-
PDF
- Split View
-
Views
-
Cite
Cite
Marketa Kozeluhova, Petr Peichl, Robert Cihak, Dan Wichterle, Vlastimil Vancura, Jan Bytesnik, Josef Kautzner, Catheter ablation of electrical storm in patients with structural heart disease, EP Europace, Volume 13, Issue 1, January 2011, Pages 109–113, https://doi.org/10.1093/europace/euq364
Close - Share Icon Share
Abstract
Electrical storm (ES) adversely affects prognosis of patients and may become a life-threatening event. Catheter ablation (CA) has been proposed for the treatment of ES. Our goal was to evaluate the efficacy of CA ablation both in acute and long-term suppression of ES.
Fifty consecutive patients with coronary artery disease (38), idiopathic dilated cardiomyopathy (5), arrhythmogenic right ventricular cardiomyopathy (6), and/or with combined aetiology (1) underwent CA for ES. Mean left ventricular ejection fraction (LVEF) was 29 ± 11%. All patients underwent electroanatomical mapping, and CA was performed to abolish all inducible ventricular arrhythmias. The ES was suppressed by CA in 84% of patients. During the follow-up of 18 ± 16 months, 24 patients had no recurrences of any ventricular tachycardia (VT; 48%). Repeated procedure was necessary to suppress the recurrent ES in 13 cases (26%). Statistical analysis revealed that low LVEF (22 ± 3 vs. 31 ± 12%; P < 0.001), increased LVend-diastolic diameter (72 ± 9.1vs. 64 ± 8.9 mm; P = 0.0135), and renal insufficiency (P < 0.001) were the univariate predictors of early mortality or necessity for heart transplantation. Recurrence of ES despite previous CA procedure was associated with a higher risk of death or heart transplant during follow-up (P < 0.05).
Catheter ablation is effective in acute suppression of ES and often represents a life-saving therapy. In the long term, it prevents recurrences of any VT in about half of the treated patients.
Introduction
Electrical storm (ES) could be characterized as a period of severe cardiac electrical instability manifested by recurrent ventricular arrhythmias. Specifically, ES is defined as the occurrence of three or more distinct episodes of ventricular tachycardia (VT) and/or ventricular fibrillation (VF) within a 24 h period. In patients with an implantable cardioverter-defibrillator (ICD), it usually leads to frequent ICD shocks. Therefore, ES represents a life-threatening condition that adversely influences short- and long-term prognosis of affected patients.1–4 The incidence of ES varies between 10 and 40% in patients with secondary preventive indication for ICD,1,5,6 and it appears to be lower in subjects with primary indication. Recently, catheter ablation (CA) has been suggested as a method of choice in management of ES.7,8 However, limited experience is available, especially on long-term outcome of this strategy. The aim of our study was to evaluate the efficacy of CA both in acute and long-term suppression of ES.
Methods
Patient population
A total of 50 consecutive patients (8 females; mean age of 59 ± 13 years) undergoing CA for ES between 2004 and 2008 in our institution were enrolled and retrospectively analysed. Electrical storm was defined as the occurrence of three or more episodes of VT/VF separated by >5 min during a 24 h period, each resulting in ICD intervention. Patients were selected from a total number of 411 patients undergoing ablation for VT/VF during the same period. The underlying diagnoses were coronary artery disease (CAD) in 38 patients (76%), idiopathic dilated cardiomyopathy (IDCM) in 5 patients (10%), arrhythmogenic right ventricular cardiomyopathy (ARVC) in 6 patients (12%), and 1 patient had combination of CAD and IDCM (2%). In patients with CAD, the mean interval between index myocardial infarction and ES reached 5.4 ± 6.1 years. The mean left ventricular ejection fraction (LVEF) was 29 ± 11%. The comorbidities that could influence total mortality in our study cohort included diabetes mellitus in 15 patients (30%), atrial fibrillation in 16 patients (32%), renal insufficiency (creatinine ≥1.5 mg/dL) in 23 patients (46%), peripheral arterial occlusive disease in 23 (46%), and stroke in 8 patients (16%). The majority of patients was symptomatic with heart failure (the mean NYHA class of 2.4 ± 0.8) and had left ventricular (LV) dilatation (end-diastolic diameter of the left ventricle of 65.8 ± 9.2 mm). Three patients were enlisted for heart transplantation before the ablation procedure.
