-
PDF
- Split View
-
Views
-
Cite
Cite
Pieter Koopman, Dieter Nuyens, Christophe Garweg, Andre La Gerche, Stijn De Buck, Lieve Van Casteren, Becker Alzand, Rik Willems, Hein Heidbuchel, Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation, EP Europace, Volume 13, Issue 10, October 2011, Pages 1386–1393, https://doi.org/10.1093/europace/eur142
Close - Share Icon Share
Abstract
Endurance sports activities have been associated with the development of atrial fibrillation (AF). Pulmonary vein isolation (PVI) by means of radiofrequency catheter ablation has been established as an effective treatment for AF. The aim of the present study was to analyse the efficacy of AF ablation in athletes.
We compared procedural outcome and median term follow-up in 94 consecutive athletes (>3 h of sports/week for ≥10 years or ≥1500 h lifetime) who underwent PVI (94% men, 51 ± 8 years, 87% paroxysmal AF, left atrial (LA) diameter 40 ± 8 mm, mean follow-up 41 months), and 41 contemporary controls. Sixty-three per cent of athletes performed endurance sports (running, cycling, swimming, and rowing). Documented focal induction of AF and failed treatment with ≥1 anti-arrhythmic drug were pre-requisites for selection of ablation treatment. Patients with long-standing persistent or permanent AF or an LA diameter ≥55 mm were not considered for ablation. Median lifetime cumulative hours of sports was 8638 (4175–13 688) in athletes vs. 450 (280–600) in controls (P < 0.001). Other baseline characteristics except for gender (94 vs. 66% men, respectively, P < 0.001) were comparable between both groups, as was the total number of ablation procedures per patient (1.2±0.5, P = 0.62). Survival analysis showed similar AF recurrence rate after a first ablation for controls and endurance athletes, though non-endurance athletes had a significantly higher AF recurrence rate (48 vs. 46 vs. 34% freedom from AF at 3 year follow-up after a single ablation, P= 0.04). Final outcome after all ablations was similar (87 vs. 84 vs. 85% freedom from AF at 3-year follow-up, P = 0.88). No other independent predictor for AF recurrence was identified.
In patients with documented focal induction of non-permanent AF and absence of structural heart disease, PVI is as effective in endurance athletes as in other patients.
Introduction
Regular sports practice, and endurance sports practice in particular, has become an increasingly popular leisure-time activity. Although benefits of regular exercise on cardiovascular health have been proven, recent data have documented a relationship between long-term endurance sports practice and the occurrence of arrhythmias such as atrial fibrillation (AF) or atrial flutter.1–11 Atrial fibrillation is mainly associated with dynamic endurance sports such as running, cycling, swimming, and rowing. This increased risk has been explained by several mechanisms that induce structural, functional, autonomic, and inflammatory alterations in the atria.11–18
In recent years, pulmonary vein isolation (PVI) has been introduced in clinical practice as an effective treatment in patients with AF, especially in patients with paroxysmal AF and without structural heart disease (SHD).19 Moreover, it is a particularly attractive option for athletes with AF, who would otherwise face a lifetime need for medical therapy, possibly interfering with exercise eligibility or tolerance.20,21 However, it remains unclear as to what extent the different changes contributing to exercise-induced AF might render the arrhythmia more refractory to ablation. The aim of the present study was to analyse the efficacy and success rate of radiofrequency catheter ablation for AF in an athlete population in comparison with contemporary controls.
Methods
Study population
We included 94 athletes and 41 control patients from a series of 210 consecutive patients younger than 65 years and without SHD, referred to our centre between May 2000 and December 2009 for radiofrequency catheter ablation of AF. Inclusion required (i) symptomatic AF, (ii) paroxysmal, or persistent for <1 year, (iii) refractory to at least one anti-arrhythmic drug, and (iv) focally induced as documented on Holter monitoring by showing frequent atrial pre-mature beats or runs of atrial tachycardia inducing bouts of AF (Figure 1). Furthermore, all pulmonary veins (PV) had to be targeted for ablation. Other exclusion criteria were missing sports questionnaire, missing data, or ablation procedures performed in other centres. Patients with important underlying atrial structural changes [i.e. left atrial (LA) diameter ≥55 mm] or permanent AF were not considered for ablation in our centre. Informed consent was obtained from all participants. The study protocol was approved by the Institutional Ethics Committee.

Flow chart of selection process. SHD, structural heart disease; AF, atrial fibrillation; AAD, anti-arrhythmic drugs; PV, pulmonary veins.
In a detailed questionnaire, patients were asked about their lifetime physical activity. Each type of regular sports activity was assessed, including number of years of participation and average number of hours per week, and a quantification of sports activity before and after ablation was calculated. Athletes were defined as those engaged in sports activities classified into category IB, IIA, or higher according to the Bethesda classification of sports.22 Those practicing class IA sports activities were not considered athletes and accordingly were classified in the control group. Sports had to be performed for ≥3 h per week during ≥10 years or for a total of ≥1500 h after the age of 14 years, based on its reported association with AF.2,13 At least some form of (self-) organized training was required to ensure adequate sports intensity. Light, purely recreational activity was not considered. Endurance athletes were defined as those having performed a lifetime activity of ≥1500 h of running, cycling, swimming, or rowing, even when other sports were practised beside it.
Transthoracic echocardiography was performed to rule out SHD and to measure LA and left ventricular diameters and left ventricular ejection fraction (LVEF). Oral anticoagulants were given for at least 4–6 weeks before and 6 weeks after ablation, bridged with low-molecular-weight heparin periprocedurally.
