-
PDF
- Split View
-
Views
-
Cite
Cite
Jing Sun, Hanfei Sang, Changbin Yang, Hailong Dong, Chong Lei, Yan Lu, Yulin Ma, Xiaodong Zhou, Xiqing Sun, Lize Xiong, Electroacupuncture improves orthostatic tolerance in healthy individuals via improving cardiac function and activating the sympathetic system, EP Europace, Volume 15, Issue 1, January 2013, Pages 127–134, https://doi.org/10.1093/europace/eus220
Close - Share Icon Share
Abstract
Orthostatic intolerance (OI) is a common clinical problem; however, effective and applicable clinical prevention/treatment is limited. The aim of this study was to investigate whether electroacupuncture (EA) is a novel effective treatment in attenuating OI in healthy individuals.
This study used a randomized, controlled, crossover design using two protocols. Orthostatic intolerance was induced with a combination of head-up tilt (HUT) and lower body negative pressure (LBNP). Twenty healthy individuals in Protocol 1 and 10 healthy individuals in Protocol 2 received no EA, EA at PC-6 acupuncture points (acupoint), and EA at a non-acupoint in a random order with an interim of 1 week. Electroacupuncture was administered prior to HUT/LBNP in Protocol 1 and simultaneously during HUT/LBNP in Protocol 2. Electroacupuncture at PC-6 administered either before or during HUT/LBNP postponed the occurrence of pre-syncopal symptoms, improved haemodynamic responses to HUT/LBNP (including increased diastolic blood pressure, stroke volume, and total peripheral resistance and a decreased heart rate), blunted decreases of maximum velocity and velocity time integral of blood flow in the middle cerebral artery, and increased plasma noradrenalin and adrenalin concentrations. In addition, heart rate variability analysis revealed that EA at PC-6 either before or during HUT/LBNP decreased high-frequency ranges of R–R interval while increasing low-frequency ranges of R-R interval, which indicates an elevated heart sympathetic tone.
Electroacupuncture at PC-6 is effective in improving orthostatic tolerance. Cardiac function improvement and sympathetic activation are responsible for the improved orthostatic tolerance after EA. EA represents a novel intervention against OI.
Introduction
Orthostatic intolerance (OI) is a significant clinical problem, occurring not only in patients with autonomic dysfunction but also in healthy individuals, such as astronauts returning from space or persons immediately after bed rest.1,2 Orthostatic intolerance affects an estimated 500 000 Americans and causes a wide range of symptoms including headaches, nausea, diminished concentration, and even syncope, due to inadequate cerebral perfusion on upright posture.3 Frequent syncope requires therapeutic measures. Unfortunately, currently available pharmacoloical treatments (and even pacemakers) do not result in significant symptomatic improvement.
Although underlying pathophysiological mechanisms remain incompletely understood, the symptoms of OI are largely due to acute cerebral hypoperfusion resulting in cerebral ischaemia.4 Therefore, inhibiting the decrease of cerebral blood flow during upright posture via sympathetic activation may be useful in improving orthostatic tolerance. Current therapeutic measures, including increasing fluid and sodium intake, physical counter manoeuvres, and administration of sympathomimetic medications, demonstrated that sympathetic activation could improve orthostatic tolerance.5–7 In addition, decreased sympathetic nerve activity has been identified as a pathophysiological factor of neurogenic OI.8
Recent studies have demonstrated that electroacupuncture (EA) could activate the cardiovascular sympathetic system and vascular pressure responses by somatosympathetic reflex.9 Clinical evidence indicated that acupuncture or EA has therapeutic effect on certain types of hypotension,10 arrhythmias,11 and myocardial ischaemia.12,13 A recent clinical study has shown that EA could reduce the severity of hypotension after spinal anaesthesia in patients undergoing a caesarean section.14 However, whether EA is capable of improving orthostatic tolerance via activating the sympathetic system and enhancing cardiac function has not been studied.
The objective of the present study was to test the hypothesis that EA pre-treatment was a clinically applicable and effective therapeutic intervention in improving orthostatic tolerance. Considering variable clinical situations, we designed two protocols of EA pre-treatment. Healthy individuals were subjected to (i) a 30 min EA before OI induced by a combination of head-up tilt testing (HUT) and lower body negative pressure (LBNP) (Protocol 1) and (ii) simultaneous EA stimulation during HUT/LBNP (Protocol 2).