Electrical storm occurred despite chronic antiarrhythmic therapy comprising amiodarone in 36%, beta-blockers in 84%, and sotalol in 2% of subjects. Before the occurrence of ES, 38 patients (76%) were implanted with an ICD due to both secondary (33 patients; 66%) and primary (5 patients; 10%) preventive indications. The mean time interval between implantation and ES occurrence reached 29 ± 33 months. Coronary angiography was performed in 17 of 50 patients (34%) in whom myocardial ischaemia was suspected. In four of them, percutaneous coronary intervention was performed with no effect on arrhythmia occurrence corresponding to the fact that the majority of ES was caused by monomorphic VTs. In nine patients, ES was caused by runs of polymorphic VT/VF that were triggered by monomorphic ventricular ectopy. Catheter ablation in this series of patients was reported previously.9 The mean interval between the beginning of ES and CA was 5.2 ± 7.0 days. Electrical storm could not be suppressed by antiarrhythmic drug therapy. Specifically, all subjects received beta-blockers, 21 patients were treated by i.v. amiodarone (1.2 g/day; 42%), and 5 patients were treated by lidocaine infusion (10%). Patients received on an average 14.6 ± 16.7 shocks per day during ES. In 10 patients, ES led to haemodynamic deterioration that necessitated infusion of pressor agents (20%). A total number of 11 patients were deeply sedated and mechanically ventilated (Table 1).
| Age, years (mean ± SD) | 59 ± 13 |
| Gender, M/F | 42/8 |
| LV ejection fraction, % (mean ± SD) | 29 ± 11 |
| NYHA class (mean ± SD) | 2.4 ± 0.8 |
| End-diastolic diameter of LV, mm (mean ± SD) | 65.8 ± 9.2 |
| Underlying heart disease, n (%) | |
| Coronary artery disease | 38 (76) |
| Idiopathic dilated cardiomyopathy | 5 (10) |
| Combination | 1 (2) |
| Arrhytmogenic right ventricular cardiomyopathy | 6 (12) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 15 (30) |
| Atrial fibrillation | 16 (32) |
| Renal insufficiency | 23 (46) |
| Peripheral arterial occlusive disease | 23 (46) |
| Prior stroke | 8 (16) |
| Chronic medication (%) | |
| Amiodarone | 36 |
| Beta-blockers | 84 |
| Sotalol | 2 |
| ICD in primary prevention, n (%) | 5 (10) |
| ICD in secondary prevention, n (%) | 33 (66) |
| Electrical shocks per patient per day, n(mean ± SD) | 14.6 ± 16.7 |
| Time from implantation to ES, months (mean ± SD) | 29 ± 33 |
| Age, years (mean ± SD) | 59 ± 13 |
| Gender, M/F | 42/8 |
| LV ejection fraction, % (mean ± SD) | 29 ± 11 |
| NYHA class (mean ± SD) | 2.4 ± 0.8 |
| End-diastolic diameter of LV, mm (mean ± SD) | 65.8 ± 9.2 |
| Underlying heart disease, n (%) | |
| Coronary artery disease | 38 (76) |
| Idiopathic dilated cardiomyopathy | 5 (10) |
| Combination | 1 (2) |
| Arrhytmogenic right ventricular cardiomyopathy | 6 (12) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 15 (30) |
| Atrial fibrillation | 16 (32) |
| Renal insufficiency | 23 (46) |
| Peripheral arterial occlusive disease | 23 (46) |
| Prior stroke | 8 (16) |
| Chronic medication (%) | |
| Amiodarone | 36 |
| Beta-blockers | 84 |
| Sotalol | 2 |
| ICD in primary prevention, n (%) | 5 (10) |
| ICD in secondary prevention, n (%) | 33 (66) |
| Electrical shocks per patient per day, n(mean ± SD) | 14.6 ± 16.7 |
| Time from implantation to ES, months (mean ± SD) | 29 ± 33 |
ICD, implantable cardioverter-defibrillator.