Pulmonary vein isolation
Ablation was performed under general anaesthesia with propofol and mechanical ventilation. All catheters were introduced percutaneously via femoral veins. Transseptal access was obtained using two transseptal sheaths (SR 0, St Jude Medical, Daig Division, Inc., Minnetonka, MN, USA). Intravenous heparin was administered adjusted by age and renal function, maintaining an activated clotting time of 250–350 s during the procedure.
All procedures were performed under fluoroscopic guidance with a Coroskop C biplane image intensifier system or Axiom Artis DynaCT system (Siemens, Erlangen, Germany). Since February 2008, a contrast-enhanced three-dimensional rotational angiography has been used as anatomical guidance for creation and validation of radiofrequency lesions.23 Mapping of pulmonary vein potentials was performed with a deflectable decapolar catheter with a distal ring configuration (Lasso, Biosense-Webster, Diamond Bar, CA, USA).
All four PV were targeted for ablation. In 28 patients, ablation of individual pulmonary vein ostia was performed. In all other procedures, continuous radiofrequency lesions were deployed surrounding ipsilateral PV. Radiofrequency energy was delivered through an irrigated tip thermocouple-equipped catheter (Biosense-Webster), a standard EPT catheter (EPT, Boston Scientific, EP Technologies, San Jose, CA, USA), or a 4 mm thermocouple-equipped Celsius catheter (Biosense-Webster), using temperature feedback with target temperature of 50°C and maximum power output of 30 W. Ablation was aimed at fully isolating all PV, guided by bidirectional disconnection of pulmonary vein potentials from the left atrium.
Empirical linear ablation lesions from the left inferior pulmonary vein towards the mitral valve and/or in the roof of the left atrium were applied if AF was still inducible after PVI or when underlying atrial structural changes were assumed (LA dilatation, persistent AF), aiming at substrate modification. Additional cavo-tricuspid isthmus ablation was performed in 108 patients (80%).
Follow-up
After ablation, patients were observed for 24 h. Holter recording and control transthoracic echocardiogram were obtained. Patients received aspirin and low-molecular-weight heparin until oral anti-coagulation again reached an international normalized ratio ≥2. Pre-procedural anti-arrhythmic drug therapy remained unchanged until the first ambulant visit after 6 weeks. In the absence of symptoms, anti-arrhythmic drug treatment was stopped at six weeks unless deemed preferable in the context of structural findings. Further follow-up information was obtained from scheduled outpatient visits including 12-lead electrocardiogram (ECG) and Holter monitoring at 6 months and 1 year, and yearly thereafter. Patients would present to the emergency room or the arrhythmia clinic when symptoms suggestive of recurrence occurred between visits. In such case, new long-term ECG recordings (24 h, 48 h, or 1 week) were performed.
For this study, total and documented arrhythmia recurrence were assessed. Total recurrence included both documented arrhythmia recurrence, defined as a documented episode of atrial fibrillation, atrial flutter, or intra-atrial re-entrant tachycardia occurring after the first ablation procedure and lasting for longer than 1 min, and subjective arrhythmia recurrence, defined as recurrence of symptoms or palpitations following ablation, identical to symptoms prior to the ablation procedure. Because all patients previously had symptomatic AF, recurrence of symptoms was considered a relevant endpoint in this specific population. Any symptoms suggestive of AF recurrence were therefore interpreted as such, unless otherwise proven (by negative repeat Holter or event recordings during symptoms reported by the patient). Transient arrhythmic episodes during the first 6 weeks after ablation, presumably related to atrial inflammatory processes following radiofrequency lesions, were not considered.
Primary endpoint of the study was freedom from any arrhythmia recurrence after the first ablation procedure, regardless of anti-arrhythmic medication. Secondary endpoints were freedom from documented arrhythmia recurrence after a singleablation procedure, and freedom from any arrhythmia recurrence after all subsequent ablation procedures, also regardless of residual medical therapy. A minimal follow-up of 6 months was required.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median and interquartile range for not-normally distributed variables. The Shapiro–Wilk W test was used to test for normality after excluding outliers. Comparisons between two groups were made using Student's t-test for continuous and normally distributed variables and using Mann–Whitney U test for categorical or continuous not-normally distributed variables. Differences in proportions between groups were evaluated by means of Fisher's exact or Pearson χ2 test. Comparisons between continuous and normally distributed variables in multiple groups were made by one-way analysis of variance with Scheffe post-hoc analysis. Comparisons between categorical or continuous not-normally distributed variables in multiple groups were made by Kruskal–Wallis testing. The relationship between single and multiple baseline variables and time to recurrence during follow-up was evaluated using univariable Cox proportional hazards regression analysis and multivariable Cox proportional hazards regression analysis with a backward stepwise conditional approach. Kaplan–Meier survival curves were constructed to evaluate follow-up of patients in all groups, and the differences between curves were tested for significance by log-rank statistics. For all analyses, a two-sided P value ≤0.05 was considered statistically significant. Analyses were performed using the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Demography
Baseline clinical and echocardiographic characteristics of all athletes and control patients are shown in Table 1. Mean age of all patients was 51 ± 8 years. Most patients (90%) had paroxysmal AF, only 10% had persistent AF. Symptoms had been lasting for a median of 4 years prior to the first ablation procedure. Apart from the amount of hours of sports performed, athletes and controls differed only in gender distribution (94% of athletes were male vs. 66% of controls, P < 0.001). Median lifetime sports practice in athletes was 8638 h or 4 h per week since the age of 14 years, compared to 450 h lifetime or 0.2 h per week in controls (P < 0.001). Atrial dimensions in both groups were slightly dilated (40 ± 8 mm in athletes vs. 38 ± 7 mm in controls, P = 0.54). Ventricular cavities were slightly enlarged in athletes, but not significantly (50 ± 5 mm in athletes vs. 48 ± 5 mm in controls, P = 0.19). Left ventricular ejection fraction was normal in both groups. Arterial hypertension was present in 23% of athletes and 29% of controls (P = 0.52). Of all athletes, 63% performed endurance sports. Comparison of baseline characteristics in the endurance and non-endurance sports group was not statistically different.
