-
PDF
- Split View
-
Views
-
Cite
Cite
Lucie Šedivá, Jan Petrů, Jan Škoda, Marek Janotka, Milan Chovanec, Vivek Reddy, Petr Neužil, Visually guided laser ablation: a single-centre long-term experience, EP Europace, Volume 16, Issue 12, December 2014, Pages 1746–1751, https://doi.org/10.1093/europace/euu168
Close - Share Icon Share
Abstract
Durable isolation of the pulmonary veins (PVs) remains the cornerstone of treatment for paroxysmal atrial fibrillation (PAF) and is also used in the treatment of some patients with persistent atrial fibrillation. Visually guided laser ablation (VGLA) has been proven to be safe and effective as a treatment for atrial fibrillation (AF). It has shown high levels of durable PV isolation (PVI), even in the hands of less experienced users. This paper presents the long-term clinical outcomes of all patients treated with VGLA over the course of 4 years in the world's most experienced centre: from early product feasibility work treating only PAF patients to our work using the commercially available product, when we also treated persistent AF patients.
One hundred and ninety-four patients (63 females, mean age 61 years) with either a history of drug-refractory PAF (time since initial diagnosis: 60.73 months) or persistent AF (time since initial diagnosis: 62.75 months) were treated in our laboratory with VGLA between 7 January 2009 and 17 May 2013. Follow-up of all patients was consistent with our standard clinical practice with a 7-day Holter being performed at the first clinical visit between 4 and 6 months and, for most patients, again at 12 months post-procedure. Twelve lead electrocardiograms were performed at all clinical visits. Recurrence of AF is defined as any documented AF episode >30 s. Acute procedural results show that 692 veins were acutely isolated with a mean procedure and fluoroscopy time of 226 and 20.4 min, respectively. One hundred and seventy (158 PAF and 12 persistent AF) patients reached 1 year of follow-up, 130 (82.3%) patients remained free of AF in the PAF group, and 9 (75%) in the persistent group. Eighty-seven PAF patients have now reached 24 months follow-up and 66 (75.9%) remain free of AF. Fifty-four PAF patients have reached 36 months follow-up with 41 (75.9%) remaining free of AF. Thirty-two PAF patients have reached 48 months follow-up and 24 (75%) remain free of AF. The peri-procedural complications we encountered were phrenic nerve injury in four patients (2.06%), tamponade or pericardial effusion in one patient (0.51%), stroke or transient ischaemic attack in one patient (0.514%), and vascular injury in six patients (3.09%). We experienced no cases of PV stenosis or atrio-oesophageal fistula.
Our single-centre experience using VGLA over 4 years shows that it can be used safely and effectively in normal clinical practice and gives high levels of acute PVI accompanied by good clinical outcomes, even after long-term follow-up.
long-term clinical outcomes of patients treated with VGLA over the course of 4 years in the world's most experienced centre.
the largest cohort of patients presented from a single centre.
treatment of persistent AF patients.
Introduction
Since the discovery of fast firing activity in the pulmonary veins (PVs) was shown to be a trigger for paroxysmal atrial fibrillation (PAF),1 isolation of all PVs has been the cornerstone of treatment.2 The technique was originally performed with a single-curve 4 mm tip radiofrequency (RF) ablation catheter, designed for the treatment of accessory pathways. Since the development of irrigated catheters, RF ablation has become the most accepted approach and has been shown to be superior to standard medical treatment for PAF in a prospective, multi-centre, randomized trial (Thermocool-AF trial).3 To overcome perceived limitations of a single-curve catheter, a variety of different technologies have been developed to isolate PVs, ranging from circular multi-electrode RF catheters,4,5 balloons employing differing energy sources,6,7 and other technologies to navigate, guide, or otherwise enhance the procedure when using a single-curve RF ablation catheter.8–10
Currently, the available techniques for visualization are fluoroscopy, three-dimensional electroanatomical mapping, or intracardiac echo. However, a new approach employs an endoscope for direct visualization. The visually guided laser ablation (VGLA) system has an endoscope and laser fibre located inside a compliant balloon that provides visual guidance while placing point-by-point lesions in the antrum of the PV (CardioFocus Inc.).
