-
PDF
- Split View
-
Views
-
Cite
Cite
Anton A.W. Mulder, Maurits C.E.F. Wijffels, Eric F.D. Wever, Lucas V.A. Boersma, Pulmonary vein isolation and left atrial complex-fractionated atrial electrograms ablation for persistent atrial fibrillation with phased radio frequency energy and multi-electrode catheters: efficacy and safety during 12 months follow-up, EP Europace, Volume 13, Issue 12, December 2011, Pages 1695–1702, https://doi.org/10.1093/europace/eur204
Close - Share Icon Share
Abstract
Ablation for persistent atrial fibrillation (AF) remains a difficult and time-consuming procedure with varying degrees of success. We evaluated the long-term effects of a novel approach for ablation of persistent AF using multi-electrode catheters.
In 89 patients with longstanding persistent AF (>1 year), multi-electrode ablation was performed with a pulmonary vein ablation catheter (PVAC), a multi-array septal catheter (MASC), and a multi-array ablation catheter (MAAC) for ablation of complex-fractionated atrial electrograms (CFAE) at the septum, left atrial (LA) roof, floor, posterior wall, and mitral isthmus. Follow-up was performed at 6 and 12 months with electrocardiogram, 7 days Holter, and occasionally ambulant event recordings. Average procedure and fluoroscopy times were 112 ± 32 and 21 ± 10 min. The pre-specified endpoint of pulmonary vein isolation and LA CFAE ablation was reached in all patients. No procedural complications were observed. At 12 months after a single treatment 44 of 89 (49%) remained in sinus rhythm, including direct current cardioversion in 12 patients. At 12 months, after a redo PVAC/MASC/MAAC, an additional 6 of 15 patients (40%) were free of AF. In 18 of 89 (20%) patients AF was changed to paroxysmal.
In this single centre study, ablation for longstanding persistent AF with the PVAC/MASC/MAAC resulted in 56% freedom of AF at 1 year after 1.2 ± 0.4 procedures. This approach is time efficient and has a favourable safety profile.
Introduction
Atrial fibrillation (AF) often has a progressive nature, which may finally lead to persistent arrhythmias, refractory to even direct current cardioversion (DCCV). In a chronic phase many patients may have to turn to rate control in the absence of safe and effective drug therapy. A rising treatment even for patients with persistent AF is ablation,1,2 with modification of the atrial substrate by means of linear lesions3 and/or ablation of complex-fractionated atrial electrograms (CFAE).4,5
As conventional procedures with 3-D mapping and point-by-point ablation are often time consuming and complex,3–5 radiofrequency (RF) ablation catheters, anatomically designed for ablation of specific parts of the left atrium, may become essential. A prior multi-centre pulmonary vein ablation catheter (PVAC)/multi-array septal catheter (MASC)/multi-array ablation catheter (MAAC) pilot study with 1.5 procedures in 50 patients with longstanding persistent AF demonstrated a reduction of >80% AF burden in 66% of patients, while 45% were free of AF and anti-arrhythmic drugs (AADs) at 20 months.6 In the present manuscript we provide further data on this new technology, describing the results of 1 year follow-up after treatment with the PVAC/MASC/MAAC catheters, in patients with longstanding persistent AF, in a single-centre study with an intensive follow-up programme, including multiple electrocardiogram (ECG) recordings, 7 days Holters, and event recordings.
Methods
Study population
The subjects of this study were patients with symptomatic longstanding persistent AF, resistant to at least one class I, or class III AAD, without a prior ablation procedure. Longstanding persistent AF was defined as continuous AF of >1 year duration.7 Eighty-nine patients were treated at the St Antonius Hospital, Nieuwegein, The Netherlands. The St Antonius Hospital Ethics Committee review board approved of the study and all patients gave informed consent to participate in the study.
Pre-procedural evaluation and the ablation procedure
Before the procedure all patients were screened by cardiac magnetic resonance imaging (MRI) and transthoracic echocardiography. Significant structural abnormalities including left ventricular ejection fraction <35%, mitral insufficiency >grade 2, aortic valve stenosis >grade 2, or left atrial (LA) diameter >55 mm were a contra-indication for ablation. A trans-oesophageal echocardiogram was performed within the week prior to ablation, to exclude LA appendage thrombus.
Before the procedure, patient history was taken and additional information, such as hypertension, amiodarone use, history of right atrial (RA) flutter, and RA flutter ablation were extracted from the patients’ case notes.