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| . | n = 41 . | n = 94 . | . | n = 59 . | n = 35 . | . |
| Age (years) | 52 ± 8 | 51 ± 8 | 0.27 | 51 ± 8 | 50 ± 7 | 0.37 |
| Male gender | 27 (65.9%) | 88 (93.6%) | <0.001 | 58 (98.3%) | 30 (85.7%) | <0.001 |
| AF type | 0.23 | 0.37 | ||||
| Paroxysmal | 39 (95.1%) | 82 (87.2%) | 51 (86.4%) | 31 (88.6%) | ||
| Persistent | 2 (4.9%) | 12 (12.8%) | 8 (13.6%) | 4 (11.4%) | ||
| AF duration (years) | 2 (2–4.5) | 4 (2–9) | 0.09 | 4 (2–10) | 4 (2–8) | 0.21 |
| Echocardiography | ||||||
| LAD (mm) | 38 ± 7 | 40 ± 8 | 0.22 | 40 ± 7 | 40 ± 10 | 0.54 |
| LVEDD (mm) | 48 ± 5 | 50 ± 5 | 0.07 | 50 ± 5 | 50 ± 5 | 0.19 |
| LVEF (%) | 63 ± 7 | 64 ± 7 | 0.28 | 64 ± 8 | 64 ± 7 | 0.63 |
| AHT | 12 (29.3%) | 22 (23.4%) | 0.52 | 12 (20.3%) | 10 (28.6%) | 0.52 |
| Lone AF Sports practice | 24 (58.5%) | 59 (62.8%) | 0.70 | 40 (67.8%) | 19 (54.3%) | 0.27 |
| Lifetime (hours) | 450 (280–600) | 8638 (4175–13688) | <0.001 | 10 550 (8375–16 225) | 3450 (2600–5175) | <0.001 |
| Since 14 years of age (hours/week) | 0.2 (0.1–0.3) | 4.4 (2.3–8.0) | <0.001 | 5.8 (4.1–8.6) | 1.8 (1.4–2.8) | <0.001 |
| After first ablation (hours/week) | 0 (0–1) | 3 (1–5) | <0.001 | 4 (2–7) | 2 (0–3) | <0.001 |
| PVI | ||||||
| Ipsilateral | 33 (80.5%) | 74 (78.7%) | 1.00 | 49 (83.1%) | 25 (71.4%) | 0.40 |
| Additional lines | 8 (19.5%) | 17 (18.1%) | 0.82 | 10 (16.9%) | 7 (20.0%) | 0.92 |
| Follow-up (months) | 40 (26–62) | 41 (28–57) | 0.92 | 41 (24–54) | 42 (25–82) | 0.85 |
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| . | n = 41 . | n = 94 . | . | n = 59 . | n = 35 . | . |
| Age (years) | 52 ± 8 | 51 ± 8 | 0.27 | 51 ± 8 | 50 ± 7 | 0.37 |
| Male gender | 27 (65.9%) | 88 (93.6%) | <0.001 | 58 (98.3%) | 30 (85.7%) | <0.001 |
| AF type | 0.23 | 0.37 | ||||
| Paroxysmal | 39 (95.1%) | 82 (87.2%) | 51 (86.4%) | 31 (88.6%) | ||
| Persistent | 2 (4.9%) | 12 (12.8%) | 8 (13.6%) | 4 (11.4%) | ||
| AF duration (years) | 2 (2–4.5) | 4 (2–9) | 0.09 | 4 (2–10) | 4 (2–8) | 0.21 |
| Echocardiography | ||||||
| LAD (mm) | 38 ± 7 | 40 ± 8 | 0.22 | 40 ± 7 | 40 ± 10 | 0.54 |
| LVEDD (mm) | 48 ± 5 | 50 ± 5 | 0.07 | 50 ± 5 | 50 ± 5 | 0.19 |
| LVEF (%) | 63 ± 7 | 64 ± 7 | 0.28 | 64 ± 8 | 64 ± 7 | 0.63 |
| AHT | 12 (29.3%) | 22 (23.4%) | 0.52 | 12 (20.3%) | 10 (28.6%) | 0.52 |
| Lone AF Sports practice | 24 (58.5%) | 59 (62.8%) | 0.70 | 40 (67.8%) | 19 (54.3%) | 0.27 |
| Lifetime (hours) | 450 (280–600) | 8638 (4175–13688) | <0.001 | 10 550 (8375–16 225) | 3450 (2600–5175) | <0.001 |
| Since 14 years of age (hours/week) | 0.2 (0.1–0.3) | 4.4 (2.3–8.0) | <0.001 | 5.8 (4.1–8.6) | 1.8 (1.4–2.8) | <0.001 |
| After first ablation (hours/week) | 0 (0–1) | 3 (1–5) | <0.001 | 4 (2–7) | 2 (0–3) | <0.001 |
| PVI | ||||||
| Ipsilateral | 33 (80.5%) | 74 (78.7%) | 1.00 | 49 (83.1%) | 25 (71.4%) | 0.40 |
| Additional lines | 8 (19.5%) | 17 (18.1%) | 0.82 | 10 (16.9%) | 7 (20.0%) | 0.92 |
| Follow-up (months) | 40 (26–62) | 41 (28–57) | 0.92 | 41 (24–54) | 42 (25–82) | 0.85 |
AF, atrial fibrillation; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; AHT, arterial hypertension; and PVI, pulmonary vein isolation.
