-
PDF
- Split View
-
Views
-
Cite
Cite
Akinori Sairaku, Yukiko Nakano, Noboru Oda, Yuko Makita, Kenta Kajihara, Takehito Tokuyama, Chikaaki Motoda, Mai Fujiwara, Yasuki Kihara, How many electrical cardioversions should be applied for repetitive recurrences of atrial arrhythmias following ablation of persistent atrial fibrillation?, EP Europace, Volume 13, Issue 12, December 2011, Pages 1703–1708, https://doi.org/10.1093/europace/eur244
Close - Share Icon Share
Abstract
We aimed to determine how many electrical cardioversions (ECs) should be applied to treat repetitive persistent recurrences of atrial fibrillation (AF) following ablation of persistent AF within the early post-procedural period.
A total of 40 patients with >1 episode of recurrent AF in the form of persistent atrial arrhythmias within 3 months following the ablation were recruited from 108 patients who underwent ablation for persistent or long-standing persistent AF. Electrical cardioversions were applied up to six times, if necessary, to restore sinus rhythm at clinical visits at 2-week intervals until 3 months after the ablation. Fourteen (35%) ablation failures defined as recurrences of AF identified from the 3rd month after the ablation procedure were finally diagnosed during the follow-up period (14 ± 4 month). The patients with an ablation failure more frequently required ECs than those without (3.7 ± 0.3 vs. 1.2 ± 0.2 times; P < 0.0001). A receiver-operating characteristic curve identified a number of ECs of ≥3 as the optimal cut-off value for predicting an ablation failure (area under the curve 0.91; sensitivity, 86%, and specificity, 96%; P = 0.0007). In the multivariate logistic regression analysis, a number of ECs of ≥3 was the only independent predictor of an ablation failure (odds ratio, 11.32; 95% confidence interval, 3.83–58.22; P = 0.0019).
It was difficult to maintain sinus rhythm in patients with persistent AF who required several ECs for recurrences of AF within the early post-ablation period.
Introduction
During the past decade, catheter ablation for atrial fibrillation (AF) has rapidly evolved. Since the validity and safety of ablation for paroxysmal AF have become widely recognized, its indication has recently been extended to persistent or long-standing persistent AF.1 However, the long-term outcome of the AF ablation of persistent AF is still far from being acceptable when compared with that of paroxysmal AF.2,3 Although an early recurrence of AF (ERAF) does not necessarily lead to an AF recurrence observed in the chronic phase, it is known to be a significant predictor of an ablation failure.2,4,5 Given that an ERAF is more common in patients with persistent AF undergoing ablation than in those with paroxysmal AF,2,5 the management of an ERAF for persistent AF should be of importance. As a delayed cure of AF after ablation is frequently observed in persistent AF patients,4,6 one should not give up on the maintenance of sinus rhythm even if an ERAF occurs as a persistent form. Hence, sinus conversion using electrical cardioversion (EC) or antiarrhythmic drugs should be attempted to treat an ERAF in patients who have undergone ablation of persistent AF if atrial tachyarrhythmias occurring as ERAFs do not terminate spontaneously. This should also be meaningful in terms of the well known concept of ‘AF begets AF’.7 However, there are some patients who have repeated episodes of sustained AF a few days or weeks after conversion to sinus rhythm, who consequently have to undergo repeated ECs. Of these, a considerable number of patients who are finally able to be considered as candidates for a second ablation procedure may be included. Moreover, an EC will give patients somewhat of a feeling of fear. Thus, an EC should not be performed too frequently. To resolve this dilemma, we aimed to determine how many ECs should be performed in patients with repetitive ERAFs following the ablation of persistent AF.
Methods
Study population
This study was approved by the Institutional Research Board of the Hiroshima University Hospital and by the hospital's ethics committee.
One hundred and eight consecutive patients undergoing catheter ablation for drug refractory persistent or long-standing persistent AF were eligible for inclusion in our study. The ablation procedures were performed at Hiroshima University Hospital from June 2008 to May 2010. The type of AF was defined according to generally accepted guidelines.8 For the purpose of our study, we also defined an ERAF as an episode of documented AF or atrial flutter (AFL) that occurred within 3 months of the blanking period that was recommended to be employed.8 We excluded 58 patients who had no ERAFs, eight patients who had an ERAF and achieved sinus conversion spontaneously without any therapeutic intervention, and two patients who had previously undergone AF ablation. Finally, we included the remaining 40 patients, who had an ERAF following the initial ablation procedure and required an EC to restore sinus rhythm (Figure 1).

Flowchart detailing the study design. AF, atrial fibrillation, ERAF, early recurrence of AF, EC, electrical cardioversion.