Anti-arrhythmic drugs were continued up to the time of the procedure. All patients remained on oral anticoagulation in the first 3 months after ablation, which was continued if patients had documented AF recurrence. In patients without documented AF and without symptoms of AF, continuation of aspirin or oral anticoagulation was recommended at the discretion of the referring cardiologist, based on the CHADS2 score. During the ablation procedure a standard deflectable four-polar catheter was introduced in the coronary sinus (CS) for pacing. Transseptal access was obtained with a standard puncture with a Brockenbrough needle during biplane fluoroscopy. In the first 25 cases a braided non-steerable sheath (Convoy, BScI, Natick, Massachusetts) with 9.5 F inner lumen diameter was used, while in later cases a braided steerable sheath (Channel, Bard, 12.5 F, Murray Hill, New Jersey) was used. A detailed description of the catheters and ablation procedure has been given in a previous report.6 Briefly, the PVAC catheter (10-polar, electrode length, and spacing 3 mm, diameter 25 mm, and 9.5 F) was used for PV isolation. The MASC (three arms with each four electrodes, 2 mm length and spacing) for septal CFAE ablation. The MAAC catheter (four arms with each two electrodes 2 mm length and spacing) for CFAE ablation at the LA roof, free wall, mitral isthmus, along the mitral annulus, and posterior wall. After isolation of the PVs and ablation of areas with CFAE, sinus rhythm was usually restored by DCCV. The endpoint with PVAC ablation (PV isolation) was confirmed by the absence of local potentials with PVAC mapping inside each vein combined with pacing manoeuvres from the CS (Figure 1A), and/or the PV (Figure 1B) to distinguish local potentials from far field potentials. The endpoint with MASC and MAAC was achieved when no CFAE were observed anymore at the targeted sites. (Figure 1C) Complex-fractionated atrial electrograms were defined visually as local continuous deflections with multiple components crossing the baseline.4
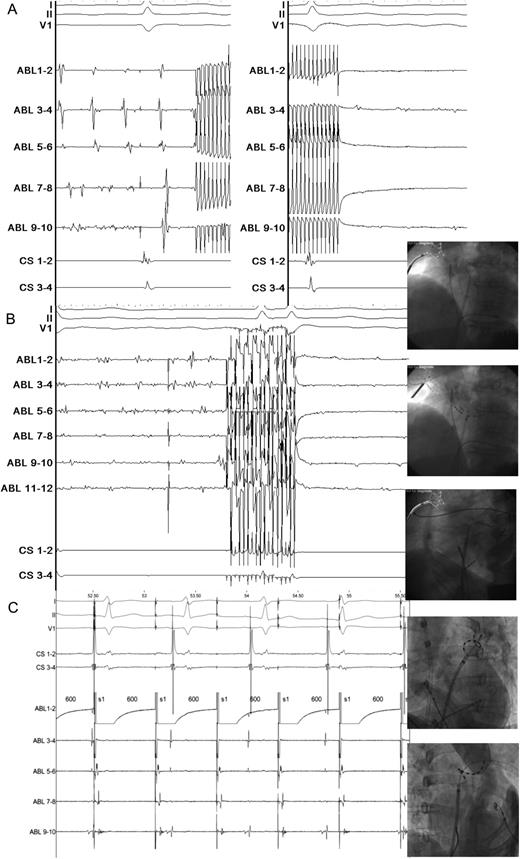
(opposite) (A) Pulmonary vein ablation catheter isolation during atrial fibrillation. Lead I, II, and V1 show electrocardiogram during atrial fibrillation, the quadripolar coronary sinus catheter is temporarily in the right ventricle for pacing purposes in case of vagal response during ablation. The five ablation (ABL) pairs show the local pulmonary vein antrum activity mapped with pulmonary vein ablation catheter. An artefact is observed when phased radiofrequency is started on all ablation pairs. After 60 s ablation at a target temperature of 60°C, the local voltage is greatly diminished. Paper speed with Prucka is 200 mm/s. (B) Complex-fractionated atrial electrograms ablation: septum, roof/floor/isthmus. Same catheter set-up as in Figure 1A. The six ABL pairs show septal activity recorded with the three armed multi-array septal catheter. During ablation the artefact persists for 60 s. After ablation local voltage abatement is observed. The fluoroscopy images show typical ablation positions with the multi-array septal catheter and multi-array ablation catheters. Paper speed with Prucka is 200 mm/s. (C) Local pulmonary vein capture, exit block. Same catheter set-up as in Figure 1A and B. The pulmonary vein ablation catheter catheter tip is positioned inside the left superior pulmonary vein. Pacing from ABL pair 1–2 shows local capture inside the pulmonary vein, while sinus rhythm continues undisturbed outside the pulmonary vein. Paper speed with Prucka is 200 mm/s.
All patients were on Coumadin therapy with a target international normalized ratio (INR), arranged by the Dutch Thrombosis Service to be between 2 and 2.5 at the day of the procedure. During the procedure, a bolus of heparin of 10.000.IU was administered IV, after transseptal puncture, followed by another 5000.IU of heparin if LA ablation lasted longer than 75 min. Oral anticoagulation was restarted directly after the procedure, and low-molecular-weight heparin was given only as bridging therapy until the INR was >2.5.
Follow-up
During the procedure and in the following 7 days the occurrence of any adverse events such as stroke, phrenic nerve palsy, groin haematoma, tamponade, or pulmonary and gastrointestinal complications was scored.
Pulmonary vein stenosis was determined, on moderate stenosis (diameter reduction of 50–69%) or significant stenosis (≥70%), during a second procedure with PV angiography or in a random selection of patients with MRI at 6 months.8
The same AADs (class I and III) as before the ablation were continued during the first 3 months after the procedure, with exception of amiodarone which was discontinued immediately after the procedure. If patients remained free of AF, their AADs were discontinued. If patients had AF recurrence, they continued using drugs in combination with a DCCV, until the time that they were free of AF, and drugs could be stopped. Patients who were not free of AF, regardless of using anti-arrhythmic drugs, were either re-scheduled for invasive treatment, or were considered as a failure for the primary endpoint, being free of AF without AADs. The choice of a treatment strategy in case of recurrence was left to the preference of the patient and each of the five referring electrophysiologists or fellows at our institute.
