-
PDF
- Split View
-
Views
-
Cite
Cite
Thorsten Lewalter, Christian Weiss, Sebastian Spencker, Werner Jung, Wilhelm Haverkamp, Stephan Willems, Thomas Deneke, Josef Kautzner, Michael Wiedemann, Jürgen Siebels, Heinz Friedrich Pitschner, Ellen Hoffmann, Gerd Hindricks, Markus Zabel, Ernst Vester, Harald Schwacke, Erica Mittmann-Braun, Lars Lickfett, Sabine Hoffmeister, Jochen Proff, Christian Mewis, Wolfgang Bauer, for the AURUM 8 Study Investigators, Gold vs. platinum–iridium tip catheter for cavotricuspid isthmus ablation: the AURUM 8 study, EP Europace, Volume 13, Issue 1, January 2011, Pages 102–108, https://doi.org/10.1093/europace/euq339
Close - Share Icon Share
Abstract
Gold electrodes have the theoretical advantage of creating bigger lesions than platinum–iridium (Pt–Ir) electrodes. We performed a prospective randomized study to compare the clinical efficacy of standard 8 mm Pt–Ir tip catheter (control) and 8 mm gold-tip catheters in the ablation of the cavotricuspid isthmus (CTI)-dependent atrial flutter.
A total of 463 patients undergoing CTI ablation in 19 clinical centres were randomized to receive the treatment by gold-tip or control catheter. The primary endpoint was cumulative radiofrequency (RF) application duration until achieving bidirectional CTI block. It did not differ significantly for the two catheters. The gold-tip catheter was, however, associated with a higher ablation success rate (94.3 vs. 89.0%, P = 0.042) and a substantially lower incidence of char and coagulum formation (4.8 vs. 37.9%, P < 0.001), which required exchange of 1 gold-tip (0.4%) and 10 control catheters (4.6%, P = 0.005). The gold-tip catheter delivered more mean power (52 ± 12 W) than the control catheter (48 ± 13 W, P < 0.001). Both mean and maximum temperatures measured by the thermocouple integrated in the catheter tip were statistically significantly lower in the gold (mean: 53.2 ± 4.7°C, max: 68.7 ± 6.6°C) than in the control catheter (54.3 ± 5.2 and 70.2 ± 7.0°C, respectively, P < 0.05). Fluoroscopy time, procedure duration, procedural-related complications, and arrhythmia recurrence during 6 months of follow-up did not differ between the two catheters.
Owing to a higher primary ablation success rate and reduced incidence of char/coagulum formation, gold may be preferred over Pt–Ir as electrode material for 8 mm tip catheters for CTI ablation.
ClinicalTrials.gov: NCT00326001 (http://clinicaltrials.gov/ct2/show/NCT00326001).
Introduction
Catheters with the 8 mm platinum–iridium (Pt–Ir) tip electrode are used to ablate the cavotricuspid isthmus (CTI)-dependent atrial flutter (AFL).1–4 Difficult AFL ablation occurs in approximately one-fourth of the patients.2–8 It is hypothesized that the substitution of Pt–Ir with a high thermal conductivity material such as gold may increase convective cooling and heat conduction from the electrode to the tissue, to defer excessive temperature rise at the electrode surface.9–13 Catheters delivering more radiofrequency (RF) power before the temperature limit is reached are capable of creating broader and deeper ablation lesions, potentially improving ablation efficacy.4,9–15 Conversely, temperature excess at the ablation electrode leads to char and/or coagulum formation and to a rapid impedance rise that hinders efficient transmission of RF.16,17
In ex vivo pig heart tissue, a gold ablation electrode resulted in 112% higher power delivery and in 64% deeper lesions than the identically designed Pt–Ir electrode.10 To evaluate clinical relevance of this observation, we conducted the prospective, non-blinded, randomized ‘Ablation of the Cavotricuspid Isthmus in Patients with Atrial Flutter Using an 8 mm Gold-Alloy Tip Electrode’ (AURUM 8) study. The study evaluated if gold-tip catheters can: (i) shorten the cumulative RF application duration time to bidirectional CTI block (primary outcome measure); (ii) increase ablation success rate; (iii) deliver more power; (iv) decrease the number of RF applications; and reduce (v) the incidence of charring/coagulum/thrombus formation, (vi) fluoroscopy time, (vii) procedure duration, (viii) procedure-related complications, and (ix) arrhythmia recurrence during 6 months of follow-up.
Methods
Study population
A total of 463 patients were enrolled in the study from June 2004 to March 2007 in 19 centres in Germany and the Czech Republic. The patients had to fulfil the following inclusion criteria: (i) at least one electrocardiogram (ECG)-documented, symptomatic, typical AFL episode with either negative, sawtooth-shaped P-waves in leads II, III, and aVF, or positive P-waves in leads II, III, and aVF; (ii) at least one persistent, typical AFL episode over 2 h, documented in the patient file and/or ECG; and (iii) a stable medication that had to be administered for at least 3 months before patient enrolment in the case of concomitant supraventricular and/or ventricular tachyarrhythmia.
The patients were not admitted to the study if any of the following criteria were present: previous CTI ablation, acute coronary syndrome or myocardial infarction within the last 3 months, severe cardiac valvular defects, tricuspid valve replacement, atrial septum defect, cardiovascular surgery scheduled within the next 6 months, NYHA class IV, age <18 years, pregnant or breastfeeding women, abuse of drugs, or exceptionally high surgical procedure risk when compared with normal patient group. All patients provided written informed consent. Following that, patients were randomized (block-based procedure) to be ablated by either Pt–Ir or gold-tip catheter.
Electrophysiological evaluation
To confirm CTI participation in AFL, a 20-pole catheter (ViaCath 20 XL, Biotronik GmbH & Co. KG, Berlin, Germany) was placed alongside the tricuspid valve annulus. An additional multipolar catheter was inserted into the coronary sinus. In patients with ongoing AFL, isthmus dependency was verified by activation mapping and concealed entrainment pacing. Thirteen patients with atrial fibrillation (AF) were cardioverted to sinus rhythm (SR) prior to the procedure.