| Age, years (mean ± SD) | 59 ± 13 |
| Gender, M/F | 42/8 |
| LV ejection fraction, % (mean ± SD) | 29 ± 11 |
| NYHA class (mean ± SD) | 2.4 ± 0.8 |
| End-diastolic diameter of LV, mm (mean ± SD) | 65.8 ± 9.2 |
| Underlying heart disease, n (%) | |
| Coronary artery disease | 38 (76) |
| Idiopathic dilated cardiomyopathy | 5 (10) |
| Combination | 1 (2) |
| Arrhytmogenic right ventricular cardiomyopathy | 6 (12) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 15 (30) |
| Atrial fibrillation | 16 (32) |
| Renal insufficiency | 23 (46) |
| Peripheral arterial occlusive disease | 23 (46) |
| Prior stroke | 8 (16) |
| Chronic medication (%) | |
| Amiodarone | 36 |
| Beta-blockers | 84 |
| Sotalol | 2 |
| ICD in primary prevention, n (%) | 5 (10) |
| ICD in secondary prevention, n (%) | 33 (66) |
| Electrical shocks per patient per day, n(mean ± SD) | 14.6 ± 16.7 |
| Time from implantation to ES, months (mean ± SD) | 29 ± 33 |
| Age, years (mean ± SD) | 59 ± 13 |
| Gender, M/F | 42/8 |
| LV ejection fraction, % (mean ± SD) | 29 ± 11 |
| NYHA class (mean ± SD) | 2.4 ± 0.8 |
| End-diastolic diameter of LV, mm (mean ± SD) | 65.8 ± 9.2 |
| Underlying heart disease, n (%) | |
| Coronary artery disease | 38 (76) |
| Idiopathic dilated cardiomyopathy | 5 (10) |
| Combination | 1 (2) |
| Arrhytmogenic right ventricular cardiomyopathy | 6 (12) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 15 (30) |
| Atrial fibrillation | 16 (32) |
| Renal insufficiency | 23 (46) |
| Peripheral arterial occlusive disease | 23 (46) |
| Prior stroke | 8 (16) |
| Chronic medication (%) | |
| Amiodarone | 36 |
| Beta-blockers | 84 |
| Sotalol | 2 |
| ICD in primary prevention, n (%) | 5 (10) |
| ICD in secondary prevention, n (%) | 33 (66) |
| Electrical shocks per patient per day, n(mean ± SD) | 14.6 ± 16.7 |
| Time from implantation to ES, months (mean ± SD) | 29 ± 33 |
ICD, implantable cardioverter-defibrillator.
Mapping and ablation strategy
The procedure was performed predominantly under local anaesthesia, except for 11 patients who were on mechanical ventilation due to frequent repeated arrhythmias and haemodynamical instability. A quadripolar diagnostic catheter was introduced via femoral vein into the right ventricular apex. If the VT was not incessant, the programmed stimulation protocol from the two sites and up to three extrastimuli was applied to induce clinical VT and ascertained to determine the ease of inducibility for subsequent testing. The left ventricle was accessed retrogradely across the aortic valve in all but one patient in whom the transseptal approach was used. The mapping was performed using an electroanatomical mapping system CARTO XP (Biosense Webster, Diamond Bar, CA, USA) in 48 patients or EnSite NavX (St Jude Medical, St Paul, MN, USA) in 2 patients, respectively. For CA, a 3.5 mm, saline-irrigated tip ablation catheter (Navistar Thermocool, Biosense Webster or Celsius Thermocool, Biosense Webster) was used. Simultaneous recordings of ventricular electrograms (bandpass filtered 50–500 Hz) and 12-lead surface electrocardiogram (ECG) were stored digitally (Prucka Cardiolab, GE Medical Systems, Milwaukee, WI, USA).