All patients were seen at the outpatient clinic at 3, 6, and 12 months for ECG (also after the second treatment). If consecutive ECG recordings showed sinus rhythm, patients were scheduled for 7 days Holter at 6 and 12 months (in accordance with the expert consensus statement of 2007).7 If 7 days Holter showed sinus rhythm, while patients remained symptomatic; an event recording was performed for up to 6 weeks. Additionally, referring physicians were asked to report any recording with AF. Patients were encouraged to visit the emergency room for an ECG in case of palpitations.
Success was defined when (i) AF, atrial flutter, or atrial tachycardia lasting >30 s were absent on any of the recordings after the 3 months blanking period by a repeated 12-lead ECG, event monitoring and Holter and (ii) patients were off class I or III AADs.7 There was also an active search for a documented episode outside the planned follow-up in our hospital as well as in other hospitals by ECG and event recording. The follow-up restarted after patients received a second treatment and in the further follow-up they were seen as single-procedure failures.
Statistical analyses
Categorical variables are presented by percentages. Numerical variables are expressed as mean, standard deviation (SD), and range. The χ2 test was used for categorical variables. The Kaplan–Meier method was used for the event-free survival curve. The measure of effect was the odds-ratio (OR) and as measure of precision the 95% confidence interval (95% CI) were calculated. All univariate predictors with P< 0.25 were tested in a multivariate model. A P value <0.05 was considered statistically significant. Data analyses were performed using the SPSS (Windows version 17.0, Chicago, Illinois).
Results
Patient characteristics
Patient characteristics are given in Table 1. The mean age was 59 years (range 37–75 years), 24% of the patients were female. Mean LA diameter was 42 mm. Mitral regurgitation grade 2 was seen in 11% of the patients, while 48% of the patients were known for hypertension. The mean number of AADs used prior to ablation was 2.2 ± 0.9, while 40 of the 89 patients (45%) had used amiodarone. In 12 of the 89 (13%) patients, amiodarone was used until the day of the procedure. Right atrial flutter ablation had been previously performed in 6 of 89 patients, as this was deemed clinically relevant at that time. It held no direct relation to the ablation for AF that was indicated later. On MRI most patients had four separate PVs, except for 10 with a common left PV, and 24 with separate right middle PV (Table 2).
| Number of patients . | 89 . | Success at 12 monthsa . | Failure at 12 monthsa . | OR for successa . | |
|---|---|---|---|---|---|
| . | . | n = 32 patients . | n = 57 patients . | ||
| . | n = patients . | (%) . | (%) . | OR . | 95% CI . |
| Female | 21 | 24 | 24 | 1.39 | 0.50–3.93 |
| Hypertension | 43 | 47 | 49 | 0.91 | 0.38–2.18 |
| Left ventricular ejection fraction <50% | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Mitral regurgitation grade 2 | 10 | 9 | 12 | 0.74 | 0.18–3.08 |
| History of right atrial flutter | 22 | 28 | 23 | 1.32 | 0.49–3.56 |
| History of right atrial flutter ablation (previous) | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Amiodarone use | 40 | 41 | 47 | 0.76 | 0.32–1.83 |
| Mean + SD (range) | Mean + SD (range) | Mean + SD (range) | OR | 95% CI | |
| Age (year) | 59 ± 8 (37–75) | 60 ± 9 (37–75) | 59 ± 7 (43–75) | 1.02 | 0.97–1.08 |
| Body mass index (kg/m2) | 28 ± 5 (21–48) | 28 ± 5 (21–41) | 28 ± 5 (21–48) | 0.99 | 0.90–1.09 |
| Left atrial size, parasternal long-axis view (mm) | 42 ± 4 (31–54) | 41 ± 4 (33–49) | 43 ± 5 (31–54) | 0.51 | 0.18–1.42 |
| Duration of atrial fibrillation (year) | 6 ± 7 (1–46) | 6 ± 8 (1–46) | 7 ± 6 (1–28) | 0.83 | 0.52–1.32 |
| Number of patients . | 89 . | Success at 12 monthsa . | Failure at 12 monthsa . | OR for successa . | |
|---|---|---|---|---|---|
| . | . | n = 32 patients . | n = 57 patients . | ||
| . | n = patients . | (%) . | (%) . | OR . | 95% CI . |
| Female | 21 | 24 | 24 | 1.39 | 0.50–3.93 |
| Hypertension | 43 | 47 | 49 | 0.91 | 0.38–2.18 |
| Left ventricular ejection fraction <50% | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Mitral regurgitation grade 2 | 10 | 9 | 12 | 0.74 | 0.18–3.08 |
| History of right atrial flutter | 22 | 28 | 23 | 1.32 | 0.49–3.56 |
| History of right atrial flutter ablation (previous) | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Amiodarone use | 40 | 41 | 47 | 0.76 | 0.32–1.83 |
| Mean + SD (range) | Mean + SD (range) | Mean + SD (range) | OR | 95% CI | |
| Age (year) | 59 ± 8 (37–75) | 60 ± 9 (37–75) | 59 ± 7 (43–75) | 1.02 | 0.97–1.08 |
| Body mass index (kg/m2) | 28 ± 5 (21–48) | 28 ± 5 (21–41) | 28 ± 5 (21–48) | 0.99 | 0.90–1.09 |
| Left atrial size, parasternal long-axis view (mm) | 42 ± 4 (31–54) | 41 ± 4 (33–49) | 43 ± 5 (31–54) | 0.51 | 0.18–1.42 |
| Duration of atrial fibrillation (year) | 6 ± 7 (1–46) | 6 ± 8 (1–46) | 7 ± 6 (1–28) | 0.83 | 0.52–1.32 |
aFor definition of success and failure see text.