Methods
Subjects
Thirty healthy individuals without a history of spontaneous syncope were recruited in the study. Our inclusion criteria were: age between 18 and 25 years; body mass index (BMI) range from 18 to 25 kg/m2; a normal physical exam and electrocardiogram (ECG) measurement. Exclusion criteria were: psychiatric disturbances; history of surgery, hernia, or epilepsy; systemic disease with or without any functional limitation; visual or acoustic dysfunction; lower-limb diseases; pregnancy as determined by urine pregnancy test; active smoking or <1 year of smoking cessation; alcohol use (consuming >0.5L/day of wine or equivalent); any use of medications during the past 7 days; antibiotic use within 48 h; female in menstrual period; being enrolled in another research study. The study was approved by the Medical Ethical Committee of Fourth Military Medical University, Xi'an, Shaanxi, China. Enrolled individuals provided written informed consent.
Experimental protocol
To investigate the effect of EA on improving orthostatic tolerance, we conducted two experimental protocols. In Protocol 1, 20 healthy subjects were subjected to HUT/LBNP immediately after a 30 min EA treatment. In Protocol 2, 10 healthy subjects were subjected to HUT/LBNP while EA treatment was simultaneously administered (Figure 1A and B).
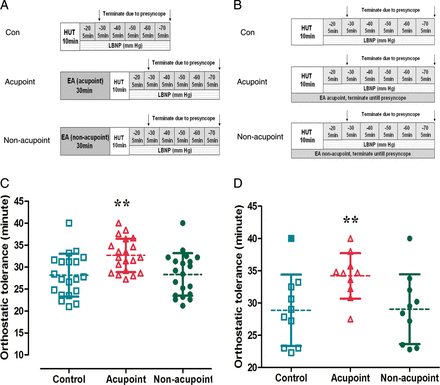
Schematic illustration for experimental protocol and changes of orthostatic tolerance. (A) Schematic illustration of Protocol 1. Electroacupuncture was administered for 30 min just before head-up tilt/lower body negative pressure. (B) Schematic illustration of Protocol 2. Electroacupuncture was simultaneously administered during head-up tilt/lower body negative pressure. (C) Changes in orthostatic tolerance in Protocol 1. Compared with the control and electroacupuncture at non-acupoint, electroacupuncture at PC-6 just before head-up tilt/lower body negative pressure significantly prolonged the onset of orthostatic tolerance. (D) Changes of orthostatic tolerance in Protocol 2. Compared with the control and electroacupuncture at non-acupoint, electroacupuncture at PC-6 during head-up tilt/lower body negative pressure significantly prolonged the onset of orthostatic tolerance. EA, electronic acupuncture; HUT, head-up tilt; LBNP, lower body negative pressure (**P< 0.01 compared with Con or non-acupoint groups).
A randomized, controlled, crossover design was employed in both protocols. Each individual was subjected to HUT/LBNP plus (i) no acupuncture (Con); (ii) bilateral EA at the Neiguan (PC-6) of both forearms (Acupoint); and (iii) bilateral EA at a non-acupoint of both shoulders (non-acupoint) on three separate days in a random order. Thus, by the end of the study, each volunteer had received all the three different treatments. There was an interim period of 1 week between each phase of the study.
Electroacupuncture
The EA was performed using small-sized (1.5 cm) cutaneous electrode pads placed bilaterally at the PC-6 points of both forearms or non-acupoints of the shoulders (SupplementarySupplementary DataSupplementary Data). The intensity of the electrical stimulation was adjusted to produce the most tolerable electrical sensation without muscle contraction or uncomfortable feelings at a frequency of 50 Hz using the Hwato electronic acupuncture treatment instrument (Model No. SDZ-II; Suzhou Medical Appliances Co., Ltd, Suzhou, China).
Assessment of orthostatic tolerance
Tests were performed in a climate-controlled room at an ambient air temperature of 24–26°C. After overnight fasting, test individuals were placed on a tilt table with the right arm at heart level. A combination of HUT and LBNP was used to provoke mediated OI. The head-up position of individuals was tilted to 70° for 10 min followed by gradual decreases during LBNP. Lower body negative pressure began at −20 mmHg for 5 min followed by a decrease of 10 mmHg every 5 min until −70 mmHg (total duration =40 min) were achieved or individuals developed presyncope. Presyncope was defined as either a decrease in systolic blood pressure (SBP; finger blood pressure) to <80 mmHg, or a decrease in SBP to < 90 mmHg plus symptoms of light headedness, nausea, sweating, or diaphoresis, or progressive symptoms of presyncope accompanied by a request from the subjects to terminate the test.15 Communication between the physician who performed EA and the commander who determined the termination of the tilt study based on cardiovascular responses were not allowed. In addition, haemodynamic responses at the end of the HUT/LBNP study were evaluated independently by two investigators who were blinded to treatments.