A 5000 IU bolus of heparin was administered intravenously, followed by additional boluses to maintain the activated clotting time at above 250 s, as needed. Except in the patients with incessant VT, initial mapping was performed during sinus rhythm. A three-dimensional voltage map of the left or right ventricle was obtained, and the scar area was identified as a region of electrograms <1.5 mV in amplitude.10 Areas of dense scar were annotated in grey in the map by dedicated software when no local bipolar pacing capture was observed at 10 mA.11 Regions with late potentials and zones of slow conduction were annotated using colour tags. Pace mapping was used in these areas to identify the critical components of the circuit in greater detail. Whenever haemodynamically stable VT was induced or occurred spontaneously, limited remap during arrhythmia was used with additional annotations of sites of entrainment pacing and/or mid-diastolic potentials, or these key points were annotated in the voltage map obtained during sinus rhythm. In subjects with ES due to polymorphic VT/VF, triggering ectopy was mapped using activation sequence mapping and annotation of sites of interest in limited electroanatomical map. In case of induction of monomorphic VT after ablation of focal trigger, the above method was used to identify substrate for such arrhythmia.
Radiofrequency (RF) lesions were created either during sinus rhythm within the region identified to be critical for the sustenance of clinical or other inducible VTs, and/or during haemodynamically tolerated VT within the central to exit zones of the circuit as identified by entrainment pacing. In patients with polymorphic VT/VF, RF current was applied focally in the site of the earliest ventricular activation (predominantly in His-Purkinje network). Ablation CA was performed in the power control mode with the mean power set up to 35 W, temperature of 43°C, and a flow of 30 mL/min. Radiofrequency energy was applied for a maximum of 60 s per target site. In four patients, clinical VT could not be abolished from endocardium and the epicardial approach was employed.12 As for the endocardial mapping, the isthmus of slow conduction was identified by activation and entrainment mapping in the case of tolerated VT, or based on pace mapping during sinus rhythm. Coronary angiography was performed before epicardial RF application in all patients to avoid damage to the epicardial coronary vessels.
After the modification of VT substrate with series of RF lesions, programmed stimulation was performed in an attempt to reinitiate VT. If another VT was induced, either additional mapping during the arrhythmia, if tolerated, or additional pace mapping in sinus rhythm was performed. The procedure ended if no monomorphic VT was inducible and/or when the clinical VT could not be ablated. In patients with runs of polymorphic VT/VF triggered by monomorphic extrasystoles, the primary endpoint of the ablation procedure was abolition of the triggering ventricular premature beats.
Patients were monitored after the procedure for several days, and all patients without previous ICD implant were implanted with an ICD before discharge. Antiaggregation or anticoagulation therapy was initiated based on the estimated risk of thromboembolism. Amiodarone treatment was initiated in 13 patients (26%).
Statistical analysis
Continuous variables are expressed as the mean value ± SD and compared using Student's t-test and Mann–Whitney test. Discrete variables were compared using χ2 test with Yates’ correction. Statistical significance was considered at P < 0.05.
For creating a model for prediction of the group with deceased patients, the multivariable logistic regression was used. Variables included in the multivariable model were sex, age, LVEF, end-diastolic diameter of the LV, coronary disease, diabetes mellitus, renal insufficiency, atrial fibrillation, peripheral arterial occlusive disease, and stroke. Survivorship curves were estimated by Kaplan–Meier analysis, their conformity was assessed by log-rank test.
Results and follow-up
Out of 50 patients, VT was induced by programmed electrical stimulation in 35 (70%) and was incessant in additional 15 (22%). During the programmed stimulation, a mean number of 2.8 ± 1.8 VTs were induced per patient. Clinical tachycardia based on morphological analysis of stored ICD electrograms and/or comparison with recorded 12-lead ECG was observed during the procedure in 26 cases. Ventricular tachycardia was not haemodynamically tolerated in 22 of them. Monomorphic VT was induced during 40 procedures, polymorphic VT during 5, and VF during 7 procedures. Mapping of the LV was performed in 43 patients, whereas solitary right ventricular mapping was used in 4 cases. In three subjects, both ventricles were mapped. Total procedure time was 197 ± 51 min with fluoroscopy time of 15.8 ± 15.4 min. The number of RF applications was 35 ± 22 per procedure, and the total time of one application reached 1724 ± 1102 s. No significant complications occurred during the procedure (Table 2).
