| WBC, ×106/L | 1.45 | 0.94–2.14 | 0.565 | |||
| SUA, mg/dL | 2.24 | 1.80–2.79 | <0.001 | 1.96 | 1.49–2.59 | <0.001 |
| hsCRP | 1.44 | 0.97–1.85 | 0.087 | 1.05 | 0.88–2.11 | 0.137 |
| eGFR | 0.76 | 0.58–1.34 | 0.342 | |||
| Follow-up parameters | ||||||
| Early recurrence | 13.5 | 6.7–27.4 | <0.001 | 4.34 | 1.90–9.95 | 0.001 |
| Variables . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Clinical parameters | ||||||
| Age, years | 1.04 | 1.01–1.07 | 0.012 | 1.03 | 0.98–1.07 | 0.245 |
| Male gender | 0.52 | 0.26–1.04 | 0.065 | 0.89 | 0.54–1.12 | 0.434 |
| Hypertension | 1.38 | 0.71–2.69 | 0.347 | |||
| Diabetes mellitus | 1.55 | 0.65–3.75 | 0.326 | |||
| Dyslipidaemia | 1.61 | 0.77–3.35 | 0.206 | |||
| BMI | 1.44 | 0.83–2.13 | 0.342 | |||
| CAD | 2.10 | 0.92–4.82 | 0.079 | 1.56 | 0.87–2.43 | 0.548 |
| Smoking | 2.23 | 1.15–4.34 | 0.018 | 1.23 | 0.54–2.86 | 0.622 |
| Alcohol | 2.61 | 1.08–3.31 | 0.032 | 1.65 | 0.84–2.12 | 0.532 |
| Duration of AF, years | 1.12 | 1.07–1.19 | <0.001 | 1.08 | 1.02–1.14 | 0.013 |
| EHRA score | 1.87 | 1.03–3.39 | 0.039 | 0.98 | 0.78–1.57 | 0.850 |
| CHADS2 score | 1.17 | 0.89–1.55 | 0.257 | |||
| CHA2DS2-vasc score | 1.03 | 0.82–1.29 | 0.779 | |||
| Echocardiographic parameters | ||||||
| LVEF, % | 0.98 | 0.90–1.06 | 0.608 | |||
| LAD, mm | 1.09 | 1.03–1.16 | 0.004 | 1.11 | 1.04–1.19 | 0.002 |
| Laboratory parameters | ||||||
| WBC, ×106/L | 1.45 | 0.94–2.14 | 0.565 | |||
| SUA, mg/dL | 2.24 | 1.80–2.79 | <0.001 | 1.96 | 1.49–2.59 | <0.001 |
| hsCRP | 1.44 | 0.97–1.85 | 0.087 | 1.05 | 0.88–2.11 | 0.137 |
| eGFR | 0.76 | 0.58–1.34 | 0.342 | |||
| Follow-up parameters | ||||||
| Early recurrence | 13.5 | 6.7–27.4 | <0.001 | 4.34 | 1.90–9.95 | 0.001 |
AF recurrence is the dependent variable. AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; EHRA, European Heart Rhythm Association; HR, hazard ratio; LA, left atrium; LAD, left atrial diameter; LAVmax, left atrial maximum volume; LVEF, left ventricular ejection fraction; SUA, serum uric acid.
In receiver operating characteristic (ROC) curve analysis, a cut-off level of 6.37 for pre-procedural SUA level predicted AF recurrence during follow-up with a sensitivity of 85.7% and a specificity of 83.7% (AUC: 0.88, 95% CI: 0.83–0.92; P = 0.001) (Figure 1). Patients with a pre-ablation SUA level of > 6.37 had a 17.8-fold increased risk of developing AF recurrence after cryoablation (HR: 17.8, 95% CI: 8.8–36.1; P < 0.001). The overall predictive performance of pre-procedural SUA level, LA diameter, and duration of AF history was calculated by comparing their area under ROC curves in which the pre-procedural SUA level was well predicted AF recurrence than other parameters (AUC: 0.881, negatif probability ratio = 0.17, P < 0.05) (Figure 1).