Amiodarone and warfarin (targeted prothrombin international normalized ratio; 2.0–3.0) were administered no less than one month before the procedure and were continued throughout the periprocedural period without interruption. The patients underwent transoesophageal and transthoracic echocardiography prior to the ablation.
Cardiac computed tomography
Cardiac computed tomography (CT) was performed using a 64-slice CT scanner (Lightspeed VCT; GE Healthcare, Waukesha, WIS, USA) within 24 h before the procedure. The lengths of the major and minor axes of each pulmonary vein (PV) ostium were measured and the average was used for the analyses. The PV anatomy was evaluated as previously described.9 In brief, a common trunk was defined as a superior and inferior PV that join proximal to the left atrium (LA) resulting in a single atriopulmonary venous junction. An accessory PV was defined as a supernumerary vein directly entering the LA. A typical PV anatomy was defined as single right and left superior and inferior PVs that drained into the LA without any accessory PVs. The LA volume was calculated using the biplane dimension-length formula: LA volume = 4/3π × (anteroposterior diameter/2) × (longitudinal diameter/2) × (transversal diameter/2).9
Catheter ablation
Written informed consent for the ablation was obtained from each patient. The details of the double Lasso catheter-guided extensive encircling PV isolation performed in our study have been described previously.10 In brief, a 6-French decapolar catheter (St Jude Medical, St Paul, MN, USA) was positioned in the coronary sinus. After transseptal catheterization, two 7-French decapolar Lasso catheters (Biosense Webster, Diamond Bar, CA, USA) were placed within the ipsilateral superior and inferior PVs guided by selective PV angiography. After constructing three-dimensional electroanatomical maps using a non-fluoroscopic navigation system (CARTO, Biosense Webster), circumferential ablation lines were created around the left- and right-sided ipsilateral PVs using a 3.5 mm-tip irrigated catheter (ThermoCool, Biosense Webster) at a maximum power of 35 W for 20 s at each site. The temperature was limited to 50°C. The endpoint of the PV isolation was either the elimination or dissociation of the PV potentials recorded from Lasso catheters placed within the ipsilateral PVs and exit block from the PVs. Subsequently, an additional linear lesion connecting the superior aspects of the left and right upper PV isolation lesions was created. If AF was still present after the LA ablation, EC was performed to restore sinus rhythm. Finally, the cavotricuspid isthmus was ablated with an end point of bi-directional conduction block using a 4.0 mm tip temperature controlled non-irrigated catheter (EPT; Boston Scientific Corporation, Natick, MA, USA) at a maximum power of 50 W and maximum electrode-tissue interface temperature of 60°C. Intravenous heparin was administered to maintain an activated clotting time of 300–400 s during the procedure.
When the ablation procedure was over, a blood sample was obtained through the sheaths inserted in the femoral vein, and the plasma level of the high-sensitive C-reactive protein (JCA-DM 2250; Nihondenshi, Tokyo, Japan) and interleukin-6 (Multiscan JX; Thermo Fisher Scientific, Vantaa, Finland) were measured to assess for any state of inflammation in each patient.
Follow-up and electrical cardioversion
The patients were discharged from the hospital 2 days after the ablation and were followed up at the outpatient clinic every 2 weeks until 3 months, and thereafter, every month until 6 months, and finally 12 months after the ablation. Twelve-lead electrocardiograms (ECGs) were obtained at all follow-up visits and 24-h Holter monitoring was performed at 3 month intervals during the follow-up period. An episode of AF or AFL detected by the 12-lead ECG or Holter monitoring was considered a recurrence of AF if it had a duration of 30 s or more. All enrolled patients underwent EC because of a first episode of an ERAF as a persistent form. When patients had sustained AF or AFL again at the next follow-up visit, an additional EC was performed. In the same fashion, if necessary, patients were cardioverted up to six times within 3 months after the procedures (Figure 1). When the patients were seen for symptoms due to the atrial arrhythmias, they were cardioverted even though it was not a scheduled clinical visit.
Electrical cardioversion was performed using a step-up method with biphagic shock energies of 50, 100, 150, and 200 J as required. In principle, amiodarone and oral anticoagulants were discontinued if the patients remained free of AF for three consecutive months. However, long-term warfarin treatment was continued for the patients with a CHADS2 score of ≥2 even though they had no recurrence of AF.
End points
The end points were as follows: (i) ablation failure defined as a recurrence of AF occurring 3 months or more after the ablation8; (ii) the number of ECs performed, not the number of shock energies delivered to restore sinus rhythm; and (iii) time from the ablation procedure to the first clinical visit that three consecutive months of freedom from AF recurrence was achieved.
