-
PDF
- Split View
-
Views
-
Cite
Cite
Pilar Merlos, Eva Rumiz, Ricardo Ruiz-Granell, Ángel Martínez, Maria Teresa Izquierdo, Ángel Ferrero, Eloy Dominguez, Juan Miguel Sánchez, Salvador Morell, Roberto García-Civera, Outcome of patients with syncope beyond the implantable loop recorder, EP Europace, Volume 15, Issue 1, January 2013, Pages 122–126, https://doi.org/10.1093/europace/eus264
Close - Share Icon Share
Abstract
The implantation of an implantable loop recorder (ILR) leads to the diagnosis in about 35% of patients with syncope of unknown origin. Information on outcome of patients in whom a diagnosis is not reached during the lifetime of the device is scarce. The aim of our study is to determine the outcome of these patients in terms of syncope recurrence and survival.
An ILR was implanted to 97 patients with syncope of unknown origin. Patients were classified in groups A and B depending on their high or low risk, respectively, of having arrhythmic syncope. Diagnosis had not been reached in 60 patients (62%) when the ILR battery reached end operational life. Five patients were lost to follow up. During a median follow-up of 48 months after ILR explantation (interquartile range 36–56), 22 patients (40%) had recurrence of syncope (32% in group A vs. 48% in group B, P = 0.3). Syncopes with no neurally mediated profile were more frequent in group A (18 vs. 0%, P = 0.05) and neurally mediated profile syncopes were more frequent in group B (44 vs. 11%, P = 0.007). Five patients died, four of them in group A and 1 in group B (P = 0.4). No sudden or cardiac deaths were detected during follow-up. All deaths were due to non-cardiac causes.
Recurrent syncope is common in patients in whom a diagnosis is not established after the full battery life of an ILR. The prognosis of these patients seems to be good, without observed sudden or cardiac death.
Introduction
Prolonged electrocardiographic monitoring, especially with implantable loop recorders (ILRs), is a useful tool in the diagnosis of syncope, and Guidelines of the European Society of Cardiology for the diagnosis and treatment of syncope encourage its early use.1 With the implantation of an ILR the diagnosis is reached in an average of 35% of patients with syncope of unknown origin over an observation period generally <18 months,2–10 but little is known about the outcome of those patients in whom a diagnosis is not reached during the lifetime of the device. The aim of our study is to determine the outcome in terms of syncope recurrence and survival of these patients.
Methods
Study group
We prospectively included 97 consecutive patients in whom we implanted an ILR according to our study protocol of syncope from July 1998 to February 2007. These patients with syncope underwent an initial evaluation that consisted of clinical history, physical examination, 12-lead electrocardiogram (ECG), 24-h Holter-ECG recording, carotid sinus massage, active ortostatic tests, and other cardiac explorations (echocardiogram and treadmill stress test) if indicated. After a negative initial evaluation an ILR was implanted following two main conditions: (i) patients with suspected arrhythmic syncope (patients presenting with one or more of the following criteria: family history of sudden death, structural heart disease, palpitations immediately after or before syncope, abnormal ECG or significant arrhythmias during monitoring or Holter recording), and no indications for implantation of a cardioverter defibrillator (ICD) for primary prevention of sudden death; these patients underwent electrophysiological study (EPS) when indicated prior to ILR implantation; (ii) an ILR was also implanted in patients who, while not having arrhythmic risk criteria, had a high rate of syncope recurrence, clinical presentation not typical for neurally mediated syncope, recurrent severe body harm or belonged to risk professions (professional drivers, working at heights …). For this study, patients at risk of arrhythmic syncope (indication stated as (i)) were considered as group A and the remaining patients (indication stated as (ii)) were considered as group B.
Identifying data, clinical history, and additional tests, especially ECG, Holter monitoring, and EPS were collected, as well as data from the follow-up.
• This is one of the first studies, to our knowledge, to study the outcome of patients with syncope of unknown origin after the explantation of an ILR.
• Recurrent syncope is common in patients in whom a diagnosis is not established after the full battery life of an ILR.