| Number of patients . | 89 . | Success at 12 monthsa . | Failure at 12 monthsa . | OR for successa . | |
|---|---|---|---|---|---|
| . | . | n = 32 patients . | n = 57 patients . | ||
| . | n = patients . | (%) . | (%) . | OR . | 95% CI . |
| Female | 21 | 24 | 24 | 1.39 | 0.50–3.93 |
| Hypertension | 43 | 47 | 49 | 0.91 | 0.38–2.18 |
| Left ventricular ejection fraction <50% | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Mitral regurgitation grade 2 | 10 | 9 | 12 | 0.74 | 0.18–3.08 |
| History of right atrial flutter | 22 | 28 | 23 | 1.32 | 0.49–3.56 |
| History of right atrial flutter ablation (previous) | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Amiodarone use | 40 | 41 | 47 | 0.76 | 0.32–1.83 |
| Mean + SD (range) | Mean + SD (range) | Mean + SD (range) | OR | 95% CI | |
| Age (year) | 59 ± 8 (37–75) | 60 ± 9 (37–75) | 59 ± 7 (43–75) | 1.02 | 0.97–1.08 |
| Body mass index (kg/m2) | 28 ± 5 (21–48) | 28 ± 5 (21–41) | 28 ± 5 (21–48) | 0.99 | 0.90–1.09 |
| Left atrial size, parasternal long-axis view (mm) | 42 ± 4 (31–54) | 41 ± 4 (33–49) | 43 ± 5 (31–54) | 0.51 | 0.18–1.42 |
| Duration of atrial fibrillation (year) | 6 ± 7 (1–46) | 6 ± 8 (1–46) | 7 ± 6 (1–28) | 0.83 | 0.52–1.32 |
| Number of patients . | 89 . | Success at 12 monthsa . | Failure at 12 monthsa . | OR for successa . | |
|---|---|---|---|---|---|
| . | . | n = 32 patients . | n = 57 patients . | ||
| . | n = patients . | (%) . | (%) . | OR . | 95% CI . |
| Female | 21 | 24 | 24 | 1.39 | 0.50–3.93 |
| Hypertension | 43 | 47 | 49 | 0.91 | 0.38–2.18 |
| Left ventricular ejection fraction <50% | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Mitral regurgitation grade 2 | 10 | 9 | 12 | 0.74 | 0.18–3.08 |
| History of right atrial flutter | 22 | 28 | 23 | 1.32 | 0.49–3.56 |
| History of right atrial flutter ablation (previous) | 6 | 9 | 5 | 1.86 | 0.35–9.82 |
| Amiodarone use | 40 | 41 | 47 | 0.76 | 0.32–1.83 |
| Mean + SD (range) | Mean + SD (range) | Mean + SD (range) | OR | 95% CI | |
| Age (year) | 59 ± 8 (37–75) | 60 ± 9 (37–75) | 59 ± 7 (43–75) | 1.02 | 0.97–1.08 |
| Body mass index (kg/m2) | 28 ± 5 (21–48) | 28 ± 5 (21–41) | 28 ± 5 (21–48) | 0.99 | 0.90–1.09 |
| Left atrial size, parasternal long-axis view (mm) | 42 ± 4 (31–54) | 41 ± 4 (33–49) | 43 ± 5 (31–54) | 0.51 | 0.18–1.42 |
| Duration of atrial fibrillation (year) | 6 ± 7 (1–46) | 6 ± 8 (1–46) | 7 ± 6 (1–28) | 0.83 | 0.52–1.32 |
aFor definition of success and failure see text.
| Procedures 89 . | Configuration . | Diameter (mm) . | RF applications . | ||
|---|---|---|---|---|---|
| . | n = patients (%) . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Left superior PV | 79 (89) | 17 ± 3 | 8–24 | 7 ± 3 | 1–28 |
| Left inferior PV | 79 (89) | 16 ± 3 | 10–27 | 5 ± 2 | 1–13 |
| Left common PV | 10 (11) | 21 ± 5 | 14–28 | 11 ± 4 | 7–18 |
| Right superior PV | 89 (100) | 18 ± 3 | 8–26 | 6 ± 3 | 2–15 |
| Right middle PV | 24 (27) | 8 ± 1 | 5–9 | 3 ± 2 | 1–6 |
| Right inferior PV | 89 (100) | 17 ± 4 | 8–31 | 6 ± 3 | 0–22 |
| Septum | — | — | — | 7 ± 2 | 2–12 |
| LA wall, roof, floor | — | — | — | 9 ± 4 | 3–25 |
| Total | — | — | — | 40 ± 10 | 20–86 |
| Procedures 89 . | Configuration . | Diameter (mm) . | RF applications . | ||
|---|---|---|---|---|---|
| . | n = patients (%) . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Left superior PV | 79 (89) | 17 ± 3 | 8–24 | 7 ± 3 | 1–28 |
| Left inferior PV | 79 (89) | 16 ± 3 | 10–27 | 5 ± 2 | 1–13 |
| Left common PV | 10 (11) | 21 ± 5 | 14–28 | 11 ± 4 | 7–18 |
| Right superior PV | 89 (100) | 18 ± 3 | 8–26 | 6 ± 3 | 2–15 |
| Right middle PV | 24 (27) | 8 ± 1 | 5–9 | 3 ± 2 | 1–6 |
| Right inferior PV | 89 (100) | 17 ± 4 | 8–31 | 6 ± 3 | 0–22 |
| Septum | — | — | — | 7 ± 2 | 2–12 |
| LA wall, roof, floor | — | — | — | 9 ± 4 | 3–25 |
| Total | — | — | — | 40 ± 10 | 20–86 |
LA, left atrial; PV, pulmonary vein; RF, radiofrequency; Unk, unknown.