Our centre was the first to use this new technology and novel approach during feasibility trials, which commenced in 2005 using the original VGLA system with a non-compliant balloon and larger laser-beam arc. Since early 2009 we have been using this technique with a redesigned catheter utilizing a fully compliant balloon and a significantly shorter arc. This method is now part of our regular clinical practice and this paper shows the total of our experience from 2009 to the present day. We present the largest cohort from a single centre with the longest follow-up.
Methods
Patient population
This retrospective study includes 27 patients who were enroled as part of a safety and feasibility study and 167 patients from regular clinical practice. The feasibility study was carried out with approval from the ethics committee of Na Homolce hospital. Informed consent was received from all patients. Consistent with the current guidelines,11 patients are classified as symptomatic, drug-refractory PAF (AF episode duration <7 days), as symptomatic persistent AF (AF episode duration of ≤365 days), or symptomatic long-standing persistent AF (AF episode duration of >365 days) (Tables 1 and 2).
| Gender female | 63 | 32.47% |
| Age | 61.84 years | (25–82) |
| Hypertension | 110 | 57.21% |
| Diabetes | 28 | 14.43% |
| Coronary artery disease | 24 | 12.37% |
| Congestive heart failure (III or IV) | 8 | 4.12% |
| LV ejection fraction | 58.6% | (30–72) |
| LA dimension | 42 mm | (30–59) |
| Gender female | 63 | 32.47% |
| Age | 61.84 years | (25–82) |
| Hypertension | 110 | 57.21% |
| Diabetes | 28 | 14.43% |
| Coronary artery disease | 24 | 12.37% |
| Congestive heart failure (III or IV) | 8 | 4.12% |
| LV ejection fraction | 58.6% | (30–72) |
| LA dimension | 42 mm | (30–59) |
| Gender female | 63 | 32.47% |
| Age | 61.84 years | (25–82) |
| Hypertension | 110 | 57.21% |
| Diabetes | 28 | 14.43% |
| Coronary artery disease | 24 | 12.37% |
| Congestive heart failure (III or IV) | 8 | 4.12% |
| LV ejection fraction | 58.6% | (30–72) |
| LA dimension | 42 mm | (30–59) |
| Gender female | 63 | 32.47% |
| Age | 61.84 years | (25–82) |
| Hypertension | 110 | 57.21% |
| Diabetes | 28 | 14.43% |
| Coronary artery disease | 24 | 12.37% |
| Congestive heart failure (III or IV) | 8 | 4.12% |
| LV ejection fraction | 58.6% | (30–72) |
| LA dimension | 42 mm | (30–59) |
| AF type . | Number of patients . | Percentage . | Mean duration . |
|---|---|---|---|
| Paroxysmal | 178 | 91.75% | 60.73 (11–300) months |
| Persistent | 16 | 8.25% | 62.75 (12–200) months |
| AF type . | Number of patients . | Percentage . | Mean duration . |
|---|---|---|---|
| Paroxysmal | 178 | 91.75% | 60.73 (11–300) months |
| Persistent | 16 | 8.25% | 62.75 (12–200) months |
| AF type . | Number of patients . | Percentage . | Mean duration . |
|---|---|---|---|
| Paroxysmal | 178 | 91.75% | 60.73 (11–300) months |
| Persistent | 16 | 8.25% | 62.75 (12–200) months |
| AF type . | Number of patients . | Percentage . | Mean duration . |
|---|---|---|---|
| Paroxysmal | 178 | 91.75% | 60.73 (11–300) months |
| Persistent | 16 | 8.25% | 62.75 (12–200) months |
The visually guided laser ablation system
The VGLA system has been described previously12–14 and consists of: a console that provides visualization from the endoscope within the balloon as well as control of the balloon size and of energy delivery; a 15F outer diameter steerable sheath that allows the users to manoeuvre the catheter into the selected vein; a 2F endoscope that is reusable and relays the images of the PV to the console for visualization; and the 12F catheter, which has a compliant and sizable balloon at the distal tip and a handle to control the placement of laser energy to create lesions.
The compliant balloon is filled with deuterium oxide fluid, which flows constantly through the system via a pump at the console. The pump has nine different speeds and increasing the speed also increases the balloon size so that the balloon can be optimized to fit all PVs. The fluid also cools the endocardial surface and allows the laser energy to pass through it with no heating effect.