Data are expressed as mean ± standard deviation, median and interquartile range (IR), percentage (%), and number of patients
aP value: athletes vs. controls
bP value: endurance athletes vs. non-endurance athletes vs. controls
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| . | n = 41 . | n = 94 . | . | n = 59 . | n = 35 . | . |
| Age (years) | 52 ± 8 | 51 ± 8 | 0.27 | 51 ± 8 | 50 ± 7 | 0.37 |
| Male gender | 27 (65.9%) | 88 (93.6%) | <0.001 | 58 (98.3%) | 30 (85.7%) | <0.001 |
| AF type | 0.23 | 0.37 | ||||
| Paroxysmal | 39 (95.1%) | 82 (87.2%) | 51 (86.4%) | 31 (88.6%) | ||
| Persistent | 2 (4.9%) | 12 (12.8%) | 8 (13.6%) | 4 (11.4%) | ||
| AF duration (years) | 2 (2–4.5) | 4 (2–9) | 0.09 | 4 (2–10) | 4 (2–8) | 0.21 |
| Echocardiography | ||||||
| LAD (mm) | 38 ± 7 | 40 ± 8 | 0.22 | 40 ± 7 | 40 ± 10 | 0.54 |
| LVEDD (mm) | 48 ± 5 | 50 ± 5 | 0.07 | 50 ± 5 | 50 ± 5 | 0.19 |
| LVEF (%) | 63 ± 7 | 64 ± 7 | 0.28 | 64 ± 8 | 64 ± 7 | 0.63 |
| AHT | 12 (29.3%) | 22 (23.4%) | 0.52 | 12 (20.3%) | 10 (28.6%) | 0.52 |
| Lone AF Sports practice | 24 (58.5%) | 59 (62.8%) | 0.70 | 40 (67.8%) | 19 (54.3%) | 0.27 |
| Lifetime (hours) | 450 (280–600) | 8638 (4175–13688) | <0.001 | 10 550 (8375–16 225) | 3450 (2600–5175) | <0.001 |
| Since 14 years of age (hours/week) | 0.2 (0.1–0.3) | 4.4 (2.3–8.0) | <0.001 | 5.8 (4.1–8.6) | 1.8 (1.4–2.8) | <0.001 |
| After first ablation (hours/week) | 0 (0–1) | 3 (1–5) | <0.001 | 4 (2–7) | 2 (0–3) | <0.001 |
| PVI | ||||||
| Ipsilateral | 33 (80.5%) | 74 (78.7%) | 1.00 | 49 (83.1%) | 25 (71.4%) | 0.40 |
| Additional lines | 8 (19.5%) | 17 (18.1%) | 0.82 | 10 (16.9%) | 7 (20.0%) | 0.92 |
| Follow-up (months) | 40 (26–62) | 41 (28–57) | 0.92 | 41 (24–54) | 42 (25–82) | 0.85 |
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| . | n = 41 . | n = 94 . | . | n = 59 . | n = 35 . | . |
| Age (years) | 52 ± 8 | 51 ± 8 | 0.27 | 51 ± 8 | 50 ± 7 | 0.37 |
| Male gender | 27 (65.9%) | 88 (93.6%) | <0.001 | 58 (98.3%) | 30 (85.7%) | <0.001 |
| AF type | 0.23 | 0.37 | ||||
| Paroxysmal | 39 (95.1%) | 82 (87.2%) | 51 (86.4%) | 31 (88.6%) | ||
| Persistent | 2 (4.9%) | 12 (12.8%) | 8 (13.6%) | 4 (11.4%) | ||
| AF duration (years) | 2 (2–4.5) | 4 (2–9) | 0.09 | 4 (2–10) | 4 (2–8) | 0.21 |
| Echocardiography | ||||||
| LAD (mm) | 38 ± 7 | 40 ± 8 | 0.22 | 40 ± 7 | 40 ± 10 | 0.54 |
| LVEDD (mm) | 48 ± 5 | 50 ± 5 | 0.07 | 50 ± 5 | 50 ± 5 | 0.19 |
| LVEF (%) | 63 ± 7 | 64 ± 7 | 0.28 | 64 ± 8 | 64 ± 7 | 0.63 |
| AHT | 12 (29.3%) | 22 (23.4%) | 0.52 | 12 (20.3%) | 10 (28.6%) | 0.52 |
| Lone AF Sports practice | 24 (58.5%) | 59 (62.8%) | 0.70 | 40 (67.8%) | 19 (54.3%) | 0.27 |
| Lifetime (hours) | 450 (280–600) | 8638 (4175–13688) | <0.001 | 10 550 (8375–16 225) | 3450 (2600–5175) | <0.001 |
| Since 14 years of age (hours/week) | 0.2 (0.1–0.3) | 4.4 (2.3–8.0) | <0.001 | 5.8 (4.1–8.6) | 1.8 (1.4–2.8) | <0.001 |
| After first ablation (hours/week) | 0 (0–1) | 3 (1–5) | <0.001 | 4 (2–7) | 2 (0–3) | <0.001 |
| PVI | ||||||
| Ipsilateral | 33 (80.5%) | 74 (78.7%) | 1.00 | 49 (83.1%) | 25 (71.4%) | 0.40 |
| Additional lines | 8 (19.5%) | 17 (18.1%) | 0.82 | 10 (16.9%) | 7 (20.0%) | 0.92 |
| Follow-up (months) | 40 (26–62) | 41 (28–57) | 0.92 | 41 (24–54) | 42 (25–82) | 0.85 |
AF, atrial fibrillation; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; AHT, arterial hypertension; and PVI, pulmonary vein isolation.