• The prognosis of these patients seems to be good, without observed sudden or cardiac death after a complete study of syncope.
Implantable loop recorder
All patients had a Medtronic Reveal®, Reveal Plus®, or Reveal XT® (Medtronic Inc., Minneapolis, MN, USA), with a nominal longevity of 18 months, implanted subcutaneously in the left paraesternal area.
The ILR was able to save up to three manual activations of 7.5 min and automatic activations that were programmed as follows: (i) rapid ventricular tachycardia (RR interval<260 ms in at least 30 of 40 consecutive beats), (ii) ventricular tachycardia (RR interval>261–340 ms in 16 consecutive beats), (iii) a pause >30 s, or (iv) bradicardia (heart rate <30 b.p.m. in 4 consecutive beats). In all cases, the sensitivity was manually adjusted.
Endpoints and follow-up
Our endpoints were syncopal recurrence and mortality. We considered syncopal recurrence as defined in current guidelines as transient loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery.1 The aetiological diagnoses of recurrences were also established according to European Society of Cardiology Guidelines.1 We assessed the cause of death (non-cardiac, cardiac, or sudden death) by reviewing medical records, and considered sudden death as defined by current guidelines as sudden and unexpected death that occurs within 1h of symptom onset if witnessed and within 24 h of the person to be seen without symptoms if it had not been witnessed.11
After ILR implantation, follow-up was performed in an outpatient clinic every 3 months until a diagnosis was reached or until the end of service of the device, the moment when the ILR was explanted. No replacement of exhausted devices was performed. After explantation, follow-up was continued in the outpatient clinic or by telephone.
Statistical analysis
The Kolmogorov–Smirnov test was used to evaluate the normal distribution of continuous data. The central tendency measures used were the mean and standard deviation (SD) or median and interquartile range (IQR) if the distribution was asymmetric. Continuous variables were analysed with the t-test if they followed a normal distribution or with the Mann–Whitney test if it was not the case. Dichotomic variables were expressed as percentages and compared with the χ2 statistic; the Fisher's exact test was used when appropriate.
Survival distributions for the time to first syncopal recurrence were estimated with the Kaplan–Meier method and the log-rank test.
A two-tailed P value <0.05 was considered to indicate a statistically significant difference. The SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) software was used.
This study complies with the Declaration of Helsinki. The local ethics committee has approved the research protocol and written informed consent has been obtained from the patients.
Results
In 60 patients (62%) no diagnosis of the cause of syncopal spells had been reached when the ILR battery went depleted due, in most of cases, to lack of symptoms during the lifetime of the device. Five patients were lost during follow-up. Thus, the study group was formed by the remaining 55. Fifty-one percent (n = 28) belonged to group A (high risk of arrhythmia), while 49% (n = 27) belonged to group B (low risk of arrhythmia).
The median duration of ILR recording was 16 months (IQR 14–18). Median follow-up from the ILR explantation was 48 months (IQR 36–56).
Baseline characteristics
Baseline characteristics of our population are listed in Table 1. Mean age of our 55 patients was 60 years, with a predominance of men (62%). Almost half were hypertensive patients whereas the prevalence of diabetes, dyslipidaemia, neurological disease, chronic obstructive pulmonary disease, or atrial fibrillation was low. No significant differences in these pathologies were found between the two groups of low and high risk of arrhythmic origin of syncope.