Ablation is carried out using a 980 nm diode laser that creates a 30° arc of energy. An aiming beam of green and red light allows the user to see where the lesion is being placed on the tissue. The laser energy can be steered independently of the balloon position at the handle of the catheter. The console also allows the visualization of the previous lesion so that lesions can be placed contiguously and overlapped to create a single circular lesion set. Energy levels can be selected by the user depending upon the level of occlusion of the vein and taking into account the patient anatomy. The default setting for the system is 8.5 W for 20 s, but the user has the option to titrate the dose with six different settings from 5.5 W for 30 s to 12 W for 20 s.
Pre-procedural imaging
All patients underwent a pre-procedural computed tomography (CT) scan (Somatom Definition flash 2 × 128, Siemens Medical Inc.), transoesophageal echo and transthoracic echo (Acuson Cypress, Siemens Medical Solutions Inc.). Patients with signs of thrombus in the left atrial (LA) appendage were excluded. We did not exclude patients with left-sided common PVs, or those with borderline-sized PVs.
Ablation procedure
The ablation procedure was performed under conscious sedation using boluses of fentanyl and midazolam as required, and dependent upon patient weight and tolerance of the procedure.
Regarding peri-procedural anticoagulation, our earlier normal clinical practice was to bridge the procedure with low weight molecular heparin. In the last 1.5 years, all patients were anticoagulated with vitamin K antagonist (VKA) to INR 2–3.
An oesophageal temperature probe was placed in all patients (5F, beta-Therm Model, G22K7MCD8, Clevertech) and energy delivery was ceased if the temperature exceeded 38.5°C.
A 6F Decapolar catheter was placed in the coronary sinus (CS) and then a single transseptal puncture was performed under both fluoroscopic and intracardiac echo guidance (10F Siemens Acuson) using a Brokenbrough needle and an 8F SL1 guiding sheath (St Jude Medical). Once transseptal puncture was achieved, a bolus of heparin was given and an activated clotting time (ACT) of 300–350 s was maintained.
An exchange length guidewire (SL Super Stiff 0.032″, St Jude) was then placed into the left superior PV (LSPV) and the transseptal sheath exchanged over the wire for the VGLA steerable sheath.
Pacing of the phrenic nerve (rate 60 b.p.m.) from the superior vena cava during ablation of the right superior PV (RSPV) was performed and diaphragmatic movement observed during energy delivery.
All veins were treated with a range of energy doses 5.5–12 W. Higher power (10–12 W) was selected for the anterior part of the lateral veins to ensure transmurality in the thicker ridge and carina areas. No more than 8.5 W was delivered to the posterior wall.
Once all veins had been treated, the VGLA catheter was removed from the steerable sheath and a circular mapping catheter introduced to the LA. Each vein was mapped to confirm isolation of the vein. Pacing of the CS and from each electrode of the circular mapping catheter from within the vein further helped to confirm PV isolation (PVI). If any vein was shown not to be isolated, the area of breakthrough was noted, the VGLA catheter re-introduced, and ablation in that area was continued. The procedure was considered complete once all veins were confirmed to be isolated. With regard to anticoagulation management, pre-procedure all patients were on warfarin and during the procedure all patients were continuously anticoagulated with heparin intravenously up to ACT 300–400 s.
Post-procedural care
A transthoracic echocardiogram was performed on all patients within 24 h of the end of the procedure to exclude pericardial effusion and pneumothorax. Our earlier practice was to use proton pump inhibitors (PPI) only for those patients whose oesophageal temperature during energy delivery exceeded 38.5°C; however, since January 2013, we have treated all ablation patients with PPI therapy for at least 2 weeks as a precaution against the possibility of oesophageal injury. Patients were usually discharged from hospital the day after the procedure. Previously ineffective antiarrhythmic drugs were continued until the first clinical visit, between 4 and 6 months post-procedure. All patients were on warfarin post-procedure per current guidelines, which state that chronic oral anticoagulation therapy with a VKA is recommended in a dose-adjusted regimen to achieve an INR range of 2.0–3.0.