| Induced VT by PES, n (%) of patients | 35 (70) |
| Incessant VT, n (%) of patients | 15 (22) |
| Mean no of VT induced per patient, (mean ± SD) | 2.8 ± 1.8 |
| Clinical tachycardia induced (n) | 26 |
| Haemodynamically, untolerated VT induced (n) | 22 |
| Monomorpfic VT induced (n) | 40 |
| Polymoprhic VT induced (n) | 5 |
| Ventricular fibrillation induced (n) | 7 |
| Mapping of LV, n (%) | 43 (86) |
| Mapping of RV, n (%) | 4 (8) |
| Mapping of RV + LV, n (%) | 3 (6) |
| Total procedure time, min (mean ± SD) | 197 ± 51 |
| Fluoroscopy time, min (mean ± SD) | 15.8 ± 15.4 |
| Radiofrequency applications per procedure (n) | 35 ± 22 |
| Total time of one application (s) | 1724 ± 1102 |
| Induced VT by PES, n (%) of patients | 35 (70) |
| Incessant VT, n (%) of patients | 15 (22) |
| Mean no of VT induced per patient, (mean ± SD) | 2.8 ± 1.8 |
| Clinical tachycardia induced (n) | 26 |
| Haemodynamically, untolerated VT induced (n) | 22 |
| Monomorpfic VT induced (n) | 40 |
| Polymoprhic VT induced (n) | 5 |
| Ventricular fibrillation induced (n) | 7 |
| Mapping of LV, n (%) | 43 (86) |
| Mapping of RV, n (%) | 4 (8) |
| Mapping of RV + LV, n (%) | 3 (6) |
| Total procedure time, min (mean ± SD) | 197 ± 51 |
| Fluoroscopy time, min (mean ± SD) | 15.8 ± 15.4 |
| Radiofrequency applications per procedure (n) | 35 ± 22 |
| Total time of one application (s) | 1724 ± 1102 |
PES, programmed electrical stimulation; VT, ventricular tachycardia.
| Induced VT by PES, n (%) of patients | 35 (70) |
| Incessant VT, n (%) of patients | 15 (22) |
| Mean no of VT induced per patient, (mean ± SD) | 2.8 ± 1.8 |
| Clinical tachycardia induced (n) | 26 |
| Haemodynamically, untolerated VT induced (n) | 22 |
| Monomorpfic VT induced (n) | 40 |
| Polymoprhic VT induced (n) | 5 |
| Ventricular fibrillation induced (n) | 7 |
| Mapping of LV, n (%) | 43 (86) |
| Mapping of RV, n (%) | 4 (8) |
| Mapping of RV + LV, n (%) | 3 (6) |
| Total procedure time, min (mean ± SD) | 197 ± 51 |
| Fluoroscopy time, min (mean ± SD) | 15.8 ± 15.4 |
| Radiofrequency applications per procedure (n) | 35 ± 22 |
| Total time of one application (s) | 1724 ± 1102 |
| Induced VT by PES, n (%) of patients | 35 (70) |
| Incessant VT, n (%) of patients | 15 (22) |
| Mean no of VT induced per patient, (mean ± SD) | 2.8 ± 1.8 |
| Clinical tachycardia induced (n) | 26 |
| Haemodynamically, untolerated VT induced (n) | 22 |
| Monomorpfic VT induced (n) | 40 |
| Polymoprhic VT induced (n) | 5 |
| Ventricular fibrillation induced (n) | 7 |
| Mapping of LV, n (%) | 43 (86) |
| Mapping of RV, n (%) | 4 (8) |
| Mapping of RV + LV, n (%) | 3 (6) |
| Total procedure time, min (mean ± SD) | 197 ± 51 |
| Fluoroscopy time, min (mean ± SD) | 15.8 ± 15.4 |
| Radiofrequency applications per procedure (n) | 35 ± 22 |
| Total time of one application (s) | 1724 ± 1102 |
PES, programmed electrical stimulation; VT, ventricular tachycardia.
