-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Rotondi, Fiore Manganelli, Emilio Di Lorenzo, Luciano Marino, Fiore Candelmo, Ferdinando Alfano, Giovanni Stanco, Giuseppe Rosato, Tako-tsubo cardiomyopathy in a patient with pacemaker syndrome, EP Europace, Volume 11, Issue 12, December 2009, Pages 1712–1714, https://doi.org/10.1093/europace/eup281
Close - Share Icon Share
Abstract
We report the case of a 71-year-old woman, with a dual chamber pacemaker (PM), in whom a PM syndrome, due to loss of atrial sensing and pacing, was associated with a tako-tsubo cardiomyopathy (TTC). The repositioning of the atrial lead immediately improved symptoms, whereas complete regression of left ventricular wall motion abnormalities occurred after 1 month. We hypothesize that haemodynamic and hormonal responses associated with a PM syndrome, such as increased levels of catecholamines, may account for TTC in our patient.
A 71-year-old woman, with a dual chamber (DDDR) pacemaker (PM) implanted 2 months earlier, was referred to our institution because of a 2 week history of asthenia and dyspnoea. She also complained of chest pain in the previous 24 h, lasting for ∼2 h. On admission, heart rate was 60 b.p.m. and the blood pressure was 90/50 mmHg. Blood test results revealed elevated levels of lactate dehydrogenase [peak 903 UI/L; normal value (nv) 250–620], aspartate aminotransferase (peak 72 UI/L; nv 15–46UI/L), creatine kinase isoenzyme MB mass (peak 4.8 ng/mL; nv 0.00–4 ng/mL), troponin I (peak 0.11 ng/mL; nv 0.00–0.06 ng/mL), epinephrine (162 pg/mL; nv 0.00–140 pg/mL), and norepinephrine (501 pg/mL; nv 0.00–450 pg/mL). An electrocardiogram (ECG) showed atrial and ventricular spikes at a rate of 60 b.p.m. with atrio-ventricular delay of 200 ms. Atrial spikes were not followed by atrial activity and the P-waves were seen on the T-waves, suggesting ventriculo-atrial retrograde conduction ( Figure 1 A ). An echocardiogram revealed depressed global left ventricular (LV) systolic function, dyskinesia of apical and mid-segments, and basal hyperkinesia ( Figure 3 A ).
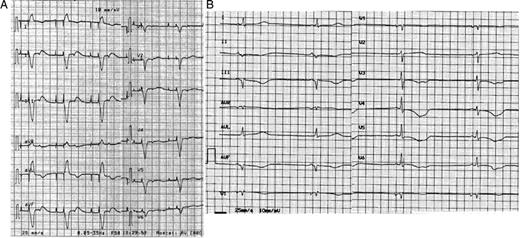
( A ) An ECG showing DDD pacing with ventriculo-atrial retrograde conduction. The atrial spikes were not followed by atrial activity. ( B ) An ECG, after reprogramming, showing VVI pacing with hysteresis at 30 beats per minute. HR = 33 bpm; QT = 0.72″, QTcF = 0.59″, T waves inversion in V3 to V6 and in II, III, aVF.
Pacemaker interrogation revealed atrial sensing and pacing failure. The PM mode was changed to VVI at a rate of 70 b.p.m. and with hysteresis at 30 b.p.m., in order to maximize spontaneous cardiac activity. Sinus bradycardia at 33 b.p.m., QTc interval prolongation (QT interval 0.72″; QTc interval according to Fridericia's formula 0.59″), and T-waves inversion in V3–V6 and in II, III, aVF appeared ( Figure 1 B ). The following day, heart rate was unchanged and the patient continued to have asthenia. Therefore, we repositioned the atrial lead. After restoration of the AAI mode, the patient reported symptomatic improvement. An ECG showed T-wave inversion in I and AVL (positive in the previous ECG) and T-wave flattening in III (negative in the previous ECG), persistence of QT interval prolongation, although the values decreased ( Figure 2 A ). A reduction in cardiac enzyme levels was observed, while echocardiographic LV abnormalities were unchanged. Coronary angiography did not show significant epicardial coronary ratery stenosis ( Figure 3 B ). She was discharged asymptomatic on therapy with aspirin and angiotensin-converting enzyme inhibitors after 2 days. At 1 month, an ECG showed AAI pacing with normal T-waves and QTc 0.44″ ( Figure 2 B ), whereas echocardiography demonstrated recovery of apical dyskinesia with normal LV systolic function. The catecholamine levels were normal.