Radiofrequency ablation
Ablation was performed using 7 F steerable quadripolar 8 mm tip catheters (AlCath, Biotronik GmbH & Co. KG), with a thermocouple integrated centrally in the catheter tip. According to randomization, either a Pt–Ir tip was selected, or a gold-tip electrode (99.99% gold). The following ranges for ablation parameters were pre-specified: maximum temperature: 60–70°C, maximum output: 60–75 W, and maximum energy delivery duration: 60–90 s. Radiofrequency deliveries in a temperature-controlled mode were repeated until a CTI conduction block was detected and could include one to three safety deliveries. The final bidirectional block test was performed 20 min after the last RF delivery, using criteria described elsewhere.18,19 Failure to achieve bidirectional CTI block after 1500 s (25 min) of energy application permitted termination of the procedure or application of an alternative catheter.
Follow-up
At patient discharge, performed within 5 days after ablation, we documented cardiac rhythm, medications, and instructed patients to record future symptoms in a patient's journal. They were told to immediately contact a physician for an unscheduled follow-up visit if experiencing symptoms suggestive of tachycardia. Scheduled follow-up examinations were performed at 3 and 6 months after ablation to evaluate: 12-lead ECG, the patient's journal, symptoms, recurrence of AFL and AF, and all-cause hospitalizations.
Statistical analysis
The primary outcome measure was cumulative duration of the RF application. The study was powered to detect a 10% difference between the two catheters, assuming a 33% standard deviation of the mean values. The sample size required to detect this difference with a statistical power of 85% at the two-sided 5% significance level was a total of 391 patients (to be randomized 1:1), or 450 patients to compensate for a drop-out of up to 15%.
Statistical analysis was performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). The normality of distribution of continuous variables was ascertained by the Kolmogorov–Smirnov test. Statistical significance of normally distributed data were assessed using two-sided t-test, and of data not normally distributed, using the Wilcoxon–Mann–Whitney (U) rank test. Continuous variables are reported as mean ± SD. Ablation procedure parameters were reported as both mean ± SD and median [interquartile range (IQR)]. Categorical data were compared using a χ2 test according to Pearson or Fisher's exact test as appropriate. Statistical significance was established as P < 0.05.
Randomized patients in whom ablation was cancelled were excluded as late drop-outs. We performed per-protocol data analysis, where successful ablation was defined as creation of a CTI block with the primary test catheter. Unsuccessful ablation was defined as failure to create a CTI block with the first catheter for any reason, including inability to continue ablation due to charring, coagulum, or impedance rise. The number, duration and cumulative energy of RF applications and fluoroscopy duration are calculated, including all the patients in whom RF delivery commenced, irrespective of the ablation success.
Results
Study population
A total of 463 patients was prospectively randomized into two groups (Table 1). The study cohort was affected by AFL for a mean of 1.4 ± 2.9 or median of 0.5 years. Patients experienced a mean of 15 ± 49 or median of three AFL episodes in the last 12 months before enrolment. Symptoms related to AFL were reported by 90% of patients.
| . | Pt–Ir tip (n = 227) . | Gold-tip (n = 236) . | P . |
|---|---|---|---|
| Mean age ± SD, year | 66 ± 11 | 66 ± 10 | 0.74 |
| Female, n (%) | 35 (15%) | 45 (19%) | 0.26 |
| Mean body surface ± SD, kg/m2 | 2.05 ± 0.21 | 2.03 ± 0.21 | 0.23 |
| Clinical AFL, n (%) | |||
| Counter-clockwise | 215 (95%) | 224 (95%) | 0.93 |
| Clockwise | 16 (7%) | 17 (7%) | 0.99 |
| Due to medication for AFB | 21 (9%) | 24 (10%) | 0.75 |
| Pre-ablation history of AFB, n (%) | 89 (39%) | 88 (37%) | 0.87 |
| Mean LAEDD ± SD, mm | 45 ± 7 | 44 ± 6 | 0.14 |
| Mean LVEF ± SD, % | 51 ± 14 | 53 ± 15 | 0.16 |
| Structural heart disease, n (%) | 102 (45%) | 97 (41%) | 40 |
| Coronary artery disease | 62 (27%) | 56 (24%) | 0.84 |
| Dilated cardiomyopathy | 9 (4%) | 11 (5%) | 0.53 |
| Left heart hypertrophy | 30 (13%) | 29 (12%) | 0.89 |
| Valvular disease | 39 (17%) | 31 (13%) | 0.23 |
| Hypertension, n (%) | 122 (54%) | 123 (52%) | 0.23 |
| Pulmonary hypertension, n (%) | 14 (6%) | 6 (3%) | 0.09 |
| Chronic obstructive pulmonary disease, n (%) | 11 (5%) | 5 (2%) | 0.11 |
| Diabetes, n (%) | 20 (9%) | 34 (14%) | 0.06 |
| Antiarrhythmics, n (%) | 198 (87%) | 203 (86%) | 0.74 |
| Anticoagulants, n (%) | 188 (83%) | 178 (75%) | 0.06 |
| . | Pt–Ir tip (n = 227) . | Gold-tip (n = 236) . | P . |
|---|---|---|---|
| Mean age ± SD, year | 66 ± 11 | 66 ± 10 | 0.74 |
| Female, n (%) | 35 (15%) | 45 (19%) | 0.26 |
| Mean body surface ± SD, kg/m2 | 2.05 ± 0.21 | 2.03 ± 0.21 | 0.23 |
| Clinical AFL, n (%) | |||
| Counter-clockwise | 215 (95%) | 224 (95%) | 0.93 |
| Clockwise | 16 (7%) | 17 (7%) | 0.99 |
| Due to medication for AFB | 21 (9%) | 24 (10%) | 0.75 |
| Pre-ablation history of AFB, n (%) | 89 (39%) | 88 (37%) | 0.87 |
| Mean LAEDD ± SD, mm | 45 ± 7 | 44 ± 6 | 0.14 |
| Mean LVEF ± SD, % | 51 ± 14 | 53 ± 15 | 0.16 |
| Structural heart disease, n (%) | 102 (45%) | 97 (41%) | 40 |
| Coronary artery disease | 62 (27%) | 56 (24%) | 0.84 |
| Dilated cardiomyopathy | 9 (4%) | 11 (5%) | 0.53 |
| Left heart hypertrophy | 30 (13%) | 29 (12%) | 0.89 |
| Valvular disease | 39 (17%) | 31 (13%) | 0.23 |
| Hypertension, n (%) | 122 (54%) | 123 (52%) | 0.23 |
| Pulmonary hypertension, n (%) | 14 (6%) | 6 (3%) | 0.09 |
| Chronic obstructive pulmonary disease, n (%) | 11 (5%) | 5 (2%) | 0.11 |
| Diabetes, n (%) | 20 (9%) | 34 (14%) | 0.06 |
| Antiarrhythmics, n (%) | 198 (87%) | 203 (86%) | 0.74 |
| Anticoagulants, n (%) | 188 (83%) | 178 (75%) | 0.06 |
AFB, atrial fibrillation; AFL, atrial flutter; LAEDD, left atrial end-diastolic diameter; LVEF, left ventricular ejection fraction; Pt–Ir, platinum–iridium.