In 22 patients (44%), there was no inducible VT at the end of the procedure (15 patients with CAD, 6 ones with ARVC, and 1 with IDCM). Non-clinical VT only was induced in other 20 patients (40%). Four patients were not tested due to haemodynamical instability after CA of incessant tachycardia.
In six patients with recurrent ES despite of CA, the rhythm was stabilized either by implantation of ventricular assist device (Thoratec Corporation, Pleasanton, CA, USA) as a bridge to the heart transplant (n = 2), by direct orthotopic heart transplant (n = 2), by surgical resection of the LV aneurysm (n = 1), and/or by urgent surgical revascularization (n = 1).
During the follow-up of 18 ± 16 months, 24 patients had no recurrences of any VT (48%). In contrast, repeated procedure was necessary to suppress the ES in 13 cases (26%), and the ES was suppressed after up to three procedures in 84% of all subjects. In the remaining seven patients, the recurrence of VT was recorded and terminated either by antitachycardia pacing (five patients) or by ICD shock (two patients). However, these patients were not scheduled for reablation.
A total number of 14 patients died during the follow-up (28%). Twelve of them died of progression of heart failure (three died within 1 week after the ablation, the LVEF of these patients was 25%, they received 985 ± 6 mL of saline during CA, and 35 ± 4.5 RF current applications were delivered). Only two subjects deceased due to recurrences of ES. Of note, all these events occurred within the first 6 months after the procedure (Figure 1).
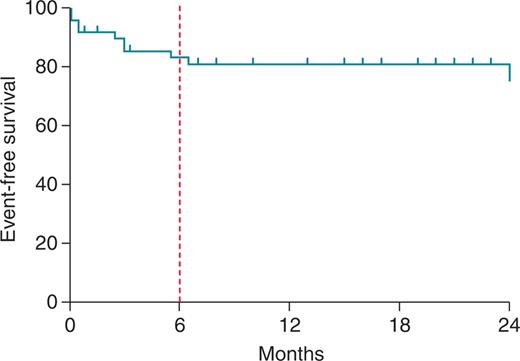
Survival free from death and heart transplant after the first ablation procedure displayed by Kaplan–Meier curve. Note the high event rate observed within the first 6 months.
Statistical analysis revealed that low LVEF (22 ± 3 vs. 31 ± 12%; P < 0.001), increased LVend-diastolic diameter (72 ± 9.1vs. 64 ± 8.9 mm; P = 0.0135), and renal insufficiency (P< 0.001) were the predictors of early mortality or necessity for heart transplantation. Additionally, recurrence of ES, but not VT, after previous CA procedure was associated with a higher risk of death or heart transplant during follow-up (P < 0.05; Figure 2).
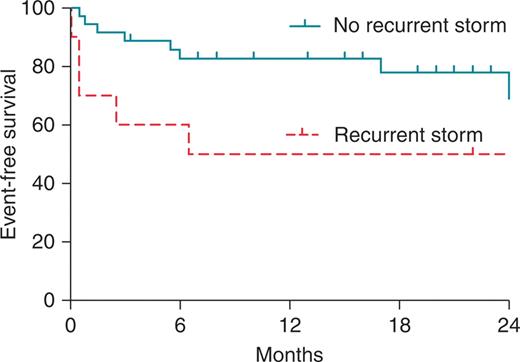
Impact of recurrent storm on the survival free from transplant and death.
Interestingly, non-inducibility of VT at the end of the procedure was not found predictive of the recurrence of ES or VT, risk of death or necessity of heart transplant. The multivariate predictors of early mortality or necessity for heart transplantation included LV end-diastolic diameter [OR: 1.18; 95% CI: (1.03; 1.35)], atrial fibrillation [OR: 8.89; 95% CI:(1.0; 83.8)], and renal insufficiency [OR: 36.8; 95% CI: (1.64; 827)].
Discussion
The main findings of this study can be summarized as follows: (i) CA is effective in suppression of ES in 84% of cases; however, repeated procedures were necessary in 13 cases of the patients. (ii) Severely depressed LVEF, a higher degree of LV dilation, renal insufficiency, and ES recurrence after previous CA procedure were identified as predictors of adverse outcome within the first 6 months after the procedure. (iii) Testing of inducibility of the VT at the end of the study was not found predictive of ES or VT recurrences during follow-up.