Data are expressed as mean ± standard deviation, median and interquartile range (IR), percentage (%), and number of patients
aP value: athletes vs. controls
bP value: endurance athletes vs. non-endurance athletes vs. controls
Procedure
Mean follow-up time was 46 ± 28 months. Athletes underwent a mean of 1.2 ± 0.4 ablation procedures vs. 1.2 ± 0.5 in the control group (Table 2). Complication rate was low (3.7%): two patients had signs of pericarditis without pericardial effusion, and three patients suffered a bleeding disorder (one urinary tract bleeding, one intracranial subarachnoidal bleeding which could be treated conservatively, and one pseudo-aneurysm with arteriovenous fistula at the puncture site). None had sequelae.
Arrhythmia recurrence evaluated at 3 years after first ablation and final outcome evaluated at 3 years after multiple ablations
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| Recurrence of arrhythmia at 3 years after first ablation | ||||||
| All recurrence (%) | 51.9 | 58.3 | 0.90 | 53.9 | 66.3 | 0.04 |
| Freedom from AF (%) | 48.1 | 41.7 | 0.90 | 46.1 | 33.7 | 0.04 |
| Documented recurrence (%) | 38.7 | 44.6 | 1.00 | 36.7 | 58.5 | 0.01 |
| Time to recurrence (months) | 2 (0–6) | 3 (0–12) | 0.52 | 5 (1–15) | 1 (0–9) | 0.16 |
| Final outcome at 3 years after multiple ablations | ||||||
| All recurrence (%) | 12.7 | 15.5 | 0.61 | 15.7 | 15.2 | 0.88 |
| Freedom from AF (%) | 87.3 | 84.5 | 0.61 | 84.3 | 84.8 | 0.88 |
| Number of procedures | 1.2 ± 0.5 | 1.2 ± 0.4 | 0.62 | 1.1 ± 0.3 | 1.3 ± 0.5 | 0.07 |
| Residual medication after multiple ablations (%) | 65.9 | 62.8 | 0.85 | 55.9 | 74.3 | 0.19 |
| Beta-blockade (%) | 53.7 | 58.9 | 0.71 | 39 | 65.7 | 0.04 |
| Calciumblockade (%) | 17.1 | 20.2 | 0.81 | 18.6 | 22.9 | 0.81 |
| Class I or III AAD (%) | 24.4 | 36.2 | 0.23 | 30.5 | 45.7 | 0.13 |
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| Recurrence of arrhythmia at 3 years after first ablation | ||||||
| All recurrence (%) | 51.9 | 58.3 | 0.90 | 53.9 | 66.3 | 0.04 |
| Freedom from AF (%) | 48.1 | 41.7 | 0.90 | 46.1 | 33.7 | 0.04 |
| Documented recurrence (%) | 38.7 | 44.6 | 1.00 | 36.7 | 58.5 | 0.01 |
| Time to recurrence (months) | 2 (0–6) | 3 (0–12) | 0.52 | 5 (1–15) | 1 (0–9) | 0.16 |
| Final outcome at 3 years after multiple ablations | ||||||
| All recurrence (%) | 12.7 | 15.5 | 0.61 | 15.7 | 15.2 | 0.88 |
| Freedom from AF (%) | 87.3 | 84.5 | 0.61 | 84.3 | 84.8 | 0.88 |
| Number of procedures | 1.2 ± 0.5 | 1.2 ± 0.4 | 0.62 | 1.1 ± 0.3 | 1.3 ± 0.5 | 0.07 |
| Residual medication after multiple ablations (%) | 65.9 | 62.8 | 0.85 | 55.9 | 74.3 | 0.19 |
| Beta-blockade (%) | 53.7 | 58.9 | 0.71 | 39 | 65.7 | 0.04 |
| Calciumblockade (%) | 17.1 | 20.2 | 0.81 | 18.6 | 22.9 | 0.81 |
| Class I or III AAD (%) | 24.4 | 36.2 | 0.23 | 30.5 | 45.7 | 0.13 |
AF, atrial fibrillation; and AAD, anti-arrhythmic drugs.