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Clinical characteristics | ||||
| Age in years, mean ± SD | 60 ± 18 | 63 ± 20 | 57 ± 16 | 0.2 |
| Female, n (%) | 32 (58%) | 20 (71%) | 12 (44%) | 0.06 |
| Hypertension, n (%) | 25 (46%) | 16 (57%) | 9 (35%) | 0.1 |
| Diabetes mellitus, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Dislipaemia, n (%) | 3 (5%) | 2 (7%) | 1 (4%) | 1 |
| Neurological disease, n (%) | 8 (15%) | 5 (18%) | 3 (11%) | 0.7 |
| Chronic pulmonary disease, n (%) | 3 (5%) | 3 (11%) | 0 | 0.2 |
| Ischaemic heart disease, n (%) | 10 (18%) | 10 (36%) | 0 | 0.001 |
| Previous myocardial infarction, n (%) | 6 (11%) | 6 (21%) | 0 | 0.02 |
| LVEF, mean ± SD | 62 ± 14 | 58 ± 16 | 68 ± 6 | 0.04 |
| Previous atrial fibrilation, n (%) | 4 (7%) | 3 (11%) | 1 (4%) | 0.3 |
| Electrocardiographic characteristics | ||||
| QRS duration in milliseconds, mean ± SD | 100 ± 25 | 111 ± 28 | 87 ± 15 | 0.001 |
| QRS axis in degrees, mean ± SD | 11 ± 43 | 2 ± 46 | 21 ± 39 | 0.1 |
| QT interval in milliseconds, mean ± SD | 404 ± 55 | 404 ± 54 | 404 ± 58 | 1 |
| Corrected QT in milliseconds, mean ± SD | 409 ± 49 | 403 ± 49 | 417 ± 48 | 0.4 |
| Syncopal burden | ||||
| Total number of syncopes, median [IQR] | 3 [1,6] | 2 [1,4] | 3 [5,20] | 0.001 |
| Number of syncopes previous year, median [IQR] | 2 [1,4] | 1.5 [1,3] | 2 [2,5] | 0.03 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Clinical characteristics | ||||
| Age in years, mean ± SD | 60 ± 18 | 63 ± 20 | 57 ± 16 | 0.2 |
| Female, n (%) | 32 (58%) | 20 (71%) | 12 (44%) | 0.06 |
| Hypertension, n (%) | 25 (46%) | 16 (57%) | 9 (35%) | 0.1 |
| Diabetes mellitus, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Dislipaemia, n (%) | 3 (5%) | 2 (7%) | 1 (4%) | 1 |
| Neurological disease, n (%) | 8 (15%) | 5 (18%) | 3 (11%) | 0.7 |
| Chronic pulmonary disease, n (%) | 3 (5%) | 3 (11%) | 0 | 0.2 |
| Ischaemic heart disease, n (%) | 10 (18%) | 10 (36%) | 0 | 0.001 |
| Previous myocardial infarction, n (%) | 6 (11%) | 6 (21%) | 0 | 0.02 |
| LVEF, mean ± SD | 62 ± 14 | 58 ± 16 | 68 ± 6 | 0.04 |
| Previous atrial fibrilation, n (%) | 4 (7%) | 3 (11%) | 1 (4%) | 0.3 |
| Electrocardiographic characteristics | ||||
| QRS duration in milliseconds, mean ± SD | 100 ± 25 | 111 ± 28 | 87 ± 15 | 0.001 |
| QRS axis in degrees, mean ± SD | 11 ± 43 | 2 ± 46 | 21 ± 39 | 0.1 |
| QT interval in milliseconds, mean ± SD | 404 ± 55 | 404 ± 54 | 404 ± 58 | 1 |
| Corrected QT in milliseconds, mean ± SD | 409 ± 49 | 403 ± 49 | 417 ± 48 | 0.4 |
| Syncopal burden | ||||
| Total number of syncopes, median [IQR] | 3 [1,6] | 2 [1,4] | 3 [5,20] | 0.001 |
| Number of syncopes previous year, median [IQR] | 2 [1,4] | 1.5 [1,3] | 2 [2,5] | 0.03 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Clinical characteristics | ||||
| Age in years, mean ± SD | 60 ± 18 | 63 ± 20 | 57 ± 16 | 0.2 |
| Female, n (%) | 32 (58%) | 20 (71%) | 12 (44%) | 0.06 |
| Hypertension, n (%) | 25 (46%) | 16 (57%) | 9 (35%) | 0.1 |
