-
PDF
- Split View
-
Views
-
Cite
Cite
Paul A. Scott, Paul R. Roberts, John M. Morgan, Trans-septal left ventricular endocardial pacing through a persistent left-sided superior vena cava, EP Europace, Volume 11, Issue 12, December 2009, Pages 1709–1711, https://doi.org/10.1093/europace/eup269
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) via the coronary sinus is not always possible. Left ventricular (LV) endocardial lead placement is a potential alternative, although its feasibility in patients with congenital heart disease is unknown. We report a case of endocardial LV pacing in a patient with a persistent left-sided superior vena cava. The procedure was successfully performed without complication, using standard equipment.
Case report
A 59-year-old gentleman was admitted to our institution for consideration of a trans-septal left ventricular (LV) endocardial lead. Three years before, he had a metallic aortic valve and root replacement for severe aortic regurgitation, with recurrent haemodynamically significant ventricular tachycardia post-operatively. His resting QRS was broad and an echocardiogram demonstrated severe LV systolic dysfunction. He therefore had a CRT-defibrillator inserted, with a bipolar LV lead (Easytrak 2 4517, Guidant, St Paul, MN, USA). During the procedure, he was noted to have a persistent left-sided superior vena cava (PLSVC) with an absent right-sided SVC ( Figure 1 ). He responded well, but unfortunately subsequently developed disabling symptoms of diaphragmatic stimulation and the LV lead was switched off.
Venogram (anteroposterior projection) demonstrating the PLSVC and absent right-sided SVC (arrowed).
Following CRT cessation, his symptoms worsened. Left ventricular lead revision was attempted but it was impossible to reposition the original lead. A venogram demonstrated the original vein was occluded with only one small target vessel remaining, and it was not possible to insert a new lead. He was therefore put forward for surgical epicardial lead placement. This was initially successful and resulted in symptomatic improvement; however, some weeks later, his symptoms deteriorated and the lead was found to have displaced. A further surgical procedure was performed with implantation of a new epicardial lead as part of a clinical trial (model 10808, Medtronic, Minneapolis, MN, USA). Although initially successful, the lead again displaced. Despite optimal medical therapy, the patient was still symptomatic and keen to be considered for a further attempt at CRT. He was therefore put forward for trans-septal LV endocardial lead placement.
In view of his metallic valve, he was converted from warfarin to an intravenous heparin infusion pre-procedurally. The infusion was stopped prior to the procedure but restarted once trans-septal access was safely achieved, and continued post-operatively until his warfarin was therapeutic.
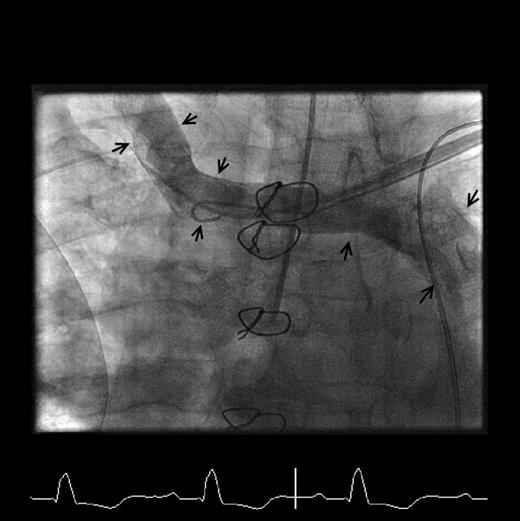
The LV lead was placed endocardially as described previously. 1 However, despite our previous experience with endocardial lead placement, the procedure was technically challenging (procedure time 265 min and screening time 64 min). Trans-septal puncture was performed from the femoral route using a radiofrequency needle (Baylis Medical, Montreal, Canada) and enlarged with a 9 Fr Channel sheath (Bard Inc., Tewkesbury, MA, USA) ( Figure 2 A ). A steerable introducer catheter (Select Site C304–L69, Medtronic) was advanced from the left subclavian vein via the PLSVC and coronary sinus to the inter-atrial septum. Under fluoroscopic guidance, the steerable introducer catheter was used to negotiate the trans-septal puncture site using a 0.035 inch angiographic guide wire as a vehicle for catheter support ( Figure 2 A ). Having gained access to the left atrium, the steerable introducer was advanced across mitral valve orifice into LV and orientated towards a pacing location on the posterior high lateral aspect of the LV endocardial wall. An active fixation polyurethane lead (Select Secure, 3830–69 cm, Medtronic) was then implanted ( Figure 2 B ).