| . | Pt–Ir tip (n = 227) . | Gold-tip (n = 236) . | P . |
|---|---|---|---|
| Mean age ± SD, year | 66 ± 11 | 66 ± 10 | 0.74 |
| Female, n (%) | 35 (15%) | 45 (19%) | 0.26 |
| Mean body surface ± SD, kg/m2 | 2.05 ± 0.21 | 2.03 ± 0.21 | 0.23 |
| Clinical AFL, n (%) | |||
| Counter-clockwise | 215 (95%) | 224 (95%) | 0.93 |
| Clockwise | 16 (7%) | 17 (7%) | 0.99 |
| Due to medication for AFB | 21 (9%) | 24 (10%) | 0.75 |
| Pre-ablation history of AFB, n (%) | 89 (39%) | 88 (37%) | 0.87 |
| Mean LAEDD ± SD, mm | 45 ± 7 | 44 ± 6 | 0.14 |
| Mean LVEF ± SD, % | 51 ± 14 | 53 ± 15 | 0.16 |
| Structural heart disease, n (%) | 102 (45%) | 97 (41%) | 40 |
| Coronary artery disease | 62 (27%) | 56 (24%) | 0.84 |
| Dilated cardiomyopathy | 9 (4%) | 11 (5%) | 0.53 |
| Left heart hypertrophy | 30 (13%) | 29 (12%) | 0.89 |
| Valvular disease | 39 (17%) | 31 (13%) | 0.23 |
| Hypertension, n (%) | 122 (54%) | 123 (52%) | 0.23 |
| Pulmonary hypertension, n (%) | 14 (6%) | 6 (3%) | 0.09 |
| Chronic obstructive pulmonary disease, n (%) | 11 (5%) | 5 (2%) | 0.11 |
| Diabetes, n (%) | 20 (9%) | 34 (14%) | 0.06 |
| Antiarrhythmics, n (%) | 198 (87%) | 203 (86%) | 0.74 |
| Anticoagulants, n (%) | 188 (83%) | 178 (75%) | 0.06 |
| . | Pt–Ir tip (n = 227) . | Gold-tip (n = 236) . | P . |
|---|---|---|---|
| Mean age ± SD, year | 66 ± 11 | 66 ± 10 | 0.74 |
| Female, n (%) | 35 (15%) | 45 (19%) | 0.26 |
| Mean body surface ± SD, kg/m2 | 2.05 ± 0.21 | 2.03 ± 0.21 | 0.23 |
| Clinical AFL, n (%) | |||
| Counter-clockwise | 215 (95%) | 224 (95%) | 0.93 |
| Clockwise | 16 (7%) | 17 (7%) | 0.99 |
| Due to medication for AFB | 21 (9%) | 24 (10%) | 0.75 |
| Pre-ablation history of AFB, n (%) | 89 (39%) | 88 (37%) | 0.87 |
| Mean LAEDD ± SD, mm | 45 ± 7 | 44 ± 6 | 0.14 |
| Mean LVEF ± SD, % | 51 ± 14 | 53 ± 15 | 0.16 |
| Structural heart disease, n (%) | 102 (45%) | 97 (41%) | 40 |
| Coronary artery disease | 62 (27%) | 56 (24%) | 0.84 |
| Dilated cardiomyopathy | 9 (4%) | 11 (5%) | 0.53 |
| Left heart hypertrophy | 30 (13%) | 29 (12%) | 0.89 |
| Valvular disease | 39 (17%) | 31 (13%) | 0.23 |
| Hypertension, n (%) | 122 (54%) | 123 (52%) | 0.23 |
| Pulmonary hypertension, n (%) | 14 (6%) | 6 (3%) | 0.09 |
| Chronic obstructive pulmonary disease, n (%) | 11 (5%) | 5 (2%) | 0.11 |
| Diabetes, n (%) | 20 (9%) | 34 (14%) | 0.06 |
| Antiarrhythmics, n (%) | 198 (87%) | 203 (86%) | 0.74 |
| Anticoagulants, n (%) | 188 (83%) | 178 (75%) | 0.06 |
AFB, atrial fibrillation; AFL, atrial flutter; LAEDD, left atrial end-diastolic diameter; LVEF, left ventricular ejection fraction; Pt–Ir, platinum–iridium.
Atrial flutter ablation results
Fifteen patients did not receive ablation for reasons listed in Figure 1. In three cases in each study arm, anatomical difficulties met the exclusion criterion of exceptionally high procedure risk, for instance, due to very large isthmus or extremely large right atrium. The seven cases of catheter defects were described by investigators as failed energy transmission (3 Pt–Ir, 1 gold), steering wire defect (1 Pt–Ir, 1 gold), and noise on ablation channels (1 gold). Data recording equipment failed in one patient in each group.
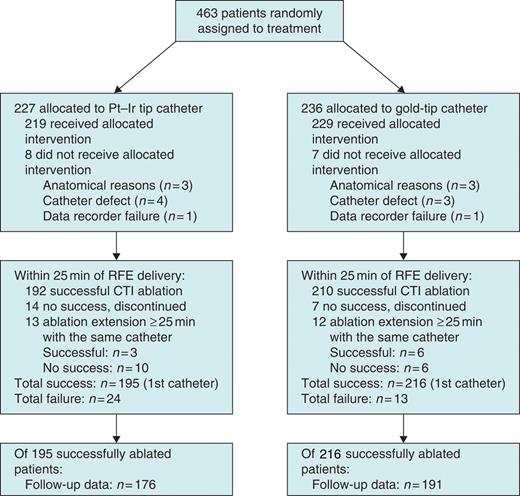
Flow diagram of a multicentre trial comparing platinum–iridium (Pt–Ir) and gold-tip catheters in radiofrequency energy (RFE) ablation of cavotricuspid isthmus (CTI)-dependent atrial flutter.