| Diabetes mellitus, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Dislipaemia, n (%) | 3 (5%) | 2 (7%) | 1 (4%) | 1 |
| Neurological disease, n (%) | 8 (15%) | 5 (18%) | 3 (11%) | 0.7 |
| Chronic pulmonary disease, n (%) | 3 (5%) | 3 (11%) | 0 | 0.2 |
| Ischaemic heart disease, n (%) | 10 (18%) | 10 (36%) | 0 | 0.001 |
| Previous myocardial infarction, n (%) | 6 (11%) | 6 (21%) | 0 | 0.02 |
| LVEF, mean ± SD | 62 ± 14 | 58 ± 16 | 68 ± 6 | 0.04 |
| Previous atrial fibrilation, n (%) | 4 (7%) | 3 (11%) | 1 (4%) | 0.3 |
| Electrocardiographic characteristics | ||||
| QRS duration in milliseconds, mean ± SD | 100 ± 25 | 111 ± 28 | 87 ± 15 | 0.001 |
| QRS axis in degrees, mean ± SD | 11 ± 43 | 2 ± 46 | 21 ± 39 | 0.1 |
| QT interval in milliseconds, mean ± SD | 404 ± 55 | 404 ± 54 | 404 ± 58 | 1 |
| Corrected QT in milliseconds, mean ± SD | 409 ± 49 | 403 ± 49 | 417 ± 48 | 0.4 |
| Syncopal burden | ||||
| Total number of syncopes, median [IQR] | 3 [1,6] | 2 [1,4] | 3 [5,20] | 0.001 |
| Number of syncopes previous year, median [IQR] | 2 [1,4] | 1.5 [1,3] | 2 [2,5] | 0.03 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Clinical characteristics | ||||
| Age in years, mean ± SD | 60 ± 18 | 63 ± 20 | 57 ± 16 | 0.2 |
| Female, n (%) | 32 (58%) | 20 (71%) | 12 (44%) | 0.06 |
| Hypertension, n (%) | 25 (46%) | 16 (57%) | 9 (35%) | 0.1 |
| Diabetes mellitus, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Dislipaemia, n (%) | 3 (5%) | 2 (7%) | 1 (4%) | 1 |
| Neurological disease, n (%) | 8 (15%) | 5 (18%) | 3 (11%) | 0.7 |
| Chronic pulmonary disease, n (%) | 3 (5%) | 3 (11%) | 0 | 0.2 |
| Ischaemic heart disease, n (%) | 10 (18%) | 10 (36%) | 0 | 0.001 |
| Previous myocardial infarction, n (%) | 6 (11%) | 6 (21%) | 0 | 0.02 |
| LVEF, mean ± SD | 62 ± 14 | 58 ± 16 | 68 ± 6 | 0.04 |
| Previous atrial fibrilation, n (%) | 4 (7%) | 3 (11%) | 1 (4%) | 0.3 |
| Electrocardiographic characteristics | ||||
| QRS duration in milliseconds, mean ± SD | 100 ± 25 | 111 ± 28 | 87 ± 15 | 0.001 |
| QRS axis in degrees, mean ± SD | 11 ± 43 | 2 ± 46 | 21 ± 39 | 0.1 |
| QT interval in milliseconds, mean ± SD | 404 ± 55 | 404 ± 54 | 404 ± 58 | 1 |
| Corrected QT in milliseconds, mean ± SD | 409 ± 49 | 403 ± 49 | 417 ± 48 | 0.4 |
| Syncopal burden | ||||
| Total number of syncopes, median [IQR] | 3 [1,6] | 2 [1,4] | 3 [5,20] | 0.001 |
| Number of syncopes previous year, median [IQR] | 2 [1,4] | 1.5 [1,3] | 2 [2,5] | 0.03 |
As follows from the definition of the groups, patients in group A (high risk) had a significantly higher prevalence of coronary artery disease and prior myocardial infarction with a significantly lower left ventricular ejection fraction. Both the number of syncopes in the year before inclusion and the total number of syncopal episodes were significantly higher in patients in group B (low risk of arrhythmic syncope), in which we observed a wide variability in the number of syncopal recurrences. QRS duration on baseline ECG was significantly higher in patients in group A.