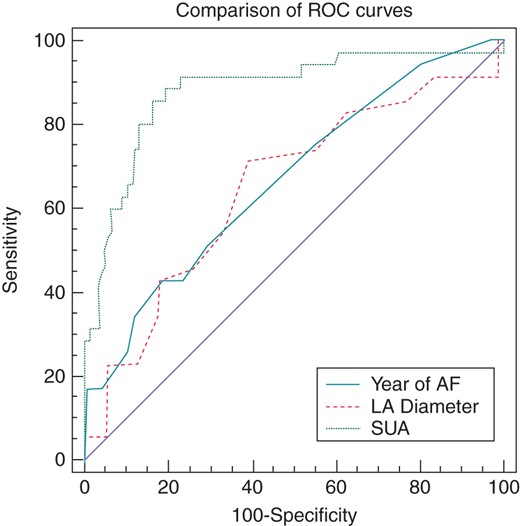
Comparison of ROC curves of pre-ablation serum uric acid level, LA diameter, and duration of AF history for predicting AF recurrence after catheter ablation (P < 0.001).
In addition, correlation analysis revealed that there was a positive correlation of pre-procedural SUA level with duration of AF history (r = 0.131, P = 0.012), hsCRP (r = 0.144, P = 0.006), and WBC count (r = 0.211, P < 0.001) (Figure 2). Figures 3 and 4 represent the Kaplan–Meier curves for the freedom from AF recurrence according to SUA cut-off level of 6.37 and SUA quartiles (log-rank, P < 0.001).
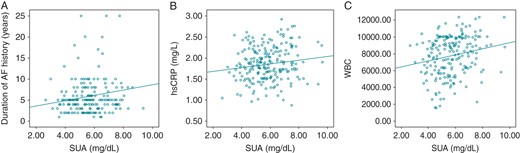
Correlation between the pre-ablation SUA level and duration of AF history (r = 0.131, P = 0.012), hsCRP (r = 0.144, P = 0.006), and WBC count (r = 0.211, P < 0.001).
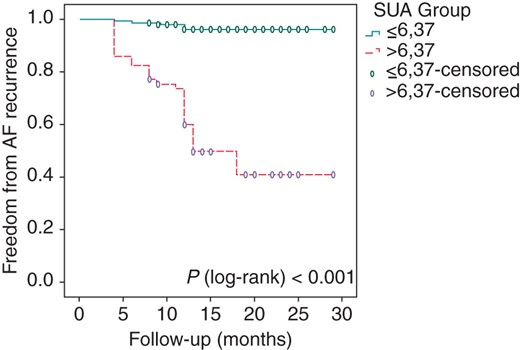
Kaplan–Meier survival estimates of AF recurrence in patients with paroxysmal AF undergoing catheter ablation stratified by the pre-ablation SUA level of ≤6.37 vs. >6.37.
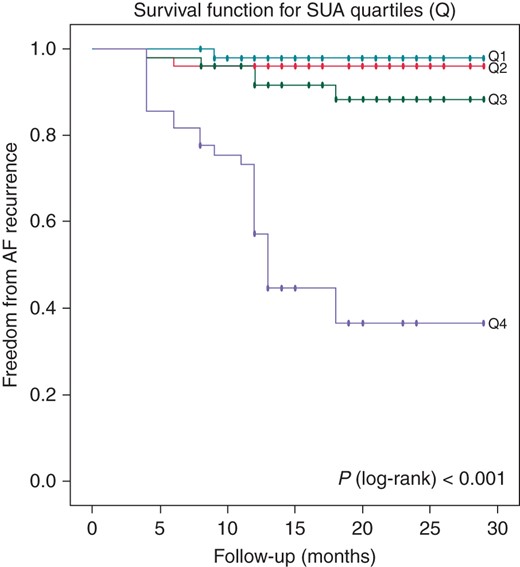
Kaplan–Meier survival estimates of AF recurrence in patients with paroxysmal AF undergoing catheter ablation stratified by pre-ablation SUA quartiles.
Discussion
In the present study, we aimed to focus on the association of pre-ablation SUA level with the development of AF recurrence during mean 19.2 ± 6.1 months follow-up. We demonstrated that SUA level is a powerful and independent predictor of AF recurrence in patients undergoing successful cryoballoon-based AF ablation. Patients in the highest quartile (Q4) of SUA were at greater risk, and SUA level >6.37 mg/dL measured before cryoablation had an 85.7% sensitivity and a 83.7% specificity in predicting AF recurrence.