| Procedures 89 . | Configuration . | Diameter (mm) . | RF applications . | ||
|---|---|---|---|---|---|
| . | n = patients (%) . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Left superior PV | 79 (89) | 17 ± 3 | 8–24 | 7 ± 3 | 1–28 |
| Left inferior PV | 79 (89) | 16 ± 3 | 10–27 | 5 ± 2 | 1–13 |
| Left common PV | 10 (11) | 21 ± 5 | 14–28 | 11 ± 4 | 7–18 |
| Right superior PV | 89 (100) | 18 ± 3 | 8–26 | 6 ± 3 | 2–15 |
| Right middle PV | 24 (27) | 8 ± 1 | 5–9 | 3 ± 2 | 1–6 |
| Right inferior PV | 89 (100) | 17 ± 4 | 8–31 | 6 ± 3 | 0–22 |
| Septum | — | — | — | 7 ± 2 | 2–12 |
| LA wall, roof, floor | — | — | — | 9 ± 4 | 3–25 |
| Total | — | — | — | 40 ± 10 | 20–86 |
| Procedures 89 . | Configuration . | Diameter (mm) . | RF applications . | ||
|---|---|---|---|---|---|
| . | n = patients (%) . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Left superior PV | 79 (89) | 17 ± 3 | 8–24 | 7 ± 3 | 1–28 |
| Left inferior PV | 79 (89) | 16 ± 3 | 10–27 | 5 ± 2 | 1–13 |
| Left common PV | 10 (11) | 21 ± 5 | 14–28 | 11 ± 4 | 7–18 |
| Right superior PV | 89 (100) | 18 ± 3 | 8–26 | 6 ± 3 | 2–15 |
| Right middle PV | 24 (27) | 8 ± 1 | 5–9 | 3 ± 2 | 1–6 |
| Right inferior PV | 89 (100) | 17 ± 4 | 8–31 | 6 ± 3 | 0–22 |
| Septum | — | — | — | 7 ± 2 | 2–12 |
| LA wall, roof, floor | — | — | — | 9 ± 4 | 3–25 |
| Total | — | — | — | 40 ± 10 | 20–86 |
LA, left atrial; PV, pulmonary vein; RF, radiofrequency; Unk, unknown.
Procedural outcomes
The average procedural and fluoroscopy time was 112 ± 32 min (range 75–240) and 21 ± 10 min (range 10–67). The procedural time decreased from 151 ± 50 to 100 ± 17 min, and fluoroscopy time decreased from 30 ± 15 to 19 ± 6 min comparing the first 20 with the last 20 patients. The average total number of applications needed for a successful procedure was 40 ± 10 (24 ± 7 PVAC, 7 ± 2 MASC, and 9 ± 4 MAAC). Application numbers, mostly with, PVAC, declined by 20% comparing the first 20 patients with the last 20 patients. All patients needed DCCV to regain sinus rhythm during the procedure, after which PV isolation was verified in all veins.
Freedom of atrial fibrillation after treatment
After a single treatment 32 of 89 (36%) patients did not have any documented recurrence of AF and were without AADs (Figure 2), while an additional 12 patients (13%) only needed DCCV at 6 ± 2 months after the procedure to remain in sinus rhythm (Table 3). There was no significant difference in efficacy between the first 20 and the last 20 treated patients in the cohort. In 15 patients (17%) the three-catheter strategy transformed longstanding persistent AF to paroxysmal AF. During follow-up after a single treatment, 57 of 89 (64%) patients restarted AADs, while 27 of 57 (47%) patients remained in sinus rhythm after DCCV or had paroxysmal AF. Seventeen per cent of the patients with paroxysmal AF had sinus rhythm after restarting previously ineffective AADs.