Syncope recurrence after implantable loop recorder end of service
Syncope recurrence after explanting the ILR in our study group was high: 22 patients (40% of our study group) had further episodes of syncope. Figure 1 and Table 2 show the characteristics of syncope recurrence and outcome in both groups. In most cases (15 patients) syncope was preceded by prodrome or accompanying vegetative symptoms suggesting a reflex mechanism, two patients had psychogenic pseudosyncopes (clinically diagnosed by their cardiologists) after the ILR was explanted and the remaining 5 patients (9% of our study group) had syncope without triggers, prodrome, or accompanying vegetative symptoms.
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Mortality, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Arrhythmic death | 0 | 0 | 0 | 1 |
| Cardiac death | 0 | 0 | 0 | 1 |
| Non-cardiac death | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Syncopal recurrence, n (%) | 22 (40%) | 9 (32%) | 13 (48%) | 0.3 |
| Non-neurally mediated profile syncope | 5 (9%) | 5 (18%) | 0 | 0.05 |
| Neurally mediated profile syncope | 15 (27%) | 3 (11%) | 12 (44%) | 0.007 |
| Pseudosyncope | 2 (4%) | 1 (4%) | 1 (4%) | 1 |
| Number of recurrences, n [IQR] | 1 [1–3.5] | 1 [1–1.75] | 2 [1–4] | 0.21 |
| Implantation of devices, n (%) | ||||
| Pacemaker | 1 (2%) | 1 (4%) | 0 | 1 |
| ICD | 3 (5%) | 3 (11%) | 0 | 0.2 |
| ILR | 1 (2%) | 0 | 1 (4%) | 0.5 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Mortality, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Arrhythmic death | 0 | 0 | 0 | 1 |
| Cardiac death | 0 | 0 | 0 | 1 |
| Non-cardiac death | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Syncopal recurrence, n (%) | 22 (40%) | 9 (32%) | 13 (48%) | 0.3 |
| Non-neurally mediated profile syncope | 5 (9%) | 5 (18%) | 0 | 0.05 |
| Neurally mediated profile syncope | 15 (27%) | 3 (11%) | 12 (44%) | 0.007 |
| Pseudosyncope | 2 (4%) | 1 (4%) | 1 (4%) | 1 |
| Number of recurrences, n [IQR] | 1 [1–3.5] | 1 [1–1.75] | 2 [1–4] | 0.21 |
| Implantation of devices, n (%) | ||||
| Pacemaker | 1 (2%) | 1 (4%) | 0 | 1 |
| ICD | 3 (5%) | 3 (11%) | 0 | 0.2 |
| ILR | 1 (2%) | 0 | 1 (4%) | 0.5 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Mortality, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Arrhythmic death | 0 | 0 | 0 | 1 |
| Cardiac death | 0 | 0 | 0 | 1 |
| Non-cardiac death | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Syncopal recurrence, n (%) | 22 (40%) | 9 (32%) | 13 (48%) | 0.3 |
| Non-neurally mediated profile syncope | 5 (9%) | 5 (18%) | 0 | 0.05 |
| Neurally mediated profile syncope | 15 (27%) | 3 (11%) | 12 (44%) | 0.007 |
| Pseudosyncope | 2 (4%) | 1 (4%) | 1 (4%) | 1 |
| Number of recurrences, n [IQR] | 1 [1–3.5] | 1 [1–1.75] | 2 [1–4] | 0.21 |
| Implantation of devices, n (%) | ||||
| Pacemaker | 1 (2%) | 1 (4%) | 0 | 1 |
| ICD | 3 (5%) | 3 (11%) | 0 | 0.2 |
| ILR | 1 (2%) | 0 | 1 (4%) | 0.5 |
| . | Total . | Group A . | Group B . | P . |
