-
PDF
- Split View
-
Views
-
Cite
Cite
Cristina Caldararu, Robert Alexandru, Daniela Bartos, Radu-Gabriel Vatasescu, Identical anatomical location of accessory pathway in a family with Wolff–Parkinson–White syndrome, EP Europace, Volume 12, Issue 4, April 2010, Pages 582–583, https://doi.org/10.1093/europace/eup403
Close - Share Icon Share
Abstract
Identical location of accessory pathways in family members with Wolff–Parkinson–White syndrome, although theoretically possible, has never been described. A 37-year-old woman and her 18-year-old son were referred for electrophysiological study due to fast rate palpitations and pre-excitation on baseline electrocardiogram. After mapping during pre-excitation, successful radiofrequency application was located at the right free wall in both patients, in an identical anatomical position, on the infero-lateral aspect of the tricuspid ring.
Introduction
Wolff–Parkinson–White (WPW) syndrome is produced by an accessory atrio-ventricular (AV) pathway, which can be the substrate for tachyarrhythmias and sometimes sudden arrhythmic death. The prevalence is 0.15–0.25% in the general population and 3.4% in first-degree relatives. 1 The accessory pathway (AP) can be localized practically anywhere along AV rings, most frequent being left free wall, postero-septal, right free wall, and right antero-septal. 1 Familial WPW syndrome has also been described. 2 However, identical anatomical location for AP for different family members has not been reported yet. We present a case of a family affected by WPW (mother and son) with identical location of AP as proved by the position of ablation catheter during successful radiofrequency (RF) application.
Case report
Case 1
A 37-year-old woman was referred to our clinic for a 3-year history of rapid palpitation episodes. History was negative for any other co-morbidities as well as clinical examination and the usual laboratory tests. The electrocardiogram (ECG) showed pre-excitation, the delta wave suggesting the presence of an AP at the free wall of right ventricle (RV) ( Figure 1 A ). The 24-h ECG monitoring identified prolonged episodes of orthodromic tachycardia. During electrophysiology study, mapping of the tricuspid annulus during pre-excitation ( Figure 1 B ) identified the AV fusion at the infero-lateral portion of the tricuspid ring ( Figure 1 C ). The pre-excitation disappeared almost instantaneously ( Figure 1 B and D ) after RF application (55 °C, 50 W) at the level of AV fusion, with no subsequent recurrence.
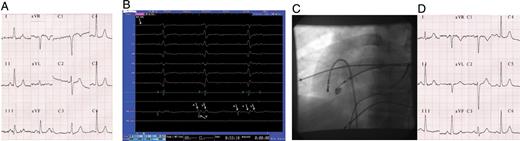
Patient 1 baseline ECG ( A ), intracardiac recordings during successful RF application ( B ), left anterior oblique (LAO) radioscopic projection of the ablation catheter position during successful RF application ( C ), and post-ablation ECG ( D ). ‘RF-on’, start of the RF application; A, local atrial electrogram; V, local ventricular electrogram; V′, far-field ventricular electrogram.
Case 2
An 18-year-old rugby player (the son of Patient 1), apparently asymptomatic, was diagnosed with pre-excitation after ECG screening. He wanted to continue to practice sports, therefore he was referred for AP ablation. The delta wave on the ECG suggested that AP was located at RV free wall ( Figure 2 A ). Electrophysiology study identified an AP with rest refractory period of 400 ms, which was reduced to 280 ms after stimulation with isoprenaline, inducing orthodromic atrio-ventricular re-entrant tachycardia. The location of the AV fusion ( Figure 2 B ) was also in infero-lateral area of the tricuspid ring ( Figure 2 C ). After <1 s of RF application (55 °C, 50 W) at this level, pre-excitation disappeared ( Figure 2 B and D ), without any inducible arrhythmias.

Patient 2 baseline ECG ( A ), intracardiac recordings during successful RF application ( B ), LAO radioscopic projection of the ablation catheter position during successful RF application ( C ), and post-ablation ECG ( D ). ‘RF-on’, start of the RF application; A, local atrial electrogram; V, local ventricular electrogram; V′, far-field ventricular electrogram.
Discussion
Pre-excitation syndrome is characterized by ECG changes as short PR interval (<0.12 ms) and delta wave. The delta wave may be permanent or intermittent and it appears as a result of ventricular activation through the AP (decremental or non-decremental) during the normal delay of AV conduction in the AV node.
Interestingly enough, both patients presented similar delta wave patterns despite different PR intervals. The polarity of delta waves in leads II, III, and aVF suggested that initial differential diagnosis should be made between right free wall and right mid-septal APs. The discordant polarity of the delta wave in leads V1 (negative) and V2 (slightly positive) is rather characteristic for the former. There are many algorithms for the localization of APs, depending on the appearance of delta waves on surface ECG. However, algorithms based on delta wave polarity on surface ECG have serious limitations due to the variable degree of pre-excitation (variable conduction over AV node and/or AP), the existence of multiple/complex APs, changes in heart position, and associated heart diseases (myocardial infarction, ventricular hypertrophy).
First-degree relatives of WPW patients have an increased prevalence of WPW syndrome. 2 Mutations in the PRKAG2 gene (7q34-q36) associated with HCM/glycogen deposits 3 or chromosome deletion (20p 12.3.) 4 associated with neuro-cognitive deficiencies have also been described in familial WPW. However, even in familial WPW APs, locations seem to be similar to sporadic WPW, 5 none of the known mutation being associated with aggregation of any particular anatomic location.
To the best of our knowledge, this is the first case of familial WPW with identical anatomical location of APs. This is even more interesting if one is considering the relatively low frequency of right free wall APs.
Conflict of interest: none declared.