Blood analysis
In Protocol 1, blood samples were withdrawn every 10 min at baseline and during EA treatment followed by withdrawal of blood every 5 min during HUT/LBNP. In Protocol 2, all the blood samples were obtained every 5 min during the whole experiment. Plasma samples were obtained by centrifugation and stored at −80°C. The plasma concentrations of catecholamines (noradrenalin and adrenalin) were assayed using a commercially available sandwich technique enzyme-linked immunosorbent assay kit (ELISA; IBL International, Hamburg, Germany) according to the test protocol of the manufacturer, and were analysed on a Fluostar Optima Microplate Reader (BMG Labtech, Offenbach, Germany).
Data acquisition and analysis
Beat-by-beat arterial blood pressure and heart rate (HR) were obtained at the finger by photo plethysmography using the Penaz principle (Finometer, TNO, The Netherlands) and recorded for 5 min, online, using Beascope 1.0 software (Finometer). To avoid disturbing theHR and SBP, variability spectral analysis and spontaneous respiration of subjects were controlled to 12 times per minute during the measurement process. For each data set, a Hann window and linear detrend of data were applied before computing the power spectral density by Fast Fourier Transform. Total power of spectra was defined in the range of 0.04–0.40 Hz, high-frequency power (HF) within 0.15–0.40 Hz, and low-frequency power (LF) within 0.04–0.15 Hz. Normalized power (n) was defined as the ratio of power in each band to LF power plus HF power. The HF n power indicated cardiac vagal modulation; the LF n power-indexed sympathetic baroreflex-modulated activity.16
Cardiac stroke volume (SV), cardiac output (CO), and total peripheral resistance (TPR) were spontaneously calculated by the machine. Off-line data were printed for visual inspection of beat-to-beat results. Cerebral blood flow velocity in the left middle cerebral artery was measured continuously by means of a 2 MHz ultrasound probe (Multi-Dop X4, TCD-8·01, DWL, Germany), kept in place by a headset. A 21-gauge catheter was placed in an antecubital or forearm vein of the left arm and anticoagulated with heparin (20 U/mL).
Spectral analysis
After the R–R interval in the ECG had been detected correctly, the Fast Fourier Transform was applied to compute the power spectral density. These calculations were made by the heart rate variability (HRV) analysing module integrated in Chart 5.5 (Adinstrument Corporation).
Statistics
All data are expressed as the mean ± SD. Intra-individual and inter-individual differences were compared by paired and unpaired t tests, respectively. Analysis of variance testing for repeated measures was used for multiple comparisons. Analyses were performed using SPSS software version 18.0 (Chicago, IL, USA). A value of P< 0.05 was considered significant.
Results
In Protocol 1 (EA for 30 min before HUT/LBNP), 12 healthy volunteers [14 men and 6 women; 22 ± 5 (Mean ± SD) years old, 24 ± 3 kg/m2 BMI, 75 ± 10 b.p.m. HR, 110 ± 18 mmHg SBP, and 68 ± 15 mmHg diastolic blood pressure (DBP)] participated.
In Protocol 2 (EA during HUT/LBNP), 10 healthy volunteers [7 men and 3 women; 24 ± 4 (Mean ± SD) years old, 24 ± 7 kg/m2 BMI, 75 ± 15 b.p.m. HR, 108 ± 16 mmHg SBP, and 70 ± 12 mmHg DBP] participated. None of the recruited individuals was ruled out from the study.
Electroacupuncture postponed symptoms of presyncope
In Protocol 1 (EA for 30 min before HUT/LBNP), 1 of the 20 individuals tolerated the entire procedure (LBNP reached −70 mmHg, total duration =40 min) and 19 of the 20 individuals developed presyncope before LBNP reached −70 mmHg. The average time of developing presyncope was 28.1 ± 4.8 min in the control (without EA) group and 28.3 ± 4.9 min in the non-acupoint group (with EA at non-acupoint). Electroacupuncture at PC-6 prolonged the time of developing presyncope in individuals. The time of developing presyncope increased to 32.7 ± 3.8 min (P< 0.01 compared with no EA and EA at non-acupoint, Figure 1C).