Statistical analysis
The clinical characteristics and end points for the patients were presented using frequencies for categorical variables and means with standard deviations for continuous variables. The differences between the patients with and without ablation failure were examined using Pearson's χ2 tests for categorical variables or t-tests for continuous variables. A Kaplan–Meier analysis was used to assess the time from the ablation procedures to the first clinical visit that three consecutive months of freedom from AF recurrence was achieved. The ability of the number of ECs to discriminate between patients with and without ablation failure was evaluated by a receiver operating characteristic (ROC) curve analysis. The optimal cut-off value was calculated by determining the number of ECs providing the greatest sum of the sensitivity and specificity. Univariate and multivariate logistic regression analyses were performed using various clinical factors to determine the predictors of ablation failure. Variables with statistical significance in the univariate analysis were included in the multivariate models. Statistical analyses were performed using JMP software version 8.0 (SAS Institute Japan, Tokyo, Japan). For all analyses, a P < 0.05 was considered statistically significant.
Results
Among the 40 enrolled patients, the mean age was 61 ± 7 years, and 23% were women. A total of 83 ECs were performed. Of those, 78 ECs were applied for persistent AF and five for persistent AFL. Seven distinct symptomatic episodes of persistent atrial arrhythmias were identified in three patients, and the remaining 76 episodes were not associated with any clear symptoms. Two episodes of ECs performed at non-scheduled clinical visits were observed. The mean time to the first EC was 10 ± 14 days after the ablation. We identified 14 (35%) patients with an ablation failure during the follow-up period of 14 ± 4 months. The clinical characteristics of the patients with and without an ablation failure are summarized in Table 1. Patients with an ablation failure were more likely to have long-standing persistent AF (93 vs. 40%; P = 0.034) and undergo frequent ECs (3.7 ± 0.3 vs. 1.2 ± 0.2 times; P < 0.0001) compared with those without (Table 1, Figure 2). There was no significant difference in the other baseline characteristics including the LA diameter and volume, PV characteristics, and levels of the inflammation markers. A Kaplan–Meier curve to assess the time taken to achieve three consecutive months of maintenance of sinus rhythm showed that 26 (65%) patients out of all the enrolled patients achieved continuous sinus rhythm until the 3rd month after the ablation, and in turn 21 of them (81%) had it restored by the 2nd month after the procedure (Figure 3). The ROC curve analysis showed that the number of ECs significantly discriminated between patients with and without an ablation failure with an area under the curve of 0.91 (P = 0.0007) (Figure 4). A number of ECs of ≥3 was identified as the optimal cut-off value to predict an ablation failure, with a sensitivity of 86% and a specificity of 96%. In the univariate logistic regression analysis, long-standing persistent AF [odds ratio (OR), 2.85; 95% confidence interval (CI), 1.14–12.6; P = 0.049] and a number of ECs of ≥3 (OR, 12.2; 95% CI, 4.24–60.67; P < 0.0001) emerged as predictors of an ablation failure. The multivariate models including those factors revealed that a number of ECs of ≥3 (OR, 11.32; 95% CI, 3.83–58.22; P = 0.0019) was the only independent predictor of an ablation failure (Table 2).