Efficacy by definitions of success in patients with long-term persistent atrial fibrillation
| Number of patients 89 . | First treatment PVAC/MASC/MAAC . | Second treatment PVAC/MASC/MAAC . | |
|---|---|---|---|
| . | n = patients/total (%) . | n = patients/total (%) . | |
| Absence of AF/flutter/tachycardiaa, and patients were off class I or III AADs, 12 months after the (last) PVAC/MASC/MAAC ablation | 32/89 (36) | 6/15 (40) | |
| Paroxysmal AF after treatment | 15/89 (17) | 4/15 (27) | |
| DCCV | 12/89 (13) | 0/15 (0) | |
| No change | 30/89 (34) | 5/15 (33) | |
| Additional procedure | 23/89 (26) | 3/15 (20) | |
| Redo PVAC/MASC/MAAC | 15/23 | – | |
| Cumulative AF freedom | Patients | n = patients/total | (%) |
| First treatment with PVAC/MASC/MAAC | n = 89 | 32/89 | 36 |
| Including DCCV | n = 12 | 44/89 | 49 |
| Second treatment with PVAC/MASC/MAAC | n = 15 | 50/89 | 56 |
| Change to paroxysmal AF (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 18 | 18/89 | 20 |
| No change (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 21 | 21/89 | 24 |
| Number of patients 89 . | First treatment PVAC/MASC/MAAC . | Second treatment PVAC/MASC/MAAC . | |
|---|---|---|---|
| . | n = patients/total (%) . | n = patients/total (%) . | |
| Absence of AF/flutter/tachycardiaa, and patients were off class I or III AADs, 12 months after the (last) PVAC/MASC/MAAC ablation | 32/89 (36) | 6/15 (40) | |
| Paroxysmal AF after treatment | 15/89 (17) | 4/15 (27) | |
| DCCV | 12/89 (13) | 0/15 (0) | |
| No change | 30/89 (34) | 5/15 (33) | |
| Additional procedure | 23/89 (26) | 3/15 (20) | |
| Redo PVAC/MASC/MAAC | 15/23 | – | |
| Cumulative AF freedom | Patients | n = patients/total | (%) |
| First treatment with PVAC/MASC/MAAC | n = 89 | 32/89 | 36 |
| Including DCCV | n = 12 | 44/89 | 49 |
| Second treatment with PVAC/MASC/MAAC | n = 15 | 50/89 | 56 |
| Change to paroxysmal AF (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 18 | 18/89 | 20 |
| No change (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 21 | 21/89 | 24 |
aLasting >30 s, on one of the recordings after the 3 month blanking period by repeated 12-lead ECG, event monitoring and Holter.
AAD, anti-arrhythmic drug; AF, atrial fibrillation; DCCV, direct current cardioversion; RF radiofrequency; PVAC, pulmonary vein ablation catheter; MASC, multi-array septal catheter; MAAC, multi-array ablation catheter.
Efficacy by definitions of success in patients with long-term persistent atrial fibrillation
| Number of patients 89 . | First treatment PVAC/MASC/MAAC . | Second treatment PVAC/MASC/MAAC . | |
|---|---|---|---|
| . | n = patients/total (%) . | n = patients/total (%) . | |
| Absence of AF/flutter/tachycardiaa, and patients were off class I or III AADs, 12 months after the (last) PVAC/MASC/MAAC ablation | 32/89 (36) | 6/15 (40) | |
| Paroxysmal AF after treatment | 15/89 (17) | 4/15 (27) | |
| DCCV | 12/89 (13) | 0/15 (0) | |
| No change | 30/89 (34) | 5/15 (33) | |
| Additional procedure | 23/89 (26) | 3/15 (20) | |
| Redo PVAC/MASC/MAAC | 15/23 | – | |
| Cumulative AF freedom | Patients | n = patients/total | (%) |
| First treatment with PVAC/MASC/MAAC | n = 89 | 32/89 | 36 |
| Including DCCV | n = 12 | 44/89 | 49 |
| Second treatment with PVAC/MASC/MAAC | n = 15 | 50/89 | 56 |
| Change to paroxysmal AF (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 18 | 18/89 | 20 |
| No change (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 21 | 21/89 | 24 |
| Number of patients 89 . | First treatment PVAC/MASC/MAAC . | Second treatment PVAC/MASC/MAAC . | |
|---|---|---|---|
| . | n = patients/total (%) . | n = patients/total (%) . | |
| Absence of AF/flutter/tachycardiaa, and patients were off class I or III AADs, 12 months after the (last) PVAC/MASC/MAAC ablation | 32/89 (36) | 6/15 (40) | |
| Paroxysmal AF after treatment | 15/89 (17) | 4/15 (27) | |
| DCCV | 12/89 (13) | 0/15 (0) | |
| No change | 30/89 (34) | 5/15 (33) | |
| Additional procedure | 23/89 (26) | 3/15 (20) | |
| Redo PVAC/MASC/MAAC | 15/23 | – | |
| Cumulative AF freedom | Patients | n = patients/total | (%) |
| First treatment with PVAC/MASC/MAAC | n = 89 | 32/89 | 36 |
| Including DCCV | n = 12 | 44/89 | 49 |
| Second treatment with PVAC/MASC/MAAC | n = 15 | 50/89 | 56 |
| Change to paroxysmal AF (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 18 | 18/89 | 20 |
| No change (cumulative after one or two treatments with PVAC/MASC/MAAC) | n = 21 | 21/89 | 24 |
aLasting >30 s, on one of the recordings after the 3 month blanking period by repeated 12-lead ECG, event monitoring and Holter.
AAD, anti-arrhythmic drug; AF, atrial fibrillation; DCCV, direct current cardioversion; RF radiofrequency; PVAC, pulmonary vein ablation catheter; MASC, multi-array septal catheter; MAAC, multi-array ablation catheter.