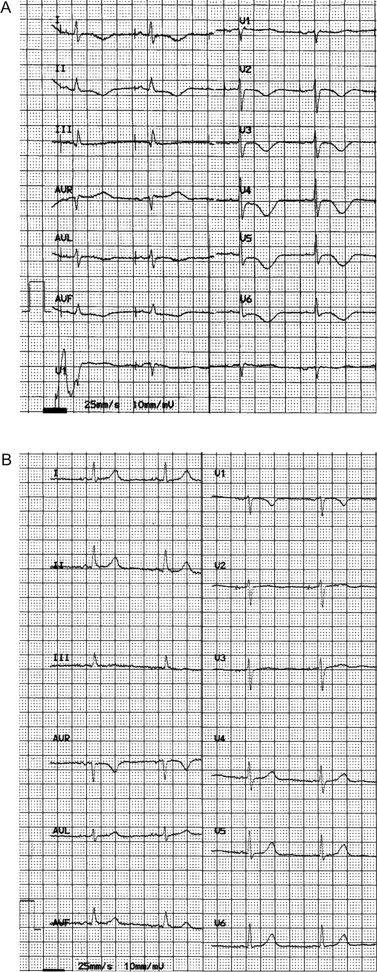
( A ) An ECG, after repositioning of the atrial lead, showing restoration of normal AAI pacing (60 bpm), QT = 0.52″, QTcF = 0.52″. T wave inversion in I and AVL (positive in the previous ECG) and T wave flattening in III (negative in the previous ECG) are seen. ( B ) At 1-month follow-up, an ECG showing AAI pacing (60 bpm), QT = 0.44″, QTcF = 0.44″ and normal T waves.
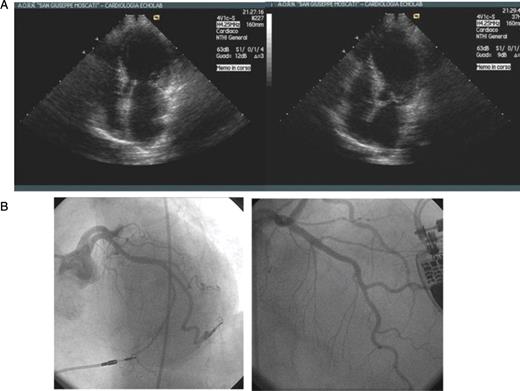
( A ) Echocardiographic 2D images from the four-chamber view showing apical, mid-septal and mid-anterior ventricular dyskinesia and basal hyperkinesia ( B ) Coronary angiography performed on third hospital day demonstrating no significant epicardial stenosis (LAO caudal and RAO cranial projection).
Discussion
The PM syndrome (PMS) is usually caused by inadequate atrio-ventricular synchronization and/or ventricular pacing with ventriculo-atrial retrograde conduction. 1 ‘Tako-tsubo’ cardiomyopathy (TTC) is characterized by transient electrocardiographic changes of ST segment with mild myocardial enzymatic release mimicking acute myocardial infarction, usually triggered by intense emotional stress. 2 The transient LV apical wall motion abnormality without coronary artery lesions presented by our patient has the features of TTC.
On the ECG immediately after the programming to VVI mode ( Figure 1 B ), the T-waves, even after recovery of spontaneous cardiac rhythm, retain the same vector as the QRS complex of paced beats, a pattern determined by the electrical cardiac memory post-pacing. 3 An ECG ( Figure 2 A ), performed 2 days after the end of ventricular stimulation, showed new T-wave inversion in I and AVL. This finding combined with the marked prolongation of the QT interval, already described in TTC, 4 is consistent with acute myocardial ischaemia rather than with cardiac memory. Moreover, persistence of borderline prolongation of QT intervals after 1 month without drugs that may influence this parameter, appears to be a characteristic of TTC. 4
To our best knowledge, the association of PMS and TTC has never been reported in the literature. Okamura et al. have described two patients with TTC after PM implantation. 5 However, in one patient, the procedure was complicated by pneumothorax and another patient experienced significant stress during implantion. In our patient, the triggering event was not the surgical procedure, because it had been uncomplicated and well tolerated. Although several pathophysiological mechanisms for TTC have been proposed, there is evidence suggesting that catecholamine-induced spasm of epicardial multivessel coronary arteries may play a role. 2 We speculate that the haemodynamic and hormonal responses associated with the PMS 1 might have precipitated TTC. Such a hypothesis is supported by the laboratory finding of a transient increase in plasma catecholamines. Our case suggests that not only ‘acute’ emotional stress but also a ‘chronic’ condition of enhanced-sympathetic activity may be responsible for this cardiomyopathy.
Conflicts of interest: none declared.