| Variables . | Ablation failure (+) . | Ablation failure (−) . | P value . |
|---|---|---|---|
| n = 14 . | n = 26 . | ||
| Age (years) | 59 ± 8 | 62 ± 6 | 0.16 |
| Female | 3 (21%) | 6 (23%) | 0.91 |
| Body mass index (kg/m2) | 24.5 ± 2.5 | 24.6 ± 3.3 | 0.91 |
| Long-standing persistent AF | 13 (93%) | 16 (40%) | 0.034 |
| Duration of AF (years) | 7.8 ± 4.5 | 8.0 ± 6.8 | 0.92 |
| Failed antiarrhythmic drugs | 2.2 ± 0.8 | 1.8 ± 0.8 | 0.13 |
| Hypertension | 9 (64%) | 11 (44%) | 0.22 |
| Diabetes | 4 (29%) | 6 (23%) | 0.75 |
| Structural heart disease | 1 (7%) | 3 (12%) | 0.66 |
| Left ventricular ejection fraction (%) | 60 ± 2 | 56 ± 2 | 0.17 |
| Left atrial diameter (mm) | 47 ± 1 | 44 ± 1 | 0.15 |
| Left atrial volume (mL) | 97 ± 9 | 95 ± 7 | 0.83 |
| Left superior PV diameter (mm) | 21.0 ± 3.9 | 19.9 ± 3.3 | 0.38 |
| Left inferior PV diameter (mm) | 15.9 ± 1.9 | 15.4 ± 2.7 | 0.59 |
| Right superior PV diameter (mm) | 21.3 ± 3.9 | 20.6 ± 4.3 | 0.59 |
| Right inferior PV diameter (mm) | 17.9 ± 5.1 | 18.1 ± 4.2 | 0.93 |
| Common PV trunks | 2 (14%) | 3 (12%) | 0.8 |
| Accessory PVs | 1 (7%) | 2 (8%) | 0.95 |
| C-reactive protein (mg/dL) | 0.047 ± 0.034 | 0.12 ± 0.19 | 0.18 |
| Interleukin-6 (pg/mL) | 9.4 ± 5.0 | 7.4 ± 4.0 | 0.17 |
| Time to early recurrence of AF (days) | 6.9 ± 7.8 | 11.4 ± 16.8 | 0.34 |
| Number of electrical cardioversions | 3.7 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
| Variables . | Ablation failure (+) . | Ablation failure (−) . | P value . |
|---|---|---|---|
| n = 14 . | n = 26 . | ||
| Age (years) | 59 ± 8 | 62 ± 6 | 0.16 |
| Female | 3 (21%) | 6 (23%) | 0.91 |
| Body mass index (kg/m2) | 24.5 ± 2.5 | 24.6 ± 3.3 | 0.91 |
| Long-standing persistent AF | 13 (93%) | 16 (40%) | 0.034 |
| Duration of AF (years) | 7.8 ± 4.5 | 8.0 ± 6.8 | 0.92 |
| Failed antiarrhythmic drugs | 2.2 ± 0.8 | 1.8 ± 0.8 | 0.13 |
| Hypertension | 9 (64%) | 11 (44%) | 0.22 |
| Diabetes | 4 (29%) | 6 (23%) | 0.75 |
| Structural heart disease | 1 (7%) | 3 (12%) | 0.66 |
| Left ventricular ejection fraction (%) | 60 ± 2 | 56 ± 2 | 0.17 |
| Left atrial diameter (mm) | 47 ± 1 | 44 ± 1 | 0.15 |
| Left atrial volume (mL) | 97 ± 9 | 95 ± 7 | 0.83 |
| Left superior PV diameter (mm) | 21.0 ± 3.9 | 19.9 ± 3.3 | 0.38 |
| Left inferior PV diameter (mm) | 15.9 ± 1.9 | 15.4 ± 2.7 | 0.59 |
| Right superior PV diameter (mm) | 21.3 ± 3.9 | 20.6 ± 4.3 | 0.59 |
| Right inferior PV diameter (mm) | 17.9 ± 5.1 | 18.1 ± 4.2 | 0.93 |
| Common PV trunks | 2 (14%) | 3 (12%) | 0.8 |
| Accessory PVs | 1 (7%) | 2 (8%) | 0.95 |
| C-reactive protein (mg/dL) | 0.047 ± 0.034 | 0.12 ± 0.19 | 0.18 |
| Interleukin-6 (pg/mL) | 9.4 ± 5.0 | 7.4 ± 4.0 | 0.17 |
| Time to early recurrence of AF (days) | 6.9 ± 7.8 | 11.4 ± 16.8 | 0.34 |
| Number of electrical cardioversions | 3.7 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
AF, atrial fibrillation; PV, pulmonary vein.