The procedural characteristics for the remaining 448 patients are presented in Table 2. Cumulative RF application duration was shorter in gold-tip catheter by 6.4% (mean 10.2 vs. 10.9 min). The difference was not statistically significant and did not reach the 10%-mark set as primary study hypothesis. Both catheters required a similar number of RF applications, fluoroscopy time, and procedure duration (from puncture to catheter out).
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Mean RF duration ± SD, min | 10.9 ± 6.9 | 10.2 ± 6.9 | 0.25 |
| Median (IQR) | 8.7 (5.7, 15.0) | 8.1 (5.0, 13.3) | |
| Mean number of RF applications ± SD, n | 12.9 ± 9.5 | 11.8 ± 8.2 | 0.21 |
| Median (IQR) | 10 (6, 17) | 10 (6, 15) | |
| Mean power delivered ± SD, W | 48 ± 13 | 52 ± 12 | <0.001 |
| Median (IQR) | 49 (40, 58) | 53 (45, 61) | |
| Mean temperature ± SD, °C | 54.3 ± 5.2 | 53.2 ± 4.7 | 0.020 |
| Median (IQR) | 53.5 (50.6, 58.3) | 52.5 (49.8, 55.7) | |
| Mean maximum temperature ± SD, °C | 70.2 ± 7.0 | 68.7 ± 6.6 | 0.021 |
| Median (IQR) | 71 (66, 75) | 69 (64, 73) | |
| Mean fluoroscopy duration ± SD, min | 17 ± 13 | 18 ± 14 | 0.78 |
| Median (IQR) | 13 (8, 22) | 14 (8, 24) | |
| Mean procedure duration ± SD, min | 84 ± 35 | 85 ± 33 | 0.88 |
| Median (IQR) | 76 (60, 99) | 77 (60, 105) |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Mean RF duration ± SD, min | 10.9 ± 6.9 | 10.2 ± 6.9 | 0.25 |
| Median (IQR) | 8.7 (5.7, 15.0) | 8.1 (5.0, 13.3) | |
| Mean number of RF applications ± SD, n | 12.9 ± 9.5 | 11.8 ± 8.2 | 0.21 |
| Median (IQR) | 10 (6, 17) | 10 (6, 15) | |
| Mean power delivered ± SD, W | 48 ± 13 | 52 ± 12 | <0.001 |
| Median (IQR) | 49 (40, 58) | 53 (45, 61) | |
| Mean temperature ± SD, °C | 54.3 ± 5.2 | 53.2 ± 4.7 | 0.020 |
| Median (IQR) | 53.5 (50.6, 58.3) | 52.5 (49.8, 55.7) | |
| Mean maximum temperature ± SD, °C | 70.2 ± 7.0 | 68.7 ± 6.6 | 0.021 |
| Median (IQR) | 71 (66, 75) | 69 (64, 73) | |
| Mean fluoroscopy duration ± SD, min | 17 ± 13 | 18 ± 14 | 0.78 |
| Median (IQR) | 13 (8, 22) | 14 (8, 24) | |
| Mean procedure duration ± SD, min | 84 ± 35 | 85 ± 33 | 0.88 |
| Median (IQR) | 76 (60, 99) | 77 (60, 105) |
IQR, interquartile range; Pt–Ir, platinum–iridium; RF, radiofrequency ablation.
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Mean RF duration ± SD, min | 10.9 ± 6.9 | 10.2 ± 6.9 | 0.25 |
| Median (IQR) | 8.7 (5.7, 15.0) | 8.1 (5.0, 13.3) | |
| Mean number of RF applications ± SD, n | 12.9 ± 9.5 | 11.8 ± 8.2 | 0.21 |
| Median (IQR) | 10 (6, 17) | 10 (6, 15) | |
| Mean power delivered ± SD, W | 48 ± 13 | 52 ± 12 | <0.001 |
| Median (IQR) | 49 (40, 58) | 53 (45, 61) | |
| Mean temperature ± SD, °C | 54.3 ± 5.2 | 53.2 ± 4.7 | 0.020 |
| Median (IQR) | 53.5 (50.6, 58.3) | 52.5 (49.8, 55.7) | |
| Mean maximum temperature ± SD, °C | 70.2 ± 7.0 | 68.7 ± 6.6 | 0.021 |
| Median (IQR) | 71 (66, 75) | 69 (64, 73) | |
| Mean fluoroscopy duration ± SD, min | 17 ± 13 | 18 ± 14 | 0.78 |
| Median (IQR) | 13 (8, 22) | 14 (8, 24) | |
| Mean procedure duration ± SD, min | 84 ± 35 | 85 ± 33 | 0.88 |
| Median (IQR) | 76 (60, 99) | 77 (60, 105) |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Mean RF duration ± SD, min | 10.9 ± 6.9 | 10.2 ± 6.9 | 0.25 |
| Median (IQR) | 8.7 (5.7, 15.0) | 8.1 (5.0, 13.3) | |
| Mean number of RF applications ± SD, n | 12.9 ± 9.5 | 11.8 ± 8.2 | 0.21 |
| Median (IQR) | 10 (6, 17) | 10 (6, 15) | |
| Mean power delivered ± SD, W | 48 ± 13 | 52 ± 12 | <0.001 |
| Median (IQR) | 49 (40, 58) | 53 (45, 61) | |
| Mean temperature ± SD, °C | 54.3 ± 5.2 | 53.2 ± 4.7 | 0.020 |
| Median (IQR) | 53.5 (50.6, 58.3) | 52.5 (49.8, 55.7) | |
| Mean maximum temperature ± SD, °C | 70.2 ± 7.0 | 68.7 ± 6.6 | 0.021 |
| Median (IQR) | 71 (66, 75) | 69 (64, 73) | |
| Mean fluoroscopy duration ± SD, min | 17 ± 13 | 18 ± 14 | 0.78 |
| Median (IQR) | 13 (8, 22) | 14 (8, 24) | |
| Mean procedure duration ± SD, min | 84 ± 35 | 85 ± 33 | 0.88 |
| Median (IQR) | 76 (60, 99) | 77 (60, 105) |
IQR, interquartile range; Pt–Ir, platinum–iridium; RF, radiofrequency ablation.