Data are expressed as mean ± standard deviation, median and interquartile range (IR), number of patients and percentage (%)
aP value: athletes vs. controls
bP value: endurance athletes vs. non-endurance athletes vs. controls
Arrhythmia recurrence evaluated at 3 years after first ablation and final outcome evaluated at 3 years after multiple ablations
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| Recurrence of arrhythmia at 3 years after first ablation | ||||||
| All recurrence (%) | 51.9 | 58.3 | 0.90 | 53.9 | 66.3 | 0.04 |
| Freedom from AF (%) | 48.1 | 41.7 | 0.90 | 46.1 | 33.7 | 0.04 |
| Documented recurrence (%) | 38.7 | 44.6 | 1.00 | 36.7 | 58.5 | 0.01 |
| Time to recurrence (months) | 2 (0–6) | 3 (0–12) | 0.52 | 5 (1–15) | 1 (0–9) | 0.16 |
| Final outcome at 3 years after multiple ablations | ||||||
| All recurrence (%) | 12.7 | 15.5 | 0.61 | 15.7 | 15.2 | 0.88 |
| Freedom from AF (%) | 87.3 | 84.5 | 0.61 | 84.3 | 84.8 | 0.88 |
| Number of procedures | 1.2 ± 0.5 | 1.2 ± 0.4 | 0.62 | 1.1 ± 0.3 | 1.3 ± 0.5 | 0.07 |
| Residual medication after multiple ablations (%) | 65.9 | 62.8 | 0.85 | 55.9 | 74.3 | 0.19 |
| Beta-blockade (%) | 53.7 | 58.9 | 0.71 | 39 | 65.7 | 0.04 |
| Calciumblockade (%) | 17.1 | 20.2 | 0.81 | 18.6 | 22.9 | 0.81 |
| Class I or III AAD (%) | 24.4 | 36.2 | 0.23 | 30.5 | 45.7 | 0.13 |
| . | Controls . | All athletes . | P valuea . | Endurance athletes . | Non-endurance athletes . | P valueb . |
|---|---|---|---|---|---|---|
| Recurrence of arrhythmia at 3 years after first ablation | ||||||
| All recurrence (%) | 51.9 | 58.3 | 0.90 | 53.9 | 66.3 | 0.04 |
| Freedom from AF (%) | 48.1 | 41.7 | 0.90 | 46.1 | 33.7 | 0.04 |
| Documented recurrence (%) | 38.7 | 44.6 | 1.00 | 36.7 | 58.5 | 0.01 |
| Time to recurrence (months) | 2 (0–6) | 3 (0–12) | 0.52 | 5 (1–15) | 1 (0–9) | 0.16 |
| Final outcome at 3 years after multiple ablations | ||||||
| All recurrence (%) | 12.7 | 15.5 | 0.61 | 15.7 | 15.2 | 0.88 |
| Freedom from AF (%) | 87.3 | 84.5 | 0.61 | 84.3 | 84.8 | 0.88 |
| Number of procedures | 1.2 ± 0.5 | 1.2 ± 0.4 | 0.62 | 1.1 ± 0.3 | 1.3 ± 0.5 | 0.07 |
| Residual medication after multiple ablations (%) | 65.9 | 62.8 | 0.85 | 55.9 | 74.3 | 0.19 |
| Beta-blockade (%) | 53.7 | 58.9 | 0.71 | 39 | 65.7 | 0.04 |
| Calciumblockade (%) | 17.1 | 20.2 | 0.81 | 18.6 | 22.9 | 0.81 |
| Class I or III AAD (%) | 24.4 | 36.2 | 0.23 | 30.5 | 45.7 | 0.13 |
AF, atrial fibrillation; and AAD, anti-arrhythmic drugs.
Data are expressed as mean ± standard deviation, median and interquartile range (IR), number of patients and percentage (%)
aP value: athletes vs. controls
bP value: endurance athletes vs. non-endurance athletes vs. controls
Outcome
Evaluated at 3 years after the first ablation procedure, AF recurrence was noted in 58% of all athletes, with documented recurrence in 42% (Table 2). Atrial fibrillation recurrence evaluated at 3 years after all procedures was 15%, thus 85% of athletes showed no recurrence of arrhythmia after all performed ablation procedures (Figure 2). In neither of the analyses, a significant difference in medical treatment after all procedures could be seen, except for the use of beta-blockers, which was significantly higher in non-endurance athletes as compared to endurance athletes (P = 0.04).
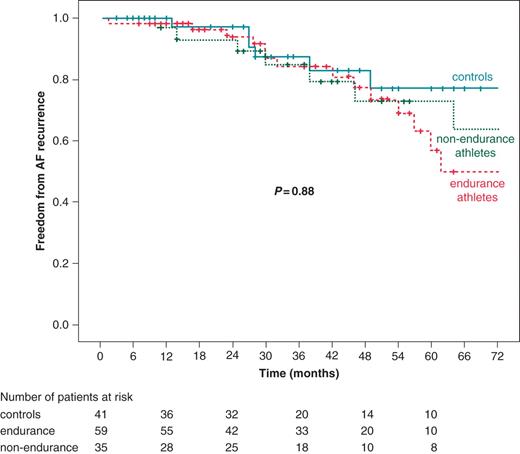
Final outcome after multiple ablations, on or off drugs. AF, atrial fibrillation. P value: log-rank P for 5-year follow-up, endurance athletes vs. non-endurance athletes vs. controls.
Compared with controls, endurance athletes had a similar proportion of total as well as documented AF recurrence after the first ablation procedure. A higher total (P = 0.04) and documented (P = 0.01) recurrence was noted in non-endurance athletes (Table 2, Figures 3A and B). They also tended to have recurrences earlier during follow-up (P = 0.16). However, the proportion of arrhythmia-free patients evaluated at 3 years after repeated ablation procedures was not statistically different for any of the analysed subgroups, revealing a total of 13% of arrhythmia recurrence in the control group compared to 16% in the endurance sports group, and 15% in the non-endurance sports group (P = 0.88, Table 2).

Freedom from atrial fibrillation recurrence after first ablation procedure. (A) All AF recurrences, AF, atrial fibrillation, P value: log-rank P for 5-year follow-up, endurance athletes vs. non-endurance athletes vs. controls. (B) Documented AF recurrences, AF, atrial fibrillation, P value: log-rank P for 5-year follow-up, endurance athletes vs. non-endurance athletes vs. controls.
Univariable and multivariable Cox regression analysis after a singleablation procedure identified no independent predictors for AF recurrence in our ablation population (Table 3). Performing endurance sports and sports burden before and after ablation tended to be (negative) predictors for AF recurrence, albeit not statistically significant.