In Protocol 2 (EA during HUT/LBNP), orthostatic tolerance was 28.9 ± 5.5 min without EA (Con) and 29 ± 5.4 min with EA at non-acupoint. Electroacupuncture at PC-6 prolonged the time of developing presyncope, which was increased to 34.2 ± 3.5 min (P< 0.01 compared with Con and EA at non-acupoint, Figure 1D).
Haemodynamic measurements
In Protocol 1, supine HR was 76 ± 27, 77 ± 27, and 74 ± 27 b.p.m. at the baseline in the Con, non-acupoint, and acupoint groups, respectively [NS; (SupplementarySupplementary DataSupplementary Data). Heart rate was not significantly changed during EA treatment, while HR gradually increased during HUT/LBNP among all groups. The increase in HR induced by HUT/LBNP was blunted in the EA acupoint group. At the end of the 10 min 70° HUT, the HR was 98 ± 45 b.p.m. (Con), 97 ± 45 b.p.m. (non-acupoint), and 88 ± 31.5 b.p.m. (acupoint, P< 0.05 compared with Con and non-acupoint, SupplementarySupplementary DataSupplementary Data]. In Protocol 2, the same trend of HR change was found as in Protocol 1. Simultaneous EA treatment during HUT/LBNP blunted the HR increase from 15 to 20 min (P< 0.05 compared with Con and non-acupoint, SupplementarySupplementary DataSupplementary Data).
In Protocols 1 and 2, SBP was decreased during HUT/LBNP. However, no significant difference among all groups was found during the whole experiment (SupplementarySupplementary DataSupplementary Data).
In Protocol 1, DBP was 69 ± 13.5, 69 ± 9, and 76 ± 13.5 mmHg at baseline in the Con, non-acupoint, and acupoint groups, respectively, which decreased during HUT/LBNP. Electroacupuncture at the acupoint significantly increased DBP. The increase in DBP was initiated at the end of EA treatment and lasted during the HUT/LBNP (from 25 to 50 min, P< 0.05 vs. Con and non-acupoint groups, SupplementarySupplementary DataSupplementary Data). At the end of the 10 min 70° HUT, DBP was 69 ± 22.5 and 70 ± 22.5 mmHg in the Con and non-acupoint, respectively, which was increased to 77 ± 45 mmHg in the acupoint group (P< 0.05, SupplementarySupplementary Data). The same trend for DBP change was found in Protocol 2. Electroacupuncture treatment increased DBP from 15 to 25 min (P< 0.05, Supplementary Figure 3D).
In Protocols 1 and 2, CO decreased during HUT/LBNP. However, no significant difference was found among the groups during the whole experiment (SupplementarySupplementary DataSupplementary Data).
In Protocol 1, HUT/LBNP induced a decrease in SV among all groups. Electroacupuncture at the acupoint significantly increased SV. The increase in SV was initiated at the end of EA treatment and lasted during HUT/LBNP (from 25 to 50 min, P< 0.05 vs. Con and non-acupoint groups, SupplementarySupplementary DataSupplementary Data). In Protocol 2, SV was increased by simultaneous EA treatment during HUT/LBNP from 15 to 25 min (P< 0.05, SupplementarySupplementary DataSupplementary Data).
In Protocol 1, TPR was increased by HUT/LBNP. At the end of EA (from 20 to 30 min), TPR was significantly increased (P< 0.05, SupplementaSupplementary DataSupplementary Data). In Protocol 2, TPR was increased by simultaneous EA treatment during HUT/LBNP from 10 to 20 min (P< 0.05, SupplementarySupplementary DataSupplementary Data).
Electroacupuncture blunted the head-up tilt/lower body negative pressure-induced decrease in cerebral blood flow velocity
In Protocol 1, EA at the acupoint increased the maximum velocity (Vmax) and velocity time integral (VTI) of blood flow in the middle cerebral artery from 25 to 50 min, which blunted the decrease of Vmax and VTI during HUT/LBNP (P< 0.05 compared with Con and non-acupoint groups, Figure 2A and C).