| Variables . | Ablation failure (+) . | Ablation failure (−) . | P value . |
|---|---|---|---|
| n = 14 . | n = 26 . | ||
| Age (years) | 59 ± 8 | 62 ± 6 | 0.16 |
| Female | 3 (21%) | 6 (23%) | 0.91 |
| Body mass index (kg/m2) | 24.5 ± 2.5 | 24.6 ± 3.3 | 0.91 |
| Long-standing persistent AF | 13 (93%) | 16 (40%) | 0.034 |
| Duration of AF (years) | 7.8 ± 4.5 | 8.0 ± 6.8 | 0.92 |
| Failed antiarrhythmic drugs | 2.2 ± 0.8 | 1.8 ± 0.8 | 0.13 |
| Hypertension | 9 (64%) | 11 (44%) | 0.22 |
| Diabetes | 4 (29%) | 6 (23%) | 0.75 |
| Structural heart disease | 1 (7%) | 3 (12%) | 0.66 |
| Left ventricular ejection fraction (%) | 60 ± 2 | 56 ± 2 | 0.17 |
| Left atrial diameter (mm) | 47 ± 1 | 44 ± 1 | 0.15 |
| Left atrial volume (mL) | 97 ± 9 | 95 ± 7 | 0.83 |
| Left superior PV diameter (mm) | 21.0 ± 3.9 | 19.9 ± 3.3 | 0.38 |
| Left inferior PV diameter (mm) | 15.9 ± 1.9 | 15.4 ± 2.7 | 0.59 |
| Right superior PV diameter (mm) | 21.3 ± 3.9 | 20.6 ± 4.3 | 0.59 |
| Right inferior PV diameter (mm) | 17.9 ± 5.1 | 18.1 ± 4.2 | 0.93 |
| Common PV trunks | 2 (14%) | 3 (12%) | 0.8 |
| Accessory PVs | 1 (7%) | 2 (8%) | 0.95 |
| C-reactive protein (mg/dL) | 0.047 ± 0.034 | 0.12 ± 0.19 | 0.18 |
| Interleukin-6 (pg/mL) | 9.4 ± 5.0 | 7.4 ± 4.0 | 0.17 |
| Time to early recurrence of AF (days) | 6.9 ± 7.8 | 11.4 ± 16.8 | 0.34 |
| Number of electrical cardioversions | 3.7 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
| Variables . | Ablation failure (+) . | Ablation failure (−) . | P value . |
|---|---|---|---|
| n = 14 . | n = 26 . | ||
| Age (years) | 59 ± 8 | 62 ± 6 | 0.16 |
| Female | 3 (21%) | 6 (23%) | 0.91 |
| Body mass index (kg/m2) | 24.5 ± 2.5 | 24.6 ± 3.3 | 0.91 |
| Long-standing persistent AF | 13 (93%) | 16 (40%) | 0.034 |
| Duration of AF (years) | 7.8 ± 4.5 | 8.0 ± 6.8 | 0.92 |
| Failed antiarrhythmic drugs | 2.2 ± 0.8 | 1.8 ± 0.8 | 0.13 |
| Hypertension | 9 (64%) | 11 (44%) | 0.22 |
| Diabetes | 4 (29%) | 6 (23%) | 0.75 |
| Structural heart disease | 1 (7%) | 3 (12%) | 0.66 |
| Left ventricular ejection fraction (%) | 60 ± 2 | 56 ± 2 | 0.17 |
| Left atrial diameter (mm) | 47 ± 1 | 44 ± 1 | 0.15 |
| Left atrial volume (mL) | 97 ± 9 | 95 ± 7 | 0.83 |
| Left superior PV diameter (mm) | 21.0 ± 3.9 | 19.9 ± 3.3 | 0.38 |
| Left inferior PV diameter (mm) | 15.9 ± 1.9 | 15.4 ± 2.7 | 0.59 |
| Right superior PV diameter (mm) | 21.3 ± 3.9 | 20.6 ± 4.3 | 0.59 |
| Right inferior PV diameter (mm) | 17.9 ± 5.1 | 18.1 ± 4.2 | 0.93 |
| Common PV trunks | 2 (14%) | 3 (12%) | 0.8 |
| Accessory PVs | 1 (7%) | 2 (8%) | 0.95 |
| C-reactive protein (mg/dL) | 0.047 ± 0.034 | 0.12 ± 0.19 | 0.18 |
| Interleukin-6 (pg/mL) | 9.4 ± 5.0 | 7.4 ± 4.0 | 0.17 |
| Time to early recurrence of AF (days) | 6.9 ± 7.8 | 11.4 ± 16.8 | 0.34 |
| Number of electrical cardioversions | 3.7 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
AF, atrial fibrillation; PV, pulmonary vein.