Electrical storm in patients with structural heart disease occurs relatively frequently and unpredictably. It has been recognized as an important predictor of subsequent cardiac death independently of ejection fraction and other prognostic variables.1–4 Electrical stormdirectly affects patient prognosis by progressive deterioration of cardiac function from prolonged low-output states, direct cell injury caused by frequent shocks,13 and/or an adverse haemodynamical effect of antiarrhythmic medication and thus, CA may become a life-saving procedure for these patients. Our data are in concordance with these results as we have demonstrated that if CA failed to prevent ES recurrence, patients had worse prognosis. Thus, it is reasonable to assume that CA should be attempted early in all eligible patients to prevent recurrence of ES.
However, in a subset of patients, the magnitude of the cardiac damage is apparently too extensive and chronic heart failure far advanced, so that the suppression of VTs does not guarantee a good prognosis and does not prevent death from progression of heart failure. In our analysis, the low LVEF, dilated LV, renal insufficiency, and recurrence of ES despite of CA of ES indicated early death or necessity of heart transplant. In such patients, other non-pharmacological approaches may be considered including arrhythmia surgery14 or mechanical support.15 The latter may be used as a bridge to cardiac transplant or only temporarily for stabilization period with subsequent CA with the support of ventricular assist device.
Catheter ablation for management of ES has been evaluated in detail by Carbucicchio et al.7 The authors included 95 patients with structural heart disease and ES refractory to antiarrhythmic medication who underwent CA. After one to three procedures, suppression of clinical VTs was achieved in 89% of patients. At median follow-up of 22 months, 92% of patients were free of ES. Importantly, in patients, where at least one clinical VT could not be abolished, ES recurred in 80% and cardiac mortality was significantly higher when compared with the patients after successful CA. In contrast to Carbucicchio et al.7 we have not found the inducibility of VTs at the end of the procedure predictive of survival, recurrence of ES, or VT. This may be explained by different reasons. It could be due to the lower number of subjects in our cohort or due to limited programmed electrical stimulation after the procedure (up to two extrastimuli) due to haemodynamical instability of many patients. Our patient cohort was also different from that reported by Carbucicchio et al.7 who studied only patients with monomorphic VTs. In contrast, we included also subjects with polymorphic VT/VF. Therefore, this patient population reflected a more real life scenario. Our data also suggest that in some patients, ES is rather an epiphenomenon of progression of heart failure and even successful CA may not influence their long-term prognosis. This may be in line with observations on a subpopulation with ES from the AVID trial in which the risk of death was greatest 3 months after ES and diminished beyond this time.16
Study limitation
One limitation could be relatively small sample size. However, there is a lack of data about the efficacy of CA in ES and even analysis of this number of cases may provide valuable information. Another limitation is that our findings are based on a retrospective analysis. Since all our patients underwent CA, we could not compare the outcome CA for ES with the outcome of conservative treatment. In the three patients who died due to progressive heart failure within 1 week after the procedure, a volume overload due to the use of the open irrigation catheter could contribute to deterioration of their heart failure.
Conclusions
Catheter ablation is effective in suppressing of ES in patients with structural heart disease, and in selected cases, it may represent a life-saving therapy. Therefore, it should be applied early in the course of ES to prevent adverse haemodynamical consequences of repeated arrhythmias. In long term, CA prevents recurrences of any VT in almost half of the treated population. The patients with very low LVEF, dilated LV, renal insufficiency, and recurrent ES despite the ablation are at high risk of death due to progression of heart failure. This study should support and encourage multicentre studies on the use of CA in treatment of ES.
Conflict of interest: J.K. has disclosed that he has received consulting fees/honoraria from Biosense Webster, Boston Scientific, Hansen Medical, and St Jude Medical. The other authors report no conflicts.
Funding
This study was partly supported by the Czech Ministry of Education, Youth and Sports (MSMT) Project Nr. 1M0510.