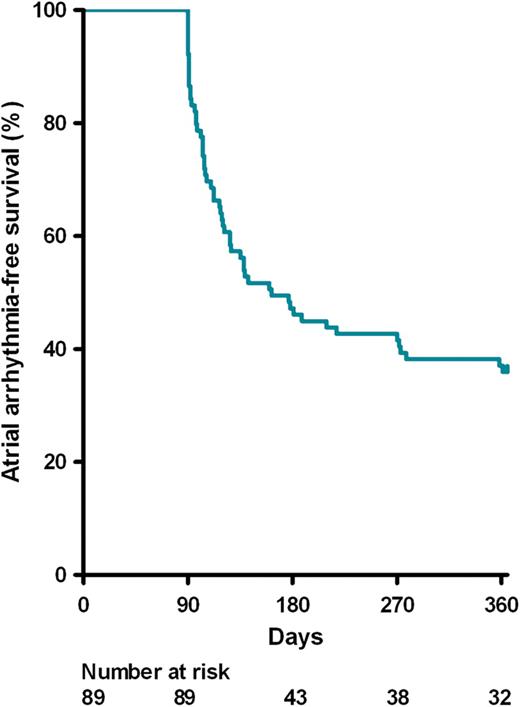
Kaplan–Meier curve demonstrating time to first atrial fibrillation/flutter/tachycardia recurrence after a single treatment with pulmonary vein ablation catheter/multi-array septal catheter/multi-array ablation catheter for longstanding persistent atrial fibrillation.
In total, 23 of 89 patients (26%) underwent an additional procedure for AF in the first year after the first treatment. A redo PVAC/MASC/MAAC treatment was performed in 15 patients, 6 of which (40%) became free of any arrhythmia at 1 year follow-up, while another 4 (27%) changed to paroxysmal AF. Patients that underwent a redo had no change of their AF pattern after the first procedure, with exception of one with paroxysmal AF who had long episodes of AF up to 1 week and was in AF at the start of the procedure. We did not perform cardioversion at the beginning of the procedure to verify PV isolation. Rather, additional antrum ablation was performed in all patients, and re-isolation was achieved and verified at the end of the procedure when patients were in sinus rhythm again. Eleven patients underwent an additional procedure with other invasive treatments at the discretion of the referring electrophysiologist and the individual patient; mini-Maze surgery in five, conventional 3-D map-guided single-tip catheter in three, full RF-Maze in one, Maze surgery in two, and one His-ablation/PM implant. Only 2 of these 11 patients (18%) became free from AF.
Patient who met the definition of success
Patients were divided into two cohorts based on whether they did, or did not, meet the definition of success. All baseline characteristics such as arrhythmia history and echocardiographic parameters were compared between groups. All analysed parameters are given in Table 1. Univariate analysis showed no clear pre-procedural predictors for success. Univariate analysis showed that patients with a pre-procedural smaller LA size, on the parasternal long-axis view, had a higher probability of a successful long-term outcome, although this did not reach statistical significance [OR 0.51 95% CI (0.18–1.42)] because of low sample size and narrow range of LA size in the total population.
Safety profile
No complications were observed during the procedure itself. Two post-procedural complications were registered within the first 24 h. In one patient an acute coronary syndrome with ST-elevation occurred, and coronary angiography showed occlusion of a post-erolateral side-branch of the circumflex artery, which was treated by a percutaneous coronary intervention with stent implantation. One patient developed a groin haematoma with spontaneous complete recovery, without need for transfusion. Two late complications were registered. One patient experienced shortness of breath, due to progressive pericardial effusion over the next 3 months, requiring successful pericardiocentesis 3 months after the procedure. Another patient had a reversible transient ischaemic attack (TIA) >1 month after the procedure.
Magnetic resonance imaging follow-up or PV angiography during a second procedure was performed in 53 of 89 patients (60%), none of them showing evidence for PV stenosis defined as >50%.
Discussion
The present study shows, that in this selected population of relatively young patients with rather preserved cardiac structure with longstanding persistent AF, the PVAC/MASC/MAAC approach seems efficient with a favourable outcome and safety profile.
Long-term success
The definitions of success were taken from the HRS/EHRA/ECAS expert consensus statement of 2007.7 In the PVAC/MASC/MAAC treatment group with longstanding persistent AF, 32 of 89 patients (36%) met the strictest criterion of total freedom of any LA arrhythmia while off AADs at 12 months after a single ablation procedure. After a second treatment with PVAC/MASC/MAAC in 15 patients, the success rate increased to 43%. Some patients only needed a DCCV in the follow-up period and were free from AF after DCCV. This resulted in a total efficacy of 56% for longstanding persistent AF after 1.2 ± 0.4 procedures. In an additional 18 of 89 patients (20%) longstanding persistent AF changed to paroxysmal.
In the multi-centre pilot study by Scharf et al.6 the single procedure success rate was not presented, nor in how many patients DCCV was used to obtain sinus rhythm. An early redo procedure was performed in 28 of 50 (56%).6 It is not clear as to how many of these became free of AF, and how many of the patients that were not retreated remained free of AF during follow-up. In that study freedom of AF without AAD after 1.5 procedures was shown to be 54% at 6 months and 45% at 20 months. The success rate of 56% after 1.2 ± 0.4 procedures at 12 months after the last ablation in our study compares favourably to the data of the multi-centre pilot trial. It is conceivable that if a redo procedure would have been done in all patients, a higher efficacy to cure longstanding persistent AF was obtained. However, a second PVAC/MASC/MAAC procedure was not always performed in the patients that failed after the first procedure. Some patient chose not to have a redo for personal reasons. In other patients other treatment modalities were elected by the referring electrophysiologist. We did not observe any differences between patients who underwent either procedure.