( A ) Fluoroscopic image (anteroposterior projection) demonstrating the Channel sheath in the right atrium via the femoral vein, and a guidewire exiting the sheath and traversing the inter-atrial septum into the left atrium (white arrows). The Channel sheath has been used to enlarge the trans-septal puncture hole and following this has been withdrawn back into the right atrium to allow access of the steerable introducing catheter into the left atrium. The steerable introducing catheter (white dotted arrow) is seen entering the right atrium from the PLSVC via the coronary sinus, and traversing the inter-atrial septum through the puncture site made by the Channel sheath. The left ventricular lead (black arrow) is seen entering the left heart through the steerable introducing catheter. ( B ) Fluoroscopic image (anteroposterior projection) demonstrating the final position of the LV lead on the lateral LV wall (white dotted arrow).
The patient responded well with improvement in functional class. After 4-month follow-up, lead parameters were stable with no complications. The final lead positions are shown in Figure 3 .
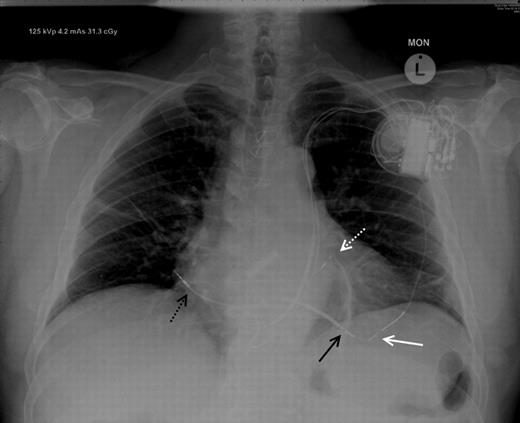
Chest X-ray (posteroanterior projection) demonstrating the final positions of the LV endocardial lead (white dotted line), right ventricular lead (black arrow), right atrial lead (black dotted arrow), and redundant epicardial LV lead (white arrow).
Discussion
The conventional approach to CRT is to use coronary sinus tributaries or place an epicardial lead surgically. Techniques have recently been developed allowing the trans-septal placement of an LV lead endocardially. 1 , 2 Such techniques are of particular value in cases such as ours, where conventional routes to LV pacing have failed or are contraindicated. The presence of a PLSVC is a common venous anomaly with an estimated prevalence of ∼0.5% in normal hearts and 3–10% of patients with congenital heart disease. 3 Its association with an absent right-sided SVC is less common. Although the implantation of a coronary sinus lead via a PLSVC has been described, to our knowledge, this is the first report of the trans-septal placement of an endocardial LV lead via this anomaly.
Left ventricular endocardial pacing does have potential limitations. The inadvertent placement of an endocardial LV lead is associated with the occurrence of systemic thrombo-embolism. 4 However, in cases of deliberate LV endocardial pacing, where patients have been carefully anticoagulated, there have been no described cases of thrombo-embolism. 1 , 2 In this case, we chose to use a polyurethane lead for LV pacing. This type of lead construction has been associated with low fibrous encroachment and thrombo-embolism risks, which is consistent with our clinical experience, and we would advocate the use of such a lead in a similar case. 1 , 5 , 6
With the long-term passage of a pacing lead across the mitral valve, there is a theoretical risk of valve damage. However, this has not been borne out by clinical data. 2 An additional concern is the difficulty in managing infection of an endocardial system. Adherent vegetations may embolize systemically and any extraction would need to be surgical. To date, there have been no reported cases of endocardial device infection.
With the growing use of CRT, coupled with the limitations of conventional LV lead access, the placement of trans-septal LV leads is likely to become increasingly important. Our case demonstrates that using conventional tools, it is possible to successfully place an endocardial LV lead trans-septally in patients with PLSVC, a common venous anomaly.
Conflicts of interest: J.M.M. and P.R.R. have received Honoraria and research grants from Medtronic, St Jude, Sorin, and Boston Scientific. P.A.S. is supported by an educational grant from Medtronic.