|---|---|---|---|---|
| (n = 55) . | (n = 28) . | (n = 27) . | ||
| Mortality, n (%) | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Arrhythmic death | 0 | 0 | 0 | 1 |
| Cardiac death | 0 | 0 | 0 | 1 |
| Non-cardiac death | 5 (9%) | 4 (14%) | 1 (4%) | 0.4 |
| Syncopal recurrence, n (%) | 22 (40%) | 9 (32%) | 13 (48%) | 0.3 |
| Non-neurally mediated profile syncope | 5 (9%) | 5 (18%) | 0 | 0.05 |
| Neurally mediated profile syncope | 15 (27%) | 3 (11%) | 12 (44%) | 0.007 |
| Pseudosyncope | 2 (4%) | 1 (4%) | 1 (4%) | 1 |
| Number of recurrences, n [IQR] | 1 [1–3.5] | 1 [1–1.75] | 2 [1–4] | 0.21 |
| Implantation of devices, n (%) | ||||
| Pacemaker | 1 (2%) | 1 (4%) | 0 | 1 |
| ICD | 3 (5%) | 3 (11%) | 0 | 0.2 |
| ILR | 1 (2%) | 0 | 1 (4%) | 0.5 |
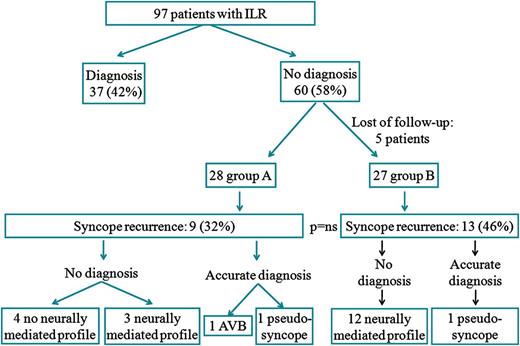
Although no significant differences in the incidence of syncope recurrence between the two groups of high and low risk were found (9 patients—32% in group A vs. 13–48% in group B, P = 0.3), there were differences in the type of recurrence. As expected, syncopes without neurally mediated profile were more frequent in group A (5–18% vs. 0, P = 0.05) and neurally mediated profile syncopes were more frequent in group B (12–44% compared with 3–11%, P = 0.007). One case of pseudosyncope was found in each group. Among the five patients with recurrences with no neurally mediated clinical profile that occurred in group A, only one patient was found to have complete atrioventricular block (AVB), and a pacemaker was implanted to this patient. In other three patients in group A an ICD was implanted during follow-up because their left ventricular function worsened, leading to fulfilment of criteria for primary prevention of sudden death. In one patient in group B, a second ILR was implanted 6 years later, due to clinical persistence of recurrences and was finally diagnosed of neurally mediated syncope with major cardioinhibitory component, and a pacemaker was implanted.
There was no difference in the number of syncopal recurrences among patients in groups A and B [1 (1–1.75) vs. 2 (1–4), P = 0.21], nor in the time to first recurrence, as shown in Figure 2.
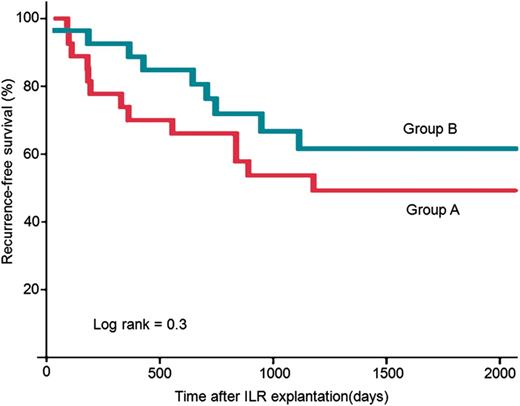
Evolution of recurrent syncope in groups A and B. Kaplan–Meier curves. We do not observe significant differences between both groups.
Mortality
After a median follow-up of 48 months, mortality in our population was 9%. Five deaths occurred in the whole population, four of them in group A and one in group B (P = 0.4). No arrhythmic or cardiac deaths were detected during follow-up. The four deaths in group A were due to: aortic dissection, thalamic linfoma, pneumonía, and sepsis. The death in group B was traumatic.
Discussion
Our data show that syncope recurrence is not uncommon after the end of service of a non-diagnostic ILR. However, most recurrences have neurally mediated clinical profile, severe causes of syncope are seldom found, and the prognosis of patients seems to be good.