Follow-up
Patients were followed up in our routine clinical manner with a 7-day Holter recording taken during the first outpatient clinical visit which takes place between 4 and 6 months post-procedure and then again at 12 months post-procedure. Anticoagulation was stopped after 6 months of follow-up in patients with no symptomatic or documented evidence of AF recurrences. Continuing follow-up of longer term patients took place at 24, 36, and 48 months post-procedure, and included 7-day Holter recordings, resting electrocardiogram, and patient history. In this cohort of patients, we found no evidence of silent asymptomatic AF during follow-up. Either patients experienced symptoms and we had documented evidence or, in patients with no symptoms, all monitoring showed stable sinus rhythm.
Repeat procedures
Patients who reported symptomatic documented recurrence of AF or were found to have episodes of AF lasting >30 s on the 7-day Holter were offered the opportunity to undergo a repeat PVI procedure.
In those patients who elected to have a second procedure, a double transseptal puncture was performed and a circular mapping catheter introduced to the LA. All veins were mapped and where re-connection was detected, ablation of the area was performed guided by either electroanatomical mapping with irrigated RF ablation catheter Biosense Webster Navistar® Thermocool® 3.5 mm (Diamond Bar), or Biosense Webster Celsius® Thermocool® 3.5 mm performed conventionally. The most frequently used power range was 20–30 W. All patients undergoing redo procedure were paroxysmal. In patients with all veins found to be isolated, more antral lines with or without mitral isthmus and LA roof lines were applied.
Results
Acute procedural results
One hundred and ninety-four patients had 698 veins targeted for PVI with VGLA. There were 23 common veins seen in this patient group. The mean procedure time was 226 min, ranging from 90 to 360 min. The average fluoroscopy time was 20.4 min, ranging from 6 to 42 min. The mean ablation time per patient was 121.6 min with the average application time per vein being 33.6 min. 99.2% of all veins targeted were acutely isolated and 95.3% of these veins were isolated at the first attempt (Table 3).
| Total number of veins targeted | 698 |
| Number of common veins | 23 |
| Procedure time | 226 (90–360) min |
| Fluoroscopy time | 20.4 (6–42) min |
| Total ablation time per patient | 121.6 min (36–157) |
| Average time application per vein | 33.6 min |
| % of veins isolated acutely | 99.2% |
| % of veins isolated at first attempt | 95.3% |
| Total number of veins targeted | 698 |
| Number of common veins | 23 |
| Procedure time | 226 (90–360) min |
| Fluoroscopy time | 20.4 (6–42) min |
| Total ablation time per patient | 121.6 min (36–157) |
| Average time application per vein | 33.6 min |
| % of veins isolated acutely | 99.2% |
| % of veins isolated at first attempt | 95.3% |
| Total number of veins targeted | 698 |
| Number of common veins | 23 |
| Procedure time | 226 (90–360) min |
| Fluoroscopy time | 20.4 (6–42) min |
| Total ablation time per patient | 121.6 min (36–157) |
| Average time application per vein | 33.6 min |
| % of veins isolated acutely | 99.2% |
| % of veins isolated at first attempt | 95.3% |
| Total number of veins targeted | 698 |
| Number of common veins | 23 |
| Procedure time | 226 (90–360) min |
| Fluoroscopy time | 20.4 (6–42) min |
| Total ablation time per patient | 121.6 min (36–157) |
| Average time application per vein | 33.6 min |
| % of veins isolated acutely | 99.2% |
| % of veins isolated at first attempt | 95.3% |
Peri-procedural complications
The most common complication we saw was vascular injury at a rate of 3.09%. Vascular injuries experienced were prolonged hospitalization due to bleeding (in four patients), vascular surgery repair (in two patients). In addition, we saw phrenic nerve injury, observed in four patients (2.06%). Diaphragmatic control recovered in all patients before the first follow-up clinical visit, which occurred between 4 and 6 months post-procedure. Tamponade was seen in only one patient (0.51%) and this occurred before a change to the catheter and the addition of a longer and softer atraumatic tip. A TIA was seen in one patient (0.51%). The patient showed symptoms of verbal confusion 2 h post-procedure, but this resolved within 24 h. No PV stenosis was seen in the 27 patients enroled in the feasibility study. The peri-procedural complications we encountered were phrenic nerve injury in four patients (2.06%), tamponade or pericardial effusion in one patient (0.51%), stroke or transient ischaemic attack (TIA) in one patient (0.51%), and vascular injury in six patients (3.09%). We experienced no cases of PV stenosis or atrio-oesophageal fistula. Patients underwent a CT scan at 3 months post-procedure to screen for stenosis. No symptomatic PV stenosis was noted in the 194 patients in our clinical practice. There was also no occurrence of atrio-oesophageal fistula in this group of patients (Table 4).