Gold-tip catheters delivered significantly more RF (mean 52 vs. 48 W, P < 0.001), whereas keeping the temperature significantly lower (Table 2). Ablation success is illustrated in Figure 2. The difference in the overall success rate (94.3% gold vs. 89.0% control), in the number of bidirectional CTI blocks achieved within 5 min (24.9% gold vs. 16.0% control), and in the number of CTI blocks achieved within 20 min (87.8% gold vs. 80.4% control) were statistically significant (Table 3). Table 3 shows the reasons for ablation failure and the duration of RF application until admitting failure, which did not differ significantly between control and gold-tip catheters (19.3 vs. 21.6 min).
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Ablation success with the first catheter, n (%) | |||
| Total | 195 (89.0%) | 216 (94.3%) | 0.042 |
| Block within 25 mina | 192 (87.7%) | 210 (91.7%) | 0.16 |
| Block within 20 mina | 176 (80.4%) | 201 (87.8%) | 0.03 |
| Block within 15 mina | 156 (71.2%) | 180 (78.6%) | 0.07 |
| Block within 10 mina | 118 (53.9%) | 134 (58.5%) | 0.32 |
| Block within 5 mina | 35 (16.0%) | 57 (24.9%) | 0.02 |
| Reasons for ablation failure, n (%) | |||
| No effect on rhythm | 11 (5.0%) | 9 (3.9%) | 0.58 |
| Unusable catheter due to excessive char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| PQ prolongation, risk of AV block | 0 | 1 (0.4%) | 1.00 |
| Bad curve steering | 2 (0.9%) | 1 (0.4%) | 0.62 |
| Repeated catheter dislocation | 1 (0.5%) | 1 (0.4%) | 1.00 |
| Mean RF duration until failure ± SD, minb | 19.3 ± 9.5 | 21.6 ± 10.2 | 0.50 |
| Median (IQR) | 21.3 (11.8, 27.7) | 26.1 (12.0, 29.3) | |
| Outcome after ablation failure, n (%) | |||
| Success with another catheter | 7 | 6 | – |
| No success with another catheter | 4 | 0 | – |
| Further procedure not documented | 13 | 7 | – |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Ablation success with the first catheter, n (%) | |||
| Total | 195 (89.0%) | 216 (94.3%) | 0.042 |
| Block within 25 mina | 192 (87.7%) | 210 (91.7%) | 0.16 |
| Block within 20 mina | 176 (80.4%) | 201 (87.8%) | 0.03 |
| Block within 15 mina | 156 (71.2%) | 180 (78.6%) | 0.07 |
| Block within 10 mina | 118 (53.9%) | 134 (58.5%) | 0.32 |
| Block within 5 mina | 35 (16.0%) | 57 (24.9%) | 0.02 |
| Reasons for ablation failure, n (%) | |||
| No effect on rhythm | 11 (5.0%) | 9 (3.9%) | 0.58 |
| Unusable catheter due to excessive char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| PQ prolongation, risk of AV block | 0 | 1 (0.4%) | 1.00 |
| Bad curve steering | 2 (0.9%) | 1 (0.4%) | 0.62 |
| Repeated catheter dislocation | 1 (0.5%) | 1 (0.4%) | 1.00 |
| Mean RF duration until failure ± SD, minb | 19.3 ± 9.5 | 21.6 ± 10.2 | 0.50 |
| Median (IQR) | 21.3 (11.8, 27.7) | 26.1 (12.0, 29.3) | |
| Outcome after ablation failure, n (%) | |||
| Success with another catheter | 7 | 6 | – |
| No success with another catheter | 4 | 0 | – |
| Further procedure not documented | 13 | 7 | – |
aDuration of RF energy application to bidirectional cavotricuspid isthmus block, including safety applications.
bDuration of RF energy application until admitting ablation failure with the test catheter.
AV, atrioventricular; IQR, interquartile range; Pt–Ir, platinum–iridium; RF, radiofrequency ablation.
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Ablation success with the first catheter, n (%) | |||
| Total | 195 (89.0%) | 216 (94.3%) | 0.042 |
| Block within 25 mina | 192 (87.7%) | 210 (91.7%) | 0.16 |
| Block within 20 mina | 176 (80.4%) | 201 (87.8%) | 0.03 |
| Block within 15 mina | 156 (71.2%) | 180 (78.6%) | 0.07 |
| Block within 10 mina | 118 (53.9%) | 134 (58.5%) | 0.32 |
| Block within 5 mina | 35 (16.0%) | 57 (24.9%) | 0.02 |
| Reasons for ablation failure, n (%) | |||
| No effect on rhythm | 11 (5.0%) | 9 (3.9%) | 0.58 |
| Unusable catheter due to excessive char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| PQ prolongation, risk of AV block | 0 | 1 (0.4%) | 1.00 |
| Bad curve steering | 2 (0.9%) | 1 (0.4%) | 0.62 |
| Repeated catheter dislocation | 1 (0.5%) | 1 (0.4%) | 1.00 |
| Mean RF duration until failure ± SD, minb | 19.3 ± 9.5 | 21.6 ± 10.2 | 0.50 |
| Median (IQR) | 21.3 (11.8, 27.7) | 26.1 (12.0, 29.3) | |
| Outcome after ablation failure, n (%) | |||
| Success with another catheter | 7 | 6 | – |
| No success with another catheter | 4 | 0 | – |
| Further procedure not documented | 13 | 7 | – |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Ablation success with the first catheter, n (%) | |||
| Total | 195 (89.0%) | 216 (94.3%) | 0.042 |
| Block within 25 mina | 192 (87.7%) | 210 (91.7%) | 0.16 |
| Block within 20 mina | 176 (80.4%) | 201 (87.8%) | 0.03 |
| Block within 15 mina | 156 (71.2%) | 180 (78.6%) | 0.07 |
| Block within 10 mina | 118 (53.9%) | 134 (58.5%) | 0.32 |
| Block within 5 mina | 35 (16.0%) | 57 (24.9%) | 0.02 |
| Reasons for ablation failure, n (%) | |||
| No effect on rhythm | 11 (5.0%) | 9 (3.9%) | 0.58 |
| Unusable catheter due to excessive char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| PQ prolongation, risk of AV block | 0 | 1 (0.4%) | 1.00 |
| Bad curve steering | 2 (0.9%) | 1 (0.4%) | 0.62 |
| Repeated catheter dislocation | 1 (0.5%) | 1 (0.4%) | 1.00 |
| Mean RF duration until failure ± SD, minb | 19.3 ± 9.5 | 21.6 ± 10.2 | 0.50 |
| Median (IQR) | 21.3 (11.8, 27.7) | 26.1 (12.0, 29.3) | |
| Outcome after ablation failure, n (%) | |||
| Success with another catheter | 7 | 6 | – |
| No success with another catheter | 4 | 0 | – |
| Further procedure not documented | 13 | 7 | – |
aDuration of RF energy application to bidirectional cavotricuspid isthmus block, including safety applications.
bDuration of RF energy application until admitting ablation failure with the test catheter.