Prolonged ECG monitoring, especially with ILR, has become an invaluable tool in the study of patients with syncope of unknown origin and current guidelines stress its early use in the study protocol of syncope. Nevertheless, its diagnostic yield is far from 100%. According to data from nine studies, which included 506 patients with unexplained syncope after a complete negative study and in whom an ILR had been implanted, diagnosis was achieved in 35%.2–10 Thus, 65% of patients who had an ILR implanted within a study protocol of syncope remain undiagnosed, which is consistent with the 62% observed in our study group.
Most of the named studies include heterogeneous groups of patients at high and low risk of arrhythmic syncope. Specifically, in the subgroup of patients at low risk, the diagnostic yield of ILR is 28%, as shown in ISSUE 1.4 For patients with bundle branch block, the diagnostic yield of ILR was 376 and 48% in the recent B4 study.12 In patients with structural heart disease, it ranges from 175 to 45%.10 However, there is little information on the evolution and prognosis of patients in whom the ILR reaches the end of service of the battery without a diagnosis of the cause of syncope. These patients, as seen above, account for >50% of patients in whom an ILR is implanted (62% in our series).
In our series, we found that syncope recurrence after ILR explantation without diagnosis is high, 32% in patients at risk of arrhythmic syncope (group A) and 46% in patients with a more benign profile (group B). In the latter, recurrences are mainly due to syncopes with neurally mediated clinical profile (92%). In group A, although 56% of recurrences (five patients) had a clinical profile not suggestive of neurally mediated syncope, only one patient was finally diagnosed of arrhythmic syncope (complete AVB requiring pacemaker implantation). We decided per protocol not to replace exhausted devices. It is not known if the probability of recording a recurrence would have been greater in case the devices had been replaced. Whereas it seems of little clinical relevance in group B patients, since most recurrences had a neurally mediated clinical profile, in patients at risk of arrhythmic syncope it would have helped to document recurrences with no such clinical profile. Anyway, most arrhythmia-related causes of syncope actually were revealed before the implantation or during the lifetime of the ILR.
According to previous studies, the incidence of sudden or cardiac death is low in patients in whom an ILR is implanted within a study protocol of syncope, even in patients at high risk of arrhythmic origin of syncope. García-Civera et al.13 reported a mortality of 2% without sudden or cardiac mortality when an ILR was implanted to this profile of patients after a negative EPS. Similar results were reported by Azocar et al.14 in a series of patients with syncope and bundle branch block in which an ILR was implanted also after a negative EPS, with no sudden mortality but with a death of coronary origin. Nevertheless, in the recently published B4 study,12 mortality in patients with bundle branch block and an ILR implanted after a negative EPS was slightly higher, 6%, with 3% of sudden death.
According to our results, this low sudden and cardiac mortality in patients with an ILR implanted after a negative EPS is maintained after the explantation of the ILR. Thus, although the size of our sample is small to draw conclusions about survival, we observed no sudden deaths or mortality of cardiac aetiology during follow-up in patients in whom the ILR did not render a diagnosis. This probably reflects that potentially lethal causes of syncope have been excluded during the diagnostic process in these patients. Non-cardiac mortality incidence was 14 and 4% in groups A and B, respectively, without significant differences between both groups, but reflecting their different risk profiles.
Limitations
This study has some limitations. The not-very-large number of patients may limit the value of some of the conclusions. In addition, except for pseudosyncopes and the case with AVB, the diagnosis of the cause of syncope after ILR explantation was only presumptive; therefore, we cannot establish a precise final cause of syncope. Besides, the duration of ILR devices available when the study was performed (median duration 16 months) was not as long as the current ones. Furukawa et al.15 recently showed that prolonging observation up to 4 years increased the diagnostic value of ILR in syncopal patients. Anyway, the results of this study show that, even after short ILR duration, with lesser diagnostic yield, prognosis of the non-diagnosed patients is good.
Conclusions
Recurrent syncope is common in patients in whom a diagnosis is not established after the full battery life of an ILR. The prognosis of these patients seems to be good, without observed sudden or cardiac death.
Conflict of interest: none declared.