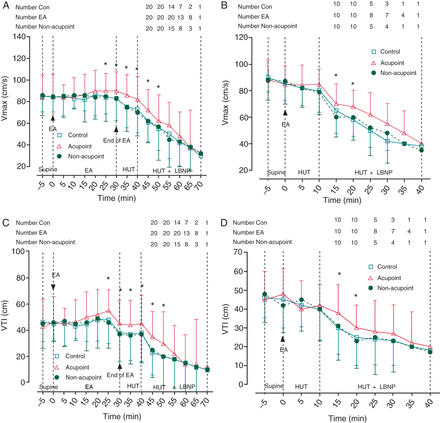
Changes in maximum velocity and velocity time integral of blood flow in the middle cerebral artery. (A and B) Changes in maximum velocity in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure blunted the head-up tilt/lower body negative pressure induced decrease of maximum velocity. (C and D) Changes in velocity time integral in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure blunted the head-up tilt/lower body negative pressure-induced decrease of velocity time integral. Vmax, maximum of velocity; VTI, velocity time integral; EA, electronic acupuncture; HUT, head-up tilt; LBNP, lower body negative pressure. Data are expressed as the Mean ± SD (*P< 0.05 compared with Con and non-acupoint groups).
In Protocol 2, EA at the acupoint blunted the decrease of Vmax and VTI during HUT and LBNP from 15 to 20 min (P< 0.05 compared with Con and non-acupoint groups, Figure 2B and D).
Electroacupuncture improved heart rate variability
In both Protocols, compared with the control and non-acupoint, EA at the acupoint decreased the HF ranges of the R–R interval and increased the LF ranges of the R–R interval in the supine position (Figure 3).
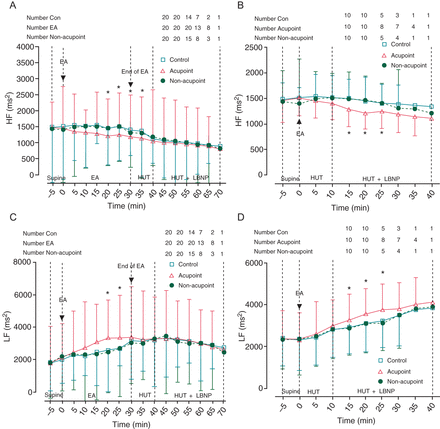
Changes of heart rate variability in the high-frequency and low-frequency ranges of R–R interval. (A and B) Changes in high-frequency ranges of R–R interval in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure decreased high-frequency ranges of R–R interval. (C and D) Changes in low-frequency ranges of R–R interval in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure increased low-frequency ranges of R–R interval. HRV, heart rate variability; HF, high-frequency; LF, low-frequency; EA, electronic acupuncture; HUT, head-up tilt; LBNP, lower body negative pressure. Data are expressed as the Mean ± SD (*P< 0.05 compared with control and non-acupoint groups).
Electroacupuncture increased the concentration of plasma noradrenalin and adrenalin
In Protocol 1, plasma noradrenalin and adrenalin concentrations were higher in the EA Acupoint group, and began rising from 20 min after EA stimulation and lasted to 50 min during HUT/LBNP (P< 0.05 compared with control and non-acupoint groups, Figure 4A and C). Similarly, in Protocol 2, plasma noradrenalin and adrenalin concentrations increased from 20 min after the onset of EA stimulation and lasted for 10 min to 30 min during HUT/LBNP (P< 0.05 compared with control and non-acupoint, Figure 4B and D).
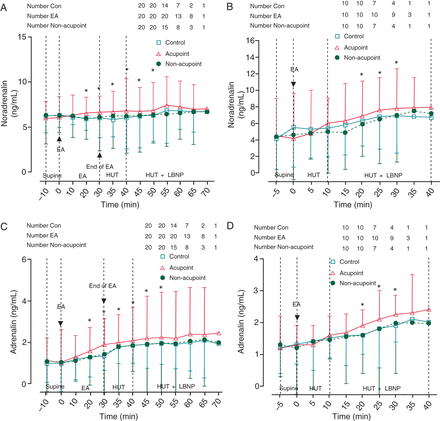
Changes in plasma noradrenalin and adrenalin concentrations. (A and B) Changes in plasma noradrenalin concentrations in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure increased plasma noradrenalin concentrations. (C and D) Changes in plasma adrenalin concentrations in Protocols 1 and 2. Electroacupuncture at PC-6 prior to or during head-up tilt/lower body negative pressure increased plasma adrenalin concentrations. EA, electronic acupuncture; HUT, head-up tilt; LBNP, lower body negative pressure. Data are expressed as the Mean ± SD (*P< 0.05 compared with Con and non-acupoint groups).