AV, atrioventricular; IQR, interquartile range; Pt–Ir, platinum–iridium; RF, radiofrequency ablation.
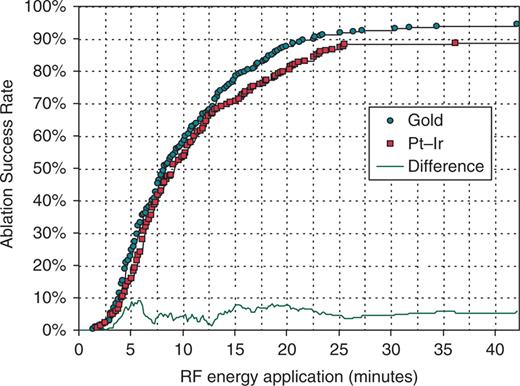
Cumulative success in achieving bidirectional cavotricuspid isthmus block as a function of duration of radiofrequency (RF) energy application for gold-tip vs. platinum–iridium (Pt–Ir) tip catheters. The absolute difference between the two success rate curves is plotted at the bottom. Statistical significance of the difference is evaluated in Table 3.
Char/coagulum was observed in 83 (37.9%) control and 11 (4.8%) gold-tip catheters (P < 0.001, Table 4). Ten Pt–Ir (4.6%) and one gold-tip catheter (0.4%, P = 0.005) had to be exchanged due to excessive charring, coagulum or thrombus formation. There were no significant differences between two catheters with respect to the impedance rise over 30 Ω, in audible ‘pop’, and in thrombus formation (Table 4).
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Impedance rise >30 Ω | 28 (12.8%) | 44 (19.2%) | 0.06 |
| Pop phenomenon | 23 (10.5%) | 24 (10.5%) | 0.97 |
| Char or coagulum formation | 83 (37.9%) | 11 (4.8%) | <0.001 |
| Char or coagulum formation on at least 50% of the electrode surface | 18 (8.2%) | 2 (0.9%) | <0.001 |
| Thrombus formation on catheter tip | 3 (1.4%) | 2 (0.9%) | 0.36 |
| Unusable catheter due to char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Impedance rise >30 Ω | 28 (12.8%) | 44 (19.2%) | 0.06 |
| Pop phenomenon | 23 (10.5%) | 24 (10.5%) | 0.97 |
| Char or coagulum formation | 83 (37.9%) | 11 (4.8%) | <0.001 |
| Char or coagulum formation on at least 50% of the electrode surface | 18 (8.2%) | 2 (0.9%) | <0.001 |
| Thrombus formation on catheter tip | 3 (1.4%) | 2 (0.9%) | 0.36 |
| Unusable catheter due to char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
Pt–Ir indicates platinum–iridium.
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Impedance rise >30 Ω | 28 (12.8%) | 44 (19.2%) | 0.06 |
| Pop phenomenon | 23 (10.5%) | 24 (10.5%) | 0.97 |
| Char or coagulum formation | 83 (37.9%) | 11 (4.8%) | <0.001 |
| Char or coagulum formation on at least 50% of the electrode surface | 18 (8.2%) | 2 (0.9%) | <0.001 |
| Thrombus formation on catheter tip | 3 (1.4%) | 2 (0.9%) | 0.36 |
| Unusable catheter due to char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Impedance rise >30 Ω | 28 (12.8%) | 44 (19.2%) | 0.06 |
| Pop phenomenon | 23 (10.5%) | 24 (10.5%) | 0.97 |
| Char or coagulum formation | 83 (37.9%) | 11 (4.8%) | <0.001 |
| Char or coagulum formation on at least 50% of the electrode surface | 18 (8.2%) | 2 (0.9%) | <0.001 |
| Thrombus formation on catheter tip | 3 (1.4%) | 2 (0.9%) | 0.36 |
| Unusable catheter due to char, coagulum or thrombus formation | 10 (4.6%) | 1 (0.4%) | 0.005 |
Pt–Ir indicates platinum–iridium.
The maximum electrode temperature in catheters with charring was significantly higher (72.3 ± 6.6°C) than in catheters without charring (68.7 ± 6.7°C, P < 0.001).
Procedural complications are listed in Table 5. The major complication, pacemaker implantation, occurred in three (1.4%) patients treated with the control catheter and five (2.2%) patients treated with the gold-tip catheter (P = non-significant). These patients exhibited atrioventricular block or sinus node dysfunction which were revealed after, or caused by, AFL ablation, which could not always be differentiated.
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Total | 5 (2.3%) | 8 (3.5%) | 0.45 |
| Pacemaker implantation | 3 (1.4%) | 5 (2.2%) | 0.73 |
| Second degree AV block | 0 | 1 (0.4%) | 1.00 |
| Transient ischaemic attack | 1 (0.5%) | 0 | 0.49 |
| Groin fistula | 1 (0.5%) | 0 | 0.49 |
| Groin haematoma | 0 | 2 (0.9%) | 0.50 |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Total | 5 (2.3%) | 8 (3.5%) | 0.45 |
| Pacemaker implantation | 3 (1.4%) | 5 (2.2%) | 0.73 |
| Second degree AV block | 0 | 1 (0.4%) | 1.00 |
| Transient ischaemic attack | 1 (0.5%) | 0 | 0.49 |
| Groin fistula | 1 (0.5%) | 0 | 0.49 |
| Groin haematoma | 0 | 2 (0.9%) | 0.50 |
AV, atrioventricular; Pt–Ir, platinum–iridium.