Although there were well-defined studies regarding the association of inflammation and oxidative stress with the pathogenesis of AF, they have a chicken–egg paradox.6,7 Whether initiation of AF promotes direct inflammatory and oxidative pathways or whether the presence of a pre-existing systemic inflammatory or pro-oxidant condition results in AF development remains unclear. Increased SUA level, which can be simply obtained from the biochemistry panel, is linked to activated both pro-inflammatory and oxidative stress pathways.8,9 Inflammation and oxidative stress, which are mechanistically inter-related, lead to calcium overload and reduces sodium channels end up with electrical remodelling, at the same time there is structural remodelling through fibroblast proliferation, inflammation, and apoptosis.10 Therefore, the association of increased uric acid level with AF may be defined by all those mechanisms. However, the predictive value of SUA level has not been investigated in paroxysmal AF patients undergoing cryoballoon-based catheter ablation.
Factors that have been identified as predictors of AF recurrence after catheter ablation, at least in some trials, include: (i) non-paroxysmal AF, (ii) sleep apnoea and obesity, (iii) increased left atrial size, (iv) increased age, (v) hypertension, (vi) inflammation, and (vii) left atrial fibrosis as detected by cardiac magnetic resonance imaging and early AF recurrence.11–14 In a retrospective study, He et al.15 enrolled 330 patients with paroxysmal AF who underwent AF ablation by using the radiofrequency technique. They showed that there was an increased risk of recurrence in subjects in the highest SUA quartile (>7.4 mg/dL) (HR: 2.8, 95% CI: 1.46–5.36, P = 0.002) and also SUA was found to be an independent predictor of recurrence (HR: 1.6, 95% CI: 1.601–1.625, P = 0.014) during 9.3 ± 3.6 months follow-up period. In our study, in addition to pre-ablation SUA level, we also found that increased LA diameter, duration of AF history, and early AF recurrence were independent predictors of AF recurrence after blanking period. It has been known that apart from the triggers, AF initiation and maintenance depend on the electrical and structural remodelling of the atria.16 So, as an assumption, we proposed that by causing electrical and structural remodelling, elevated SUA levels may play a role in AF recurrence. Moreover, our study differs from the study by He et al.15 regarding the prospective design, application of cryoballon-based ablation technique, longer follow-up period, and performing 24 h Holter monitorization independent from symptomatology of the patients during follow-up which might have effects on the success rates of catheter ablation. So, our study results should be carefully interpreted due to study population, ablation technique, and follow-up methods although study results seemed to be confirmatory to study by He et al.15
From the view point of clinical practice, detection of powerful predictors of post-ablation AF recurrence may help electrophysiologists for appropriate patient selection strategy and therefore to improve the overall success rate of the catheter ablation methods. However, it is unclear with our study whether the SUA level is a therapeutic target or simply a biomarker or a mediator molecule. Identifying new relationships and mechanisms of AF could guide to therapeutics targets in the future.17 Therefore, the potential role of specific pharmacological agents like allopurinol that modulate the SUA level also needs to be investigated.
Our study should be evaluated with some limitations. First, the study showed the results of single-centre experience with only including paroxysmal AF patients and cannot be generalized to all AF populations. Second, despite pre-defined control visits and questioning of symptoms, the detection of silent episodes of AF recurrence is very difficult. Therefore, we may have underestimated the true incidence of AF recurrence in this study. Third, oxidative stress biomarkers were not assessed. Fourth, the observational design of the study identifies only an association and only a single SUA measurement was available.
Conclusion
Pre-procedural pro-inflammatory and pro-oxidant niche has significant impact on AF recurrence after catheter ablation. As an inexpensive, easy to obtain, widely available marker of inflammation and oxidative stress, pre-ablation SUA level has well-predicted AF recurrence after cryoablation. Further large-scale, prospective studies are needed to further elucidate the role of SUA level in AF recurrence and also the effect of the alternative treatment strategies reducing SUA levels.
Acknowledgements
The authors thank Hakan Çakır for statistical assistance.
Conflict of interest: none declared.