| Complication . | Occurrence . | Percentage . |
|---|---|---|
| Stroke/TIA | 1 | 0.51% |
| Tamponade/pericardial effusion | 1 | 0.51% |
| Acute phrenic nerve injury | 4 | 2.06% |
| Persistent phrenic nerve injury (>6 months) | 0 | 0% |
| Vascular injury | 6 | 3.09% |
| PV stenosis | 0 | 0% |
| Atrio-oesophageal fistula | 0 | 0% |
| Complication . | Occurrence . | Percentage . |
|---|---|---|
| Stroke/TIA | 1 | 0.51% |
| Tamponade/pericardial effusion | 1 | 0.51% |
| Acute phrenic nerve injury | 4 | 2.06% |
| Persistent phrenic nerve injury (>6 months) | 0 | 0% |
| Vascular injury | 6 | 3.09% |
| PV stenosis | 0 | 0% |
| Atrio-oesophageal fistula | 0 | 0% |
| Complication . | Occurrence . | Percentage . |
|---|---|---|
| Stroke/TIA | 1 | 0.51% |
| Tamponade/pericardial effusion | 1 | 0.51% |
| Acute phrenic nerve injury | 4 | 2.06% |
| Persistent phrenic nerve injury (>6 months) | 0 | 0% |
| Vascular injury | 6 | 3.09% |
| PV stenosis | 0 | 0% |
| Atrio-oesophageal fistula | 0 | 0% |
| Complication . | Occurrence . | Percentage . |
|---|---|---|
| Stroke/TIA | 1 | 0.51% |
| Tamponade/pericardial effusion | 1 | 0.51% |
| Acute phrenic nerve injury | 4 | 2.06% |
| Persistent phrenic nerve injury (>6 months) | 0 | 0% |
| Vascular injury | 6 | 3.09% |
| PV stenosis | 0 | 0% |
| Atrio-oesophageal fistula | 0 | 0% |
Freedom from atrial fibrillation
In the PAF group, of the 158 patients who reached 12 months follow-up, 130 (82.3%) were free of AF; 87 patients from this group reached 24 months follow-up and 66 were free of AF (75.9%); 54 reached 36 months follow-up and 41 were free of AF (75.9%); and 32 patients reached 48 months follow-up with 24 being free of AF (75%) (Figure 1).
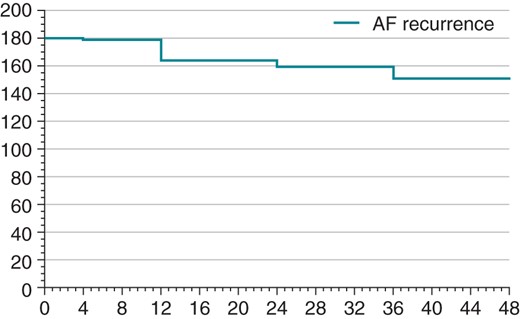
In the persistent AF group, only 12 patients had reached 12 months follow-up and 9 (75%) of these were free of AF. No patients from the persistent have reached 24 months follow-up at the time of writing.
Learning curve data
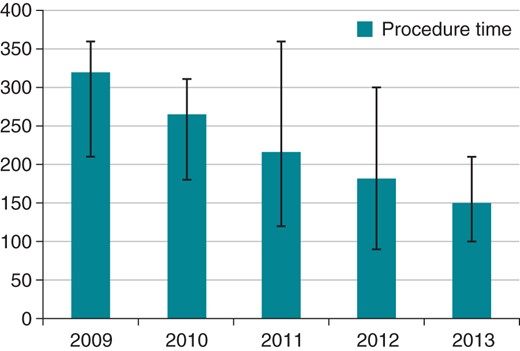
Whenever a new technique or technology is adopted, there will be an associated learning curve. Comparisons of clinical procedures (excluding data from the feasibility study) carried out in each year clearly show a significant reduction in procedure time and fluoroscopy time (Figure 2).