Discussion
The present study demonstrated that EA stimulation significantly improved orthostatic tolerance in healthy individuals. We found that EA stimulation for 30 min immediately before HUT/LBNP, and simultaneously with EA stimulation during HUT/LBNP, produced a remarkable improvement of orthostatic tolerance via improvement of cardiac performance and activation of the peripheral sympathetic nervous system.
Orthostatic intolerance is a clinical entity that is defined as an SBP decrease of at least 20 mmHg or a DBP decrease of at least 10 mmHg within 3min of standing.17 It may be idiopathic or related to diseases with autonomic failure, such as diabetes. We used a combination of HUT testing and LBNP to induce neutrally mediated presyncope in healthy individuals, which has been shown to provide a quantitative and reproducible measurement of orthostatic tolerance.18 The combination of HUT and LBNP exerts a much greater orthostatic stimulus than regular standing.
In neutrally mediated syncope, a sudden decrease of sympathetic activity results in vasodilatation and bradycardia, which compromises blood supply to vital organs, particularly to the brain.19 Many sympathomimetic agents such as midodrine (α1-adrenoreceptoragonists) and norepinephrine precursors (DL-DOPS) have been used in the treatment of OI.20 Therefore, increasing the activity of the sympathetic nervous system is effective in preventing OI.
However, associated adverse effects limit clinical application of pharmacological treatments. Previous studies have demonstrated that electric stimulation of the median nerve enhanced sympathetic activity via a somatosympathetic reflex, and consequently improved left ventricular contractility and caused a pressor response.9,21,22 The PC-6 acupoint is located close to the median nerve. Syuu et al.9 reported that EA at PC-6 mimicked the effect of median nerve stimulation during activation of the sympathetic nervous system, and induced a depressor response. Based on this information, we selected EA at the PC-6 acupoint to test our hypothesis that EA stimulation may reduce OI.
In the current study, we showed that EA stimulation for 30 min just before HUT/LBNP and during HUT/LBNP increased the concentration of plasma noradrenalin and adrenalin, improved haemodynamic responses, enhanced the ratio of LF of R–R interval to HF of R–R interval, and inhibited the decrease of cerebral blood flow velocity. These results indicate that EA stimulation improves orthostatic tolerance through improvement of cardiac performance and activation of the peripheral sympathetic nervous system, thus inhibiting the decrease of cerebral blood flow.
We therefore speculated that the sympathetic responses of EA treatment at the PC-6 acupoint may be induced through the neurohumoral activation of catecholamines and activation of the somatosympathetic reflex pathway. Previous studies have suggested that electrical stimulation-induced rhythmic muscle contractions activate the skeletal muscle pump to induce the pressor effect.23 However, in the present study, we did not observe any visible local muscle contractions. Furthermore, no protective effect was found in the non-acupoint group. Therefore, the increase in SV induced by EA at PC-6 probably results from activation of neurohumoral regulation and the somatosympathetic reflex rather than from electrical stimulation-induced muscle contractions.
In traditional Chinese medicine, acupuncture therapy has been performed for thousands of years to treat a variety of diseases and disorders. In the West, acupuncture or EA, has been used to treat pain,24 migraines,25 cocaine addiction,26 emesis,27 and stroke,28 Therefore, acupuncture or EA is a widely accepted treatment.
In summary, we have demonstrated that EA at the PC-6 acupoint improved orthostatic tolerance. Electroacupuncture stimulation produced a remarkable improvement of orthostatic tolerance via up-regulation of both the cardiac and the peripheral sympathetic nervous systems. Therefore, EA stimulation at the PC-6 acupoint immediately prior to or during the development of OI may be a novel therapeutic method in the prevention or treatment of OI. Whether this treatment can also be used as an effective treatment in those patients with severe OI warrants further investigation.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 30725039, 30930091, and 30873326).
Acknowledgements
We thank Dr Wayne Bond Lau and Professor Xinliang Ma, Thomas Jefferson University, and Professor Zhiyi Zuo, University of Virginia, for their valuable suggestions on revising the manuscript. We also thank Professor Liwen Liu and Dr Yanjun Xu, Department of Ultrasound Medicine, Xijing Hospital, for their technical assistance in the measurement of cerebral blood flow.
Conflict of interest: none declared.
References
Author notes
Contributed equally to this work.