| Variables . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P value . | OR (95% CI) . | P value . |
| Age >65 years | 0.78 (0.34 – 1.63) | 0.53 | ||
| Female | 0.95 (0.41 – 2.05) | 0.91 | ||
| Body mass index >25 kg/m2 | 1.37 (0.70 – 2.71) | 0.36 | ||
| Long-standing persistent AF | 2.85 (1.14 – 12.6) | 0.049 | 2.16 (0.49 – 15.77) | 0.36 |
| Duration of AF of >5 years | 1.35 (0.69 – 2.85) | 0.39 | ||
| Number of failed AADs of ≥2 | 1.49 (0.67 – 4.07) | 0.37 | ||
| Hypertension | 1.51 (0.78 – 3.06) | 0.23 | ||
| Diabetes | 1.13 (0.52 – 2.35) | 0.75 | ||
| Structural heart disease | 0.77 (0.17 – 2.27) | 0.66 | ||
| Left ventricular ejection fraction <50% | 1.15 (0.53 – 2.41) | 0.7 | ||
| Left atrial diameter >45 mm | 1.37 (0.71 – 2.70) | 0.35 | ||
| Left atrial volume >90 mL | 1.46 (0.76 – 2.89) | 0.26 | ||
| Mean diameter of the PVs >20mm | 0.92 (0.43 – 1.86) | 0.82 | ||
| Atypical PV anatomy | 1.07 (0.45 – 2.37) | 0.87 | ||
| Interleukin-6 > 7.0 pg/mL | 1.25 (0.65 – 2.44) | 0.51 | ||
| Time to early recurrence of AF of ≥10 days | 0.87 (0.43 – 1.69) | 0.69 | ||
| Number of electrical cardiversions of ≥3 | 12.2 (4.24 – 60.67) | <0.0001 | 11.32 (3.83 – 58.22) | 0.0019 |
| Variables . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P value . | OR (95% CI) . | P value . |
| Age >65 years | 0.78 (0.34 – 1.63) | 0.53 | ||
| Female | 0.95 (0.41 – 2.05) | 0.91 | ||
| Body mass index >25 kg/m2 | 1.37 (0.70 – 2.71) | 0.36 | ||
| Long-standing persistent AF | 2.85 (1.14 – 12.6) | 0.049 | 2.16 (0.49 – 15.77) | 0.36 |
| Duration of AF of >5 years | 1.35 (0.69 – 2.85) | 0.39 | ||
| Number of failed AADs of ≥2 | 1.49 (0.67 – 4.07) | 0.37 | ||
| Hypertension | 1.51 (0.78 – 3.06) | 0.23 | ||
| Diabetes | 1.13 (0.52 – 2.35) | 0.75 | ||
| Structural heart disease | 0.77 (0.17 – 2.27) | 0.66 | ||
| Left ventricular ejection fraction <50% | 1.15 (0.53 – 2.41) | 0.7 | ||
| Left atrial diameter >45 mm | 1.37 (0.71 – 2.70) | 0.35 | ||
| Left atrial volume >90 mL | 1.46 (0.76 – 2.89) | 0.26 | ||
| Mean diameter of the PVs >20mm | 0.92 (0.43 – 1.86) | 0.82 | ||
| Atypical PV anatomy | 1.07 (0.45 – 2.37) | 0.87 | ||
| Interleukin-6 > 7.0 pg/mL | 1.25 (0.65 – 2.44) | 0.51 | ||
| Time to early recurrence of AF of ≥10 days | 0.87 (0.43 – 1.69) | 0.69 | ||
| Number of electrical cardiversions of ≥3 | 12.2 (4.24 – 60.67) | <0.0001 | 11.32 (3.83 – 58.22) | 0.0019 |
OR, odds ratio; CI, confidence interval; AF, atrial fibrillation; AADs, antiarrhythmic drugs; PV, pulmonary vein.
| Variables . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P value . | OR (95% CI) . | P value . |
| Age >65 years | 0.78 (0.34 – 1.63) | 0.53 | ||
| Female | 0.95 (0.41 – 2.05) | 0.91 | ||
| Body mass index >25 kg/m2 | 1.37 (0.70 – 2.71) | 0.36 | ||