The peri-procedural complications we encountered were phrenic nerve injury in four patients (2.06%), tamponade or pericardial effusion in one patient (0.51%), stroke or TIA in one patient (0.51%), and vascular injury in six patients (3.09%). We experienced no cases of PV stenosis or atrio-oesophageal fistula. These complications were resolved in a timely manner and did not result in changes in the learning curve. From our data, it is not evident that there was a significant reduction in the complication rate over time. Complications occurred in both initial and alter cases and were dependent on the patient clinical status (Table 5).
| . | No. of patients . | Procedure time . | Fluoroscopy time . |
|---|---|---|---|
| 2009 | 41 | 320 (210–360) min | 22 (11–30) min |
| 2010 | 18 | 264 (180–310) min | 24 (14–32) min |
| 2011 | 36 | 216 (120–360) min | 21 (16–28) min |
| 2012 | 48 | 181 (90–300) min | 22 (7–41) min |
| 2013 | 24 | 150 (100–210) min | 13 (6–28) min |
| . | No. of patients . | Procedure time . | Fluoroscopy time . |
|---|---|---|---|
| 2009 | 41 | 320 (210–360) min | 22 (11–30) min |
| 2010 | 18 | 264 (180–310) min | 24 (14–32) min |
| 2011 | 36 | 216 (120–360) min | 21 (16–28) min |
| 2012 | 48 | 181 (90–300) min | 22 (7–41) min |
| 2013 | 24 | 150 (100–210) min | 13 (6–28) min |
| . | No. of patients . | Procedure time . | Fluoroscopy time . |
|---|---|---|---|
| 2009 | 41 | 320 (210–360) min | 22 (11–30) min |
| 2010 | 18 | 264 (180–310) min | 24 (14–32) min |
| 2011 | 36 | 216 (120–360) min | 21 (16–28) min |
| 2012 | 48 | 181 (90–300) min | 22 (7–41) min |
| 2013 | 24 | 150 (100–210) min | 13 (6–28) min |
| . | No. of patients . | Procedure time . | Fluoroscopy time . |
|---|---|---|---|
| 2009 | 41 | 320 (210–360) min | 22 (11–30) min |
| 2010 | 18 | 264 (180–310) min | 24 (14–32) min |
| 2011 | 36 | 216 (120–360) min | 21 (16–28) min |
| 2012 | 48 | 181 (90–300) min | 22 (7–41) min |
| 2013 | 24 | 150 (100–210) min | 13 (6–28) min |
Repeat procedures
Eleven patients returned for a repeat procedure and were shown to have only nine veins that had reconnected. Four patients had no veins reconnected: one of those patients was treated for atrioventricular nodal re-entrant tachycardia; and the other three had additional mitral valve isthmus and roof lines added.
Of the nine veins reconnected, four were the LSPV, two were left inferior pulmonary veins (LIPV), one was a RSPV, one was a right inferior pulmonary vein (RIPV), and one was a right common pulmonary vein (RCPV) (Table 6).
| Vein . | LSPV . | LIPV . | RSPV . | RIPV . | RCPV . |
|---|---|---|---|---|---|
| Number of veins reconnected | 4 | 2 | 1 | 1 | 1 |
| Vein . | LSPV . | LIPV . | RSPV . | RIPV . | RCPV . |
|---|---|---|---|---|---|
| Number of veins reconnected | 4 | 2 | 1 | 1 | 1 |
| Vein . | LSPV . | LIPV . | RSPV . | RIPV . | RCPV . |
|---|---|---|---|---|---|
| Number of veins reconnected | 4 | 2 | 1 | 1 | 1 |
| Vein . | LSPV . | LIPV . | RSPV . | RIPV . | RCPV . |
|---|---|---|---|---|---|
| Number of veins reconnected | 4 | 2 | 1 | 1 | 1 |
Discussion
Our retrospective study represents the largest single-centre cohort of patients treated with VGLA for AF. We also present here for the first time 4-year follow-up data from the first patients treated with VGLA. This represents the longest follow-up of patients treated with this modality. This paper also discusses the longest experience of any centre. One interesting facet of our experience with VGLA is that we have been involved with the development of this technology from a very early stage. Finally, we report for the first time on the use of VGLA in patients with persistent AF, which gives a promising insight into the role of durable PVI in the treatment of this type of patient.