Univariate Cox regression analysis, predictors for recurrence of atrial fibrillation on or off drugs after a singleablation procedure
| Covariate . | Hazard ratio . | P value . |
|---|---|---|
| Age (years) | 0.987 (0.959–1.016) | 0.39 |
| Male gender | 1.586 (0.832–3.025) | 0.16 |
| Persistent AF | 1.072 (0.533–2.156) | 0.84 |
| AF duration (years) | 1.012 (0.974–1.053) | 0.54 |
| Echocardiography | ||
| LAD | 1.006 (0.972–1.040) | 0.74 |
| LVEDD | 1.029 (0.977–1.084) | 0.28 |
| LVEF | 0.973 (0.941–1.006) | 0.11 |
| AHT | 0.874 (0.513–1.490) | 0.62 |
| Lone AF Sports practice | 1.046 (0.654–1.671) | 0.85 |
| Athlete | 0.858 (0.516–1.427) | 0.56 |
| Sports type | ||
| Non-endurance | 0.999 (0.552–1.807) | 1.00 |
| Endurance | 0.771 (0.457–1.355) | 0.37 |
| Lifetime (hours) | 1.000 (1.000–1.000) | 0.25 |
| Since 14 years of age (hours/week) | 0.976 (0.936–1.017) | 0.25 |
| After first ablation (hours/week) | 0.967 (0.903–1.037) | 0.35 |
| PVI | ||
| Ipsilateral circumferential | 1.758 (0.950–3.253) | 0.07 |
| Additional lines | 1.144 (0.626–2.091) | 0.66 |
| Covariate . | Hazard ratio . | P value . |
|---|---|---|
| Age (years) | 0.987 (0.959–1.016) | 0.39 |
| Male gender | 1.586 (0.832–3.025) | 0.16 |
| Persistent AF | 1.072 (0.533–2.156) | 0.84 |
| AF duration (years) | 1.012 (0.974–1.053) | 0.54 |
| Echocardiography | ||
| LAD | 1.006 (0.972–1.040) | 0.74 |
| LVEDD | 1.029 (0.977–1.084) | 0.28 |
| LVEF | 0.973 (0.941–1.006) | 0.11 |
| AHT | 0.874 (0.513–1.490) | 0.62 |
| Lone AF Sports practice | 1.046 (0.654–1.671) | 0.85 |
| Athlete | 0.858 (0.516–1.427) | 0.56 |
| Sports type | ||
| Non-endurance | 0.999 (0.552–1.807) | 1.00 |
| Endurance | 0.771 (0.457–1.355) | 0.37 |
| Lifetime (hours) | 1.000 (1.000–1.000) | 0.25 |
| Since 14 years of age (hours/week) | 0.976 (0.936–1.017) | 0.25 |
| After first ablation (hours/week) | 0.967 (0.903–1.037) | 0.35 |
| PVI | ||
| Ipsilateral circumferential | 1.758 (0.950–3.253) | 0.07 |
| Additional lines | 1.144 (0.626–2.091) | 0.66 |
AF, atrial fibrillation; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; SHD, structural heart disease; AHT, arterial hypertension; and PVI, pulmonary vein isolation.
Data are expressed as hazard ratio and confidence interval (CI)
Univariate Cox regression analysis, predictors for recurrence of atrial fibrillation on or off drugs after a singleablation procedure
| Covariate . | Hazard ratio . | P value . |
|---|---|---|
| Age (years) | 0.987 (0.959–1.016) | 0.39 |
| Male gender | 1.586 (0.832–3.025) | 0.16 |
| Persistent AF | 1.072 (0.533–2.156) | 0.84 |
| AF duration (years) | 1.012 (0.974–1.053) | 0.54 |
| Echocardiography | ||
| LAD | 1.006 (0.972–1.040) | 0.74 |
| LVEDD | 1.029 (0.977–1.084) | 0.28 |
| LVEF | 0.973 (0.941–1.006) | 0.11 |
| AHT | 0.874 (0.513–1.490) | 0.62 |
| Lone AF Sports practice | 1.046 (0.654–1.671) | 0.85 |
| Athlete | 0.858 (0.516–1.427) | 0.56 |
| Sports type | ||
| Non-endurance | 0.999 (0.552–1.807) | 1.00 |
| Endurance | 0.771 (0.457–1.355) | 0.37 |
| Lifetime (hours) | 1.000 (1.000–1.000) | 0.25 |
| Since 14 years of age (hours/week) | 0.976 (0.936–1.017) | 0.25 |
| After first ablation (hours/week) | 0.967 (0.903–1.037) | 0.35 |
| PVI | ||
| Ipsilateral circumferential | 1.758 (0.950–3.253) | 0.07 |
| Additional lines | 1.144 (0.626–2.091) | 0.66 |
| Covariate . | Hazard ratio . | P value . |
|---|---|---|
| Age (years) | 0.987 (0.959–1.016) | 0.39 |
| Male gender | 1.586 (0.832–3.025) | 0.16 |
| Persistent AF | 1.072 (0.533–2.156) | 0.84 |
| AF duration (years) | 1.012 (0.974–1.053) | 0.54 |
| Echocardiography | ||
| LAD | 1.006 (0.972–1.040) | 0.74 |
| LVEDD | 1.029 (0.977–1.084) | 0.28 |
| LVEF | 0.973 (0.941–1.006) | 0.11 |
| AHT | 0.874 (0.513–1.490) | 0.62 |
| Lone AF Sports practice | 1.046 (0.654–1.671) | 0.85 |
| Athlete | 0.858 (0.516–1.427) | 0.56 |
| Sports type | ||
| Non-endurance | 0.999 (0.552–1.807) | 1.00 |
| Endurance | 0.771 (0.457–1.355) | 0.37 |
| Lifetime (hours) | 1.000 (1.000–1.000) | 0.25 |
| Since 14 years of age (hours/week) | 0.976 (0.936–1.017) | 0.25 |
| After first ablation (hours/week) | 0.967 (0.903–1.037) | 0.35 |
| PVI | ||
| Ipsilateral circumferential | 1.758 (0.950–3.253) | 0.07 |
| Additional lines | 1.144 (0.626–2.091) | 0.66 |
AF, atrial fibrillation; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; SHD, structural heart disease; AHT, arterial hypertension; and PVI, pulmonary vein isolation.