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Total | 5 (2.3%) | 8 (3.5%) | 0.45 |
| Pacemaker implantation | 3 (1.4%) | 5 (2.2%) | 0.73 |
| Second degree AV block | 0 | 1 (0.4%) | 1.00 |
| Transient ischaemic attack | 1 (0.5%) | 0 | 0.49 |
| Groin fistula | 1 (0.5%) | 0 | 0.49 |
| Groin haematoma | 0 | 2 (0.9%) | 0.50 |
| . | Pt–Ir tip (n = 219) . | Gold-tip (n = 229) . | P . |
|---|---|---|---|
| Total | 5 (2.3%) | 8 (3.5%) | 0.45 |
| Pacemaker implantation | 3 (1.4%) | 5 (2.2%) | 0.73 |
| Second degree AV block | 0 | 1 (0.4%) | 1.00 |
| Transient ischaemic attack | 1 (0.5%) | 0 | 0.49 |
| Groin fistula | 1 (0.5%) | 0 | 0.49 |
| Groin haematoma | 0 | 2 (0.9%) | 0.50 |
AV, atrioventricular; Pt–Ir, platinum–iridium.
Follow-up
No significant differences in deaths and hospitalizations were observed between the study arms (Table 6). One patient in the control group died 25 days after AFL ablation due to sudden death. Two patients in the gold-tip group died 40 and 123 days after ablation due to cerebral bleeding caused by septic multiorganic failure and car accident, respectively. During the follow-up, AFL was noted in 10 (2.7%) and AF in 48 (13.1%) of the successfully ablated patients, with negligible differences between the groups (Table 6).
| . | Pt–Ir tip (n = 195) . | Gold–tip (n = 216) . | P . |
|---|---|---|---|
| Patients with FU, n (%) | 176 (90.3%) | 191 (88.4%) | 0.55 |
| Mean FU duration ± SD, months | 5.9 ± 2.1 | 5.9 ± 1.5 | 0.96 |
| Antiarrhythmics at discharge, n (%) | 146 (83%) | 159 (83%) | 0.98 |
| AFL recurrence, n (%) | 6 (3.4%) | 4 (2.1%) | 0.53 |
| AFB, n (%) | 25 (14.2%) | 23 (12.0%) | 0.54 |
| All-cause death, n (%) | 1 (0.6%) | 2 (1.0%) | 1.00 |
| Cardiovascular death, n (%) | 1 (0.6%) | 0 | 0.48 |
| All-cause hospitalization, n (%) | 12 (6.8%) | 18 (9.4%) | 0.36 |
| Cardiovascular reason, n (%) | 9 (5.1%) | 11 (5.8%) | 0.79 |
| Stroke or TIA, n (%) | 1 (0.6%) | 1 (0.5%) | 1.00 |
| Non-cardiac event, n (%) | 2 (1.1%) | 6 (3.1%) | 0.29 |
| . | Pt–Ir tip (n = 195) . | Gold–tip (n = 216) . | P . |
|---|---|---|---|
| Patients with FU, n (%) | 176 (90.3%) | 191 (88.4%) | 0.55 |
| Mean FU duration ± SD, months | 5.9 ± 2.1 | 5.9 ± 1.5 | 0.96 |
| Antiarrhythmics at discharge, n (%) | 146 (83%) | 159 (83%) | 0.98 |
| AFL recurrence, n (%) | 6 (3.4%) | 4 (2.1%) | 0.53 |
| AFB, n (%) | 25 (14.2%) | 23 (12.0%) | 0.54 |
| All-cause death, n (%) | 1 (0.6%) | 2 (1.0%) | 1.00 |
| Cardiovascular death, n (%) | 1 (0.6%) | 0 | 0.48 |
| All-cause hospitalization, n (%) | 12 (6.8%) | 18 (9.4%) | 0.36 |
| Cardiovascular reason, n (%) | 9 (5.1%) | 11 (5.8%) | 0.79 |
| Stroke or TIA, n (%) | 1 (0.6%) | 1 (0.5%) | 1.00 |
| Non-cardiac event, n (%) | 2 (1.1%) | 6 (3.1%) | 0.29 |
All percentages were calculated from patients with FU (176 and 191, respectively). AFB, atrial fibrillation; AFL, atrial flutter; FU, follow-up; Pt–Ir, platinum–iridium; TIA, transient ischaemic attack.
| . | Pt–Ir tip (n = 195) . | Gold–tip (n = 216) . | P . |
|---|---|---|---|
| Patients with FU, n (%) | 176 (90.3%) | 191 (88.4%) | 0.55 |
| Mean FU duration ± SD, months | 5.9 ± 2.1 | 5.9 ± 1.5 | 0.96 |
| Antiarrhythmics at discharge, n (%) | 146 (83%) | 159 (83%) | 0.98 |
| AFL recurrence, n (%) | 6 (3.4%) | 4 (2.1%) | 0.53 |
| AFB, n (%) | 25 (14.2%) | 23 (12.0%) | 0.54 |
| All-cause death, n (%) | 1 (0.6%) | 2 (1.0%) | 1.00 |
| Cardiovascular death, n (%) | 1 (0.6%) | 0 | 0.48 |
| All-cause hospitalization, n (%) | 12 (6.8%) | 18 (9.4%) | 0.36 |
| Cardiovascular reason, n (%) | 9 (5.1%) | 11 (5.8%) | 0.79 |
| Stroke or TIA, n (%) | 1 (0.6%) | 1 (0.5%) | 1.00 |
| Non-cardiac event, n (%) | 2 (1.1%) | 6 (3.1%) | 0.29 |
| . | Pt–Ir tip (n = 195) . | Gold–tip (n = 216) . | P . |
|---|---|---|---|
| Patients with FU, n (%) | 176 (90.3%) | 191 (88.4%) | 0.55 |
| Mean FU duration ± SD, months | 5.9 ± 2.1 | 5.9 ± 1.5 | 0.96 |
| Antiarrhythmics at discharge, n (%) | 146 (83%) | 159 (83%) | 0.98 |
| AFL recurrence, n (%) | 6 (3.4%) | 4 (2.1%) | 0.53 |
| AFB, n (%) | 25 (14.2%) | 23 (12.0%) | 0.54 |
| All-cause death, n (%) | 1 (0.6%) | 2 (1.0%) | 1.00 |
| Cardiovascular death, n (%) | 1 (0.6%) | 0 | 0.48 |
| All-cause hospitalization, n (%) | 12 (6.8%) | 18 (9.4%) | 0.36 |
| Cardiovascular reason, n (%) | 9 (5.1%) | 11 (5.8%) | 0.79 |
| Stroke or TIA, n (%) | 1 (0.6%) | 1 (0.5%) | 1.00 |
| Non-cardiac event, n (%) | 2 (1.1%) | 6 (3.1%) | 0.29 |
All percentages were calculated from patients with FU (176 and 191, respectively). AFB, atrial fibrillation; AFL, atrial flutter; FU, follow-up; Pt–Ir, platinum–iridium; TIA, transient ischaemic attack.