Patient cohort
This is the largest cohort of patients presented from a single centre. The results show excellent acute success rate and a very acceptable complication rate. This is consistent with previously published data. Our regular use of visually guided laser therapy indicates excellent possibilities for the treatment of paroxysmal AF patients, showing a short learning curve and the adaptability of VGLA for clinical usage.
Length of follow-up
Visually guided laser ablation has shown to have high rates of durable PVI at 3 months following the index procedure.15 Promising clinical success rates have also been shown at 12 months follow-up. For the first time, here we show that freedom from AF is maintained at 48 months of follow-up suggesting that durable isolation of PVs is attainable in the medium term and that this does lead to freedom from AF in the PAF patient. The average follow-up was 30.0 (4–48) months.
Repeat procedures
The low number of repeat procedures is due to several factors: first, due to the infrequent follow-up and low-level patient monitoring employed in our centre, we may often miss occurrences of asymptomatic AF; secondly, patients may elect not to have a second procedure even when AF has recurred, particularly if their symptoms are dramatically reduced or they are happy with a previously failed antiarrhythmic drug or some other medical management approach. However, the low number of reconnected veins in patients returning for a second procedure is encouraging, and may confirm the data from the remapping study showing high levels of durable isolation.
Length of experience
One interesting facet of our experience with VGLA is that we have been involved with the development of this technology from a very early stage. Our first experience of this device came in 2005 when we ran a clinical feasibility trial with a previous iteration. The catheter used then did not have a compliant balloon, came in two different sizes and the arc was 110°. In 2005, the console did not allow us to track previous lesions, which made overlapping of energy delivery difficult. Surprisingly, although the various developments and improvements in the console, catheter, and deflectable sheath have led to lower procedure and fluoroscopy times and general improvements in clinical workflow, the results are comparable with our previous work, with our early experience suggesting that the basic concept of visually guided delivery of contiguous laser energy can result in durable isolation of PVs.
We now have four experienced operators in our institution who have been through a learning curve that is acceptable in length and difficulty, and we believe that our experience shows that from a practical standpoint this technology can be implemented in routine clinical practice.
Durable pulmonary vein isolation in the persistent atrial fibrillation patient
This paper presents the first published experience of a small cohort of patients with persistent AF treated with VGLA and our results give a promising insight into the role of durable PVI in this type of patient. At present, there seems to be no consensus within the electrophysiology (EP) community on how to treat patients with persistent AF.16 Some add lines to PVI, while new mapping techniques focus on substrate mapping and modification.17 However, until durable isolation is proven it is difficult to understand the role of these additional treatment manoeuvres. We do not maintain that ablation is clearly beneficial as our study concerned only 16 patients, but 9 of these patients (79.11%) were free of AF at 12-month follow-up, and we attribute this success to a sufficiently antral isolation of PVs. We believe that these results show that there is a role for PVI alone in a subset of patients with persistent AF and consider that circumferential antral PVI ablation performed using laser balloon should be suitable even for patients with persistent AF, as the PVI lesions are suitably antral, even for those patients. There should, therefore, be no extra cost for persistent patients compared with those with PAF. Further randomized prospective studies are needed to assess this.
Limitation
This study represents observational data from only a single centre. Follow-up of the majority of patients was carried out according to our regular clinical procedures and is less rigorous than that of the initial 27 patients included in the prospective feasibility trial. For example, 7-day Holter recordings are taken routinely in our hospital at the first clinical visit after the index procedure, but are then repeated only in patients reporting symptoms, or for other clinical reasons based on the judgement of the physician. We must also assume that in this non-homogeneous population, because of the less rigorous follow-up, we may have missed patients with non-symptomatic episodes of AF.
Conclusion
In our long-term 4-year clinical follow-up, VGLA has been shown to be safe and effective in patients with paroxysmal AF. The learning curve is short in the hands of experienced electrophysiologists and the rate of complications is in line with other techniques we employ in our EP laboratory. These clinical results confirm the validity of our decision to use this technology as part of our routine clinical practice in patients with paroxysmal AF. While our early experience of treating persistent AF patients with this technology is promising, because of the very low number of patients we cannot yet draw any final conclusions for this AF group and further studies are required to confirm its role with this type of patients.