| Long-standing persistent AF | 2.85 (1.14 – 12.6) | 0.049 | 2.16 (0.49 – 15.77) | 0.36 |
| Duration of AF of >5 years | 1.35 (0.69 – 2.85) | 0.39 | ||
| Number of failed AADs of ≥2 | 1.49 (0.67 – 4.07) | 0.37 | ||
| Hypertension | 1.51 (0.78 – 3.06) | 0.23 | ||
| Diabetes | 1.13 (0.52 – 2.35) | 0.75 | ||
| Structural heart disease | 0.77 (0.17 – 2.27) | 0.66 | ||
| Left ventricular ejection fraction <50% | 1.15 (0.53 – 2.41) | 0.7 | ||
| Left atrial diameter >45 mm | 1.37 (0.71 – 2.70) | 0.35 | ||
| Left atrial volume >90 mL | 1.46 (0.76 – 2.89) | 0.26 | ||
| Mean diameter of the PVs >20mm | 0.92 (0.43 – 1.86) | 0.82 | ||
| Atypical PV anatomy | 1.07 (0.45 – 2.37) | 0.87 | ||
| Interleukin-6 > 7.0 pg/mL | 1.25 (0.65 – 2.44) | 0.51 | ||
| Time to early recurrence of AF of ≥10 days | 0.87 (0.43 – 1.69) | 0.69 | ||
| Number of electrical cardiversions of ≥3 | 12.2 (4.24 – 60.67) | <0.0001 | 11.32 (3.83 – 58.22) | 0.0019 |
| Variables . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P value . | OR (95% CI) . | P value . |
| Age >65 years | 0.78 (0.34 – 1.63) | 0.53 | ||
| Female | 0.95 (0.41 – 2.05) | 0.91 | ||
| Body mass index >25 kg/m2 | 1.37 (0.70 – 2.71) | 0.36 | ||
| Long-standing persistent AF | 2.85 (1.14 – 12.6) | 0.049 | 2.16 (0.49 – 15.77) | 0.36 |
| Duration of AF of >5 years | 1.35 (0.69 – 2.85) | 0.39 | ||
| Number of failed AADs of ≥2 | 1.49 (0.67 – 4.07) | 0.37 | ||
| Hypertension | 1.51 (0.78 – 3.06) | 0.23 | ||
| Diabetes | 1.13 (0.52 – 2.35) | 0.75 | ||
| Structural heart disease | 0.77 (0.17 – 2.27) | 0.66 | ||
| Left ventricular ejection fraction <50% | 1.15 (0.53 – 2.41) | 0.7 | ||
| Left atrial diameter >45 mm | 1.37 (0.71 – 2.70) | 0.35 | ||
| Left atrial volume >90 mL | 1.46 (0.76 – 2.89) | 0.26 | ||
| Mean diameter of the PVs >20mm | 0.92 (0.43 – 1.86) | 0.82 | ||
| Atypical PV anatomy | 1.07 (0.45 – 2.37) | 0.87 | ||
| Interleukin-6 > 7.0 pg/mL | 1.25 (0.65 – 2.44) | 0.51 | ||
| Time to early recurrence of AF of ≥10 days | 0.87 (0.43 – 1.69) | 0.69 | ||
| Number of electrical cardiversions of ≥3 | 12.2 (4.24 – 60.67) | <0.0001 | 11.32 (3.83 – 58.22) | 0.0019 |
OR, odds ratio; CI, confidence interval; AF, atrial fibrillation; AADs, antiarrhythmic drugs; PV, pulmonary vein.
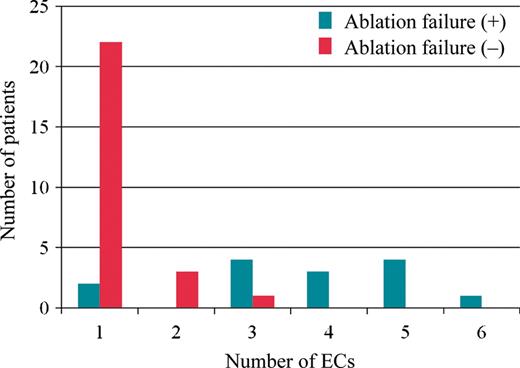
Distribution of the patients according to the number of electrical cardioversions applied in the patients with or without an ablation failure. ECs, electrical cardioversions.
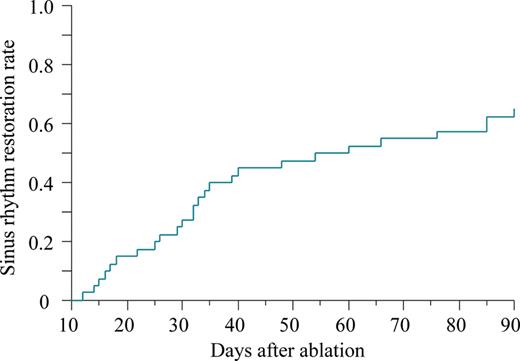
Kaplan–Meier estimate of the time to restoration of sinus rhythm for three consecutive months after ablation.
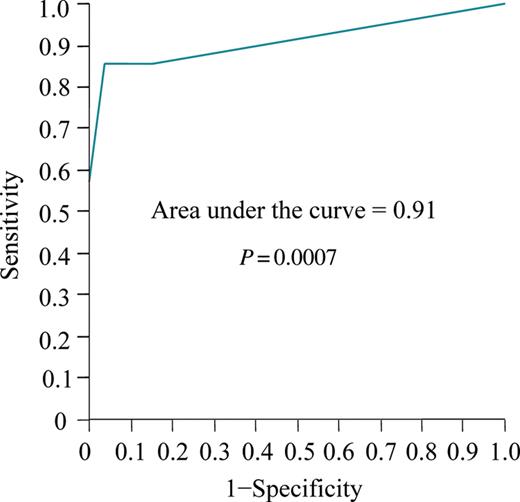
Receiver-operating characteristic curve for the number of electrical cardioversions that were applied to predict the risk of an ablation failure.