Discussion
Major findings
To our knowledge, this is the first prospective, randomized, large multicentre study to compare effects of gold and Pt–Ir tip electrodes on ablation for typical AFL. This study demonstrated that gold-tip catheters could facilitate CTI ablation by enhanced success rate and RF delivery, while keeping the electrode surface slightly cooler and substantially less affected by charring. Gold-tip catheters, however, did not significantly reduce cumulative RF application time until achieving bidirectional CTI block (primary study endpoint), fluoroscopy time, or procedure duration. Acute and follow-up data gathered in this large patient cohort imply that gold-tip catheters are as safe for use as conventional Pt–Ir tip catheters.
Previous studies with gold ablation electrodes
In vitro experiments have demonstrated that both 4 and 8 mm gold-tip catheters deliver substantially more RF power and induce deeper lesions in myocardial and liver porcine tissues than Pt–Ir tip counterparts, as long as non-irrigated catheters without active cooling are used.10,13
Stuhlinger et al.12 randomized 252 patients undergoing AVNRT ablation to 4 mm gold or 4 mm Pt–Ir electrodes. Significantly less charring or blood clotting was observed on gold electrodes (in 0.8 vs. 6.4% of patients, respectively), but gold did not increase power delivery or procedural efficacy.12 The authors suggested that several underlying reasons for seeing no benefit of gold catheters in power delivery, including the procedure-mandatory looser wall contact of the catheter in their study than in the in vitro study and high blood flow in their study that cooled both catheter materials more firmly than in in vitro conditions.
Another, single centre study did not find a clinical difference between the 8 mm gold-tip, 8 mm Pt–Ir tip, and 4 mm externally irrigated-tip catheters.14 The sample size was small (20 patients in each randomization group), and the gold and Pt–Ir catheters were from different manufacturers, thus differing in other features on top of electrode material.
Findings and implications of our study
We used identically designed catheters in both study arms, except for the electrode material. The large patient collective allowed wide matching of the randomized groups and sound statistical evaluation of the differences between the catheters. More than 20 ablation operators participated, which better reflects standard conditions and outcomes than studies at single ablation centres.
Gold-tip catheter was associated with an 8.5% reduction in the number and 6.4% reduction of the cumulative duration of RF applications. Since standard deviations for these parameters were large, ∼70% of the mean values, neither trend reached statistical significance. A similar increase in the mean power delivered (+8.3%) and a small reduction in the temperature (−2%) were statistically significant due to low standard deviations. Ablation success was consistently higher in the gold-tip arm. In 94.3% of these patients there was no need for crossover to a second catheter, compared with 89.0% in the Pt–Ir tip arm (P < 0.05). One quarter of the patients in the gold-tip arm was ablated with <5 min of RF delivery, compared with 16% in the Pt–Ir arm.
Charring was eight-fold less frequent on the gold-tip (4.8%) than on control catheter (37.9%). Our use of more aggressive target parameters, up to 70°C and 75 W, was likely the reason for more charring than previously reported in the literature on 8 mm Pt–Ir electrodes without active cooling (5–15%, at 50–60°C and 50–70 W).4,14,15,20,21 According to the temperature comparisons for charred and clean electrodes in the Results section, maximum temperature seems not to be the only underlying factor for charring, unless heat distribution across the electrode is very asymmetric.22
Less charring allows less catheter cleaning and better RF transmission. This permits the use of ‘aggressive’ ablation parameters in the right atrium with gold electrodes. Since charring can contribute to the development of thrombi and thrombo-embolic complications,23–25 application of conventional Pt–Ir tip catheters is generally limited to the right atrium (pulmonary circulation ‘filters’ out thrombi). Catheters with a saline irrigated-tip reduce charring by active cooling14,15,26 and are commonly used for ablations in the left atrium or the left ventricle, although they are more complex to handle. With their handling simplicity, low charring rate and ablation efficacy, gold-tip catheters emerge as a potential alternative to the irrigated-tip catheters for left heart applications, which warrants further study.
Theoretically, the more powerful effect of gold, presumably allowing larger ablation lesions, may increase the risk of inadvertent atrioventricular block induction during CTI ablation.20,26,27 In our study, pacemaker implantation occurred in five (2.2%) patients treated with the gold-tip catheter and three (1.4%) patients treated with the control catheter, which did not differ significantly. In some cases, a conduction disease that was pre-existent and only revealed after post-ablation restoration of SR rather then caused by ablation cannot be excluded. Another theoretical consequence of more powerful effect of gold may be a lower recurrence rate of AFL.28,29 Recurrence of AFL over 6 months was only slightly lower in gold-tip (2.1%) than control catheters (3.4%), without statistical significance.
Conclusion
The acute and follow-up data gathered in 463 patients imply that the gold-tip catheter is as safe for use as a conventional Pt–Ir tip catheter. There were no differences between the two catheters in RF application duration until achieving bidirectional CTI block, fluoroscopy time, procedure duration, and arrhythmia recurrence during 6 months of follow-up. The gold-tip catheter was, however, associated with a significantly higher primary ablation success rate, more power delivery, and less char and coagulum formation, which supports the choice of gold as preferable electrode material.
Conflict of interest: E.H., T.L., and E.V. have received sponsorship for research and honoraria for lectures from Biotronik. E.M.-B. has received sponsorship for research from Biotronik. S.H. and J.P. are employed by Biotronik. The other authors have no conflicts of interest.
Funding
This study was supported by a grant of the Deutsche Stiftung für Herzforschung.