A recent review article shows that the success of conventional catheter ablation in patients with (longstanding) persistent AF, using different treatment strategies, varies widely with efficacies ranging from 21 to 95%, including multiple procedures.9
Contemporary conventional catheter ablation techniques may be divided in the anatomical and the electrogram-guided approach. The first approach focuses on isolation of the four PVs, with or without additional linear lesions. The second approach focuses on targeting areas of CFAE. Literature on PVI combined with linear lesions shows that freedom of LA arrhythmia, after a single treatment off AADs in patients with persistent AF, efficacy varies from 21 to 45%.10–12 A recent study by O'Neill et al.5 shows that a very extensive ablation of the left and right atrium (CFAE), besides PVI in patients with longstanding persistent AF, results in freedom of LA arrhythmia off AADs in 48% of patients after a single procedure. This increased up to 80%, only with multiple procedures.
As a side effect of the extensive treatment, many arrhythmia recurrences are due to LA flutters that require additional therapy. The phased RF PVAC/MASC/MAAC approach in our series did not show in any LA flutter during follow-up. This may be explained by a preserved LA diameter. Alternatively, the less extensive ablation without (often incomplete) lines may prevent the occurrence of secondary LA reentrant arrhythmias. The targets for PVAC/MASC/MAAC ablation may have included areas with ganglionic plexi13 although we did not try to establish this in our study. Vagal response were sometimes observed, mainly when PVAC application were made at the left superior pulmonary vein antrum.
In our series a redo PVAC/MASC/MAAC procedure was successful in achieving sinus rhythm in 40% of patients. We did not perform such a second procedure in all patients with failure for variety of reasons. In some patients AF was accepted and no additional invasive treatment was done. In other patients, more conventional ablation tools or even (mini-) Maze surgery had a very low success rate in patients who failed a first ablation. Obviously, more extensive head-on-comparison is needed to appreciate the relative merits of surgery vs. extensive ablation with three catheters and phased RF.
Safety
The worldwide survey on the methods and safety of conventional catheter ablation shows an incidence of major complications of 4.5%.14 The multi-centre pilot study from Scharf et al.6 reported a procedural complication rate of 8% with the PVAC/MASC/MAAC treatment strategy. In our study group with PVAC/MASC/MAAC, including a second PVAC/MASC/MAAC in 15 patients, peri-procedural complications occurred in only 2%, while a late complication was seen in 1%. Of the few complications observed, groin haematomas, a TIA after 1 month, and a pericardial effusion several months after the ablation procedure, may be related to the fact that a procedure was done, but are not likely due to the specific ablation technology used.
Procedural characteristics
The average procedural and fluoroscopy times in our study (112 ± 32 and 21 ± 10 min) were shorter than in the multi-centre pilot trial by Scharf et al.6 (155 ± 40 and 55 ± 35 min), which is probably the result of the increasing experience with the system. These procedural data also compare favourably with other conventional procedures using point-by-point ablation with 3-D navigation systems: Linear lesions 240 and 71 min;3 CFAE ablation 186 ± 51 and 15 ± 5 min;4 Stepwise ablation 255 ± 69 and 86 ± 28 min.5
Study limitations
This prospective study is observational with inherent limitations
The preliminary data show that in this selected group of 89 patients, the safety profile was favourable compared with a prior publication on a similar study by Scharf et al.6 and in agreement with other publications on safety with phased RF and PVAC ablation.15,16 Additional studies in larger populations are needed to determine how safe this new technology is. In our study, neurological evaluation with MRI was only performed in case of symptoms of stroke, and there was no active search for silent ischaemia with diffusion-weighted–MRI. Arrhythmia follow-up was very intensive, but episodes of AF may have been missed, as recording was not continuous such as with an implantable loop recorder. Still, the current follow-up was more intense than that in studies performed before publication of the HRS/EHRA/ECAS expert consensus statement of 2007. This makes it difficult to compare this technology to historical data with other technologies. A prospective randomized multi-centre trial will be needed to assess the relative value of this technology compared with conventional strategies.
Conclusion
This single-centre study shows that an ablation strategy in selected patients with longstanding persistent AF using the PVAC, MASC, and MAAC has a 56% efficacy to restore and maintain sinus rhythm after 1 year of follow-up (1.2 ± 0.4 procedures). In an additional 20% of the patients this strategy transforms longstanding persistent AF to paroxysmal AF. The procedure is standardized, can be performed relatively time-efficient, and has a favourable safety profile. These data will have to be confirmed in larger multi-centre trials.
Conflict of interest: L.V.A.B. is a consultant for Medtronic, and a prior stockholder of Ablation Frontiers. The Cardiology Department has received grant support for research from Ablation Frontiers, Inc. M.C.E.F.W., E.F.D.W., and A.A.W.M. have no conflict of interest.
Funding
This work was supported by the Cardiology Department, St Antonius Hospital, Nieuwegein, The Netherlands.
Acknowledgements
The authors would like to thank Ms. J.C.M. van Weverwijk of the R&D Cardiology, St Antonius Hospital for her friendly assistance with the 7 days Holters in the study.