Discussion
The major findings of our study were as follows: (i) the persistent or long-standing persistent AF patients with an ablation failure required more frequent ECs as treatments for ERAFs than those without; (ii) a frequent episode of ECs that were applied to treat ERAFs was the only independent predictor of an ablation failure for the patients with persistent or long-standing persistent AF.
As a likely mechanism of an ERAF, the following has been provided: (i) transient stimulatory effects of radiofrequency energy applications; (ii) imbalances in the autonomic nervous system leading to increased sympathetic activity or reduced parasympathetic activity; (iii) incomplete PV isolation or recovery of conduction in a previously isolated PV; and (iv) arrhythmogenic foci outside the PVs.4,11 The latter two causes of an ERAF would be expected to result in recurrent AF throughout the follow-up period, not just within the first several weeks after the ablation. Accordingly, multiple repetitive recurrences of persistent atrial arrhythmias may have resulted from the same two causes and thereby the patients requiring frequent ECs after the ablation may be considered as good candidates for further ablation procedures. It is well accepted that the development of AF requires not only a trigger but also an arrhythmogenic substrate characterized by electrical and structural remodelling of the atrium.8 Therefore, it is possible that the substrate in those patients was too strong to be sufficiently modified by the ablation strategies applied in our study, and consequently they experienced repeated episodes of atrial arrhythmias during the early post-ablation period.
O'Donnell et al.6 showed that a delayed cure of AF can occur in a significant number of patients despite an ERAF after PV isolation. The authors stated that this phenomenon was most commonly observed in patients with persistent AF and atrial structural and electric abnormalities. We demonstrated that 81% of the patients who had experienced an ERAF but were finally diagnosed with an ablation success restored continuous sinus rhythm within 2 months after the ablation, suggesting that such patients will need that period of time to complete a ‘delayed cure’.
In our study, most of the enrolled patients had no symptoms resulting from the ERAF. Hence, recurrences of AF are highly likely to be missed unless patients following ablation of persistent AF are willingly checked for any recurrences. The ERAFs following ablation for persistent AF much more commonly took the form of persistent atrial arrhythmias than a transient form in our study. Accordingly, given the high incidence of asymptomatic recurrences of AF, atrial arrhythmias occurring as ERAFs will persist without interruption until they are recognized at clinical visits, which again should cause atrial remodelling making AF further less curable despite a short-lived restoration of sinus rhythm achieved by an ablation procedure.7,12 As Baman et al.13 addressed in their work, the shorter the duration of persistent recurrent AF is, the higher the probability of long-term maintenance of sinus rhythm will be for the patients who underwent ablation of persistent AF. Therefore, an intense clinical follow-up to check for a recurrence of AF should be desirable for those patients at least during the blanking period even though they had no symptoms.
Many large-scale studies recruiting patients with paroxysmal AF or those studying patients with paroxysmal or persistent AF3 have clearly shown that LA enlargement and long-standing persistent AF are predictors of a recurrence of AF after ablation. However, in studies enrolling only patients with persistent or long-standing persistent AF, some investigators14 reported that the LA enlargement was an independent predictor of ablation failure, while others15,16 failed to show that, such as in our study. Thus, it seems to be controversial whether or not the LA size influences the outcomes after the ablation of persistent AF. Those studies were all relatively small scale and at single centres; therefore, in order to clarify this issue, multicentre studies with large numbers of subjects that recruit only patients with non-paroxysmal AF should be conducted.
Clinical implications
The number of ECs that will be performed for ERAFs cannot be known prior to an AF ablation and thereby might not be a predictor of an ablation failure in a strict sense. However, unlike most operations, the success and failure of the AF ablation are measured a few months after the procedure rather than immediately after it. Accordingly, the data we presented could have yielded important information to determine the post-procedural management of patients with persistent AF. Specifically, we suggest performing additional ablation procedures or giving up restoration of sinus rhythm in patients with persistent AF who required several ECs for recurrences of atrial arrhythmias within the early post-procedural period.
Limitations
Oral et al.1 reported that amiodarone enabled 58% of the patients with persistent AF to maintain sinus rhythm for 1 year, suggesting that pharmacotherapy with amiodarone alone should be effective for some patients with persistent AF of a relatively short duration. Hence, because all the patients enrolled in our study were taking amiodarone for at least 3 months after the ablation, the number of ECs applied to treat the ERAFs in that period may have been affected by the individual variability in the response to amiodarone; the patients who responded to amiodarone may have received less frequent ECs. Finally, this was a retrospective single centre study with a small number of patients and consequently the statistical power was limited.
Conflict of interest: none declared.
Acknowledgements
The authors thank John Martin for his proofreading the English.