Data are expressed as hazard ratio and confidence interval (CI)
Discussion
Pulmonary vein isolation has been established as an effective treatment for AF, with success rates ranging from 30 to 85%.24 Until now, it was unclear as to whether radiofrequency catheter ablation is as effective in athletes at it is in a general population. Previously, a small study by Furlanello et al.21 demonstrated high efficacy of repeated ablation procedures in a population of elite athletes. However, the athletes underwent an average of 2.3 ± 0.4 procedures, even when asymptomatic, and were not compared with controls. Hence, the study did not provide evidence with regard to whether outcome was influenced by sports history. Calvo et al.25 reported high efficacy of circumferential pulmonary vein ablation in endurance athletes with lone AF, but there is a confounding presence of endurance athletes in the control group due to the unusual classification of subgroups based on lone AF.
Our present data compare AF recurrence after a first ablation procedure between endurance and non-endurance athletes and a control group. Rate of AF recurrence after a singleablation procedure was similar for endurance athletes compared with controls. Equal results were obtained when only documented AF recurrence was considered. Somewhat surprisingly, a higher AF recurrence for both total and documented arrhythmia was noted in athletes performing non-endurance sports. Recurrences in non-endurance athletes seem to occur especially early during follow-up.
Due to the inclusion of only paroxysmal forms of AF and exclusion of SHD, our study population included a high proportion of athletes, confirming endurance sports as the likely etiologic factor.12 Our data indicate that although endurance sports may promote AF, it does not affect the outcome of AF ablation in our study cohort, i.e. the ablation procedure modified the arrhythmia mechanism in a similar manner. This could be attributed to similar and homogenous selection criteria for PVI in our centre, including only patients with proven focally-induced AF (documentation of AF induced by abundant atrial ectopy presumably from the PV) and excluding patients with permanent AF or major structural atrial changes. Our results could therefore be influenced by this selection-bias. It is unclear as to whether a difference in AF recurrence would be present if no selection was made before admitting endurance athletes for ablation. We have no obvious explanation for non-endurance athletes having a higher AF recurrence rate, and therefore results of the sub-analysis should be interpreted with caution.
We found no evidence for cardiac structural differences between endurance or non-endurance athletes and controls, irrespective of the type of sports practice and the duration or intensity of sports activities. Nevertheless, the number of lifetime hours of sports activities was significantly different. Echocardiographic LA diameter was not different between athletes and controls, even though there were more female patients with a significantly lower atrial diameter in the control group. Several explanations could be proposed: (i) the control group comprises a slightly higher proportion of patients with hypertension, although not statistically significant, (ii) sports practice could result in a different substrate for AF due to cellular and interstitial microstructural changes without macroscopic atrial dilatation, as has been described in certain animal models,26 (iii) LA dilatation could originate secondary to AF itself, (iv) values for LA diameter in the endurance group could be influenced by selection-bias as only endurance athletes with focally-induced AF and smaller atria were admitted for ablation, whereas in a normal athlete population a larger atrial size would be expected as part of physiologic adaptation to exercise conditioning,27 and (v) groups were too small to detect a difference. Further studies are needed to elucidate these findings.
Earlier studies reported LA diameter, LVEF, long-standing AF, non-paroxysmal AF, SHD, age, and hypertension as potential predictors for AF recurrence.25,28–31 Applying Cox proportional hazards regression analysis to our data, none of these variables could be identified as an independent predictor for AF recurrence. A recent meta-analysis among patients with approximately normal LA diameter showed moderate level of evidence that LA size was not an independent predictor for arrhythmia recurrence,28 which is consistent with our findings. Type of AF was not a predictor for recurrence either, probably also because patients with permanent or long-standing persistent AF were ‘a priori’ not considered for ablation in our centre. It is remarkable that neither the burden nor intensity of sports practice were predictors for AF recurrence. Also, our data could not demonstrate a relation between sports continuation after ablation and AF recurrence. Therefore, at least in athletes with evidence of focally induced AF from pulmonary vein origin, ablation seems to be an effective treatment option.
Limitations
One limitation of our study derives from the follow-up method whereby asymptomatic arrhythmias between follow-up visits could have been missed. This limitation seems unlikely to be significant, since all patients were highly symptomatic before the first ablation procedure was performed and would be likely to experience similar symptoms. Moreover, our analysis is not different for total compared to documented AF recurrence. Another limitation is that the control group includes a higher percentage of female patients. Comparison between both male and female control patients was statistically identical for all variables except for LA diameter. This would, however, favour a positive outcome in control patients, thus being more likely to exaggerate a possible difference between controls and athletes. Finally, the number of patients included in our study is still relatively small. Therefore, results have to be interpreted with caution, especially concerning the differences between endurance and non-endurance athletes. No definite conclusions can be drawn in this regard and further prospective randomized controlled studies with a larger number of patients are required.
Conclusion
Our data indicate that the outcome of PVI for AF in endurance athletes seems to be no different than in other patients, when selection occurs based on focal induction of AF and absence of large atrial size or permanent AF. These data add to available evidence that there is no reason to withhold ablation therapy from such athletes with AF.
Conflict of interest:None declared.



