-
PDF
- Split View
-
Views
-
Cite
Cite
Krystle Lander, Priyanka Thakeria, Sachin Nayyar, Prophylactic anticoagulation in sinus rhythm for stroke prevention in cardiovascular disease: contemporary meta-analysis of large randomized trials, European Journal of Preventive Cardiology, Volume 28, Issue 17, December 2021, Pages 1939–1948, https://doi.org/10.1093/eurjpc/zwab113
Close - Share Icon Share
Abstract
Anticoagulation with non-vitamin K oral anticoagulants (NOACs) to prevent stroke is a mainstay of atrial fibrillation (AF) management. However, multiple cardiovascular diseases (CVDs) are associated with elevated ischaemic stroke risk even in sinus rhythm. In this meta-analysis, we assess efficacy and safety of prophylactic NOAC agents for stroke prevention in patients without AF.
A search was conducted for randomized controlled trials (RCTs) that evaluated an NOAC and control drug (placebo or antiplatelet) in non-AF patients with mixed CVD. The primary efficacy and safety outcomes were ischaemic stroke and major bleeding, respectively. Results were stratified based on primary- and mini-NOAC doses. Thirteen RCTs were identified with a total of 89 383 patients with CVD in sinus rhythm (53 778 on NOAC, 35 605 on control drug; mean age 65.5 ± 2.7 years). Over a mean follow-up of 18.3 months, 1429 (1.6%) ischaemic strokes occurred. Use of NOAC was associated with 26% reduction in stroke [odds ratio (OR) 0.74, 95% confidence interval (CI) 0.62–0.87; 1.1 vs. 1.8 events per 100 person-years], with numbers needed to treat of 153 patients to prevent one stroke. Major bleeding was increased with NOAC (OR 1.74, 95% CI 1.44–2.09; 2.1 vs. 1.0 events per 100 person-years). The weighted net clinical benefit (wNCB, composite of ischaemic stroke and bleeding) did not suggest a favourable effect with any NOAC dose (wNCB for primary-dose: −0.35; mini-dose: −0.06).
Current evidence does not support use of NOACs for stroke prevention in non-AF CVD population as risk of major bleeding still exceeds ischaemic stroke benefit.
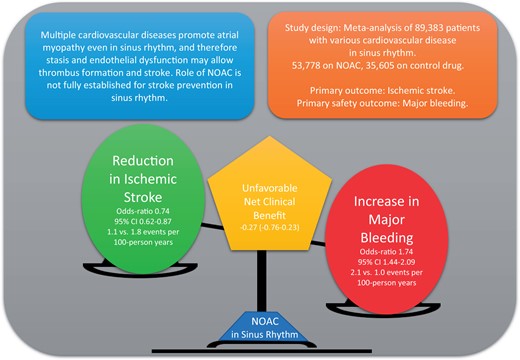
Multiple CVDs can promote thrombogenic state even in the absence of AF, which may cause ischaemic stroke. This meta-analysis of 89 383 patients with mixed CVD in sinus rhythm compares reduction in ischaemic stroke rate with the use NOACs in comparison to placebo or antiplatelet drugs. We found a significant reduction (26%, OR 0.74 95% CI 0.62—0.87) in stroke rate but there was harm from major bleeding and intracranial haemorrhage with both primary- and mini-dose of NOACs. Future studies should test safety of NOACs in highest stroke risk patients such as those with atrial cardiopathy in sinus rhythm.
Introduction
Stroke is a disastrous occurrence that can often be fatal or produce immense public morbidity. Oral anticoagulation is a mainstay treatment to prevent ischaemic stroke in the presence of atrial fibrillation (AF) and cardiovascular disease (CVD).1,2 In the last 10 years, large, randomized controlled trials (RCTs) comparing use of non-vitamin K oral anticoagulant (NOAC) agents and warfarin in non-valvular AF have demonstrated non-inferiority of NOAC in stroke prevention and more importantly a decisive reduction in risk of bleeding as against warfarin.3–6
The question of whether oral anticoagulation in sinus rhythm will reduce stroke is pertinent given the increasing evidence to support that AF is a marker of atrial cardiopathy and fibrosis, and potentially is a bystander to stroke, rather than the causative agent of it.7,8 Patients with established CVD may have conditions that fulfil Virchow’s triad for thrombogenesis even in sinus rhythm.9 In fact, in the pre-NOAC era, investigations into the utility of warfarin in patients with mixed CVD in sinus rhythm showed a 50% reduction in the risk of ischaemic stroke. The clinical benefit of warfarin was, however, offset by several fold increased risk of major bleeding compared to an antiplatelet drug. The overall evidence therefore did not support that warfarin is superior to antiplatelets or placebo for patients in sinus rhythm.10,11 Most recently, the COMMANDER HF trial2,12,13 was conducted in patients with heart failure (HF) in sinus rhythm, comparing rivaroxaban with placebo. While negative for its composite primary outcome, a post hoc analysis showed a reduction in the ischaemic stroke with rivaroxaban and more importantly similar rate of bleeding compared with placebo. In contrast, the results of embolic stroke of undetermined source (ESUS) trials (which compared NOAC alone to aspirin alone in patients with embolic source of unknown source) did not show a positive advantage with NOAC and were associated with a higher risk of bleeding.14,15
It is in this context that this meta-analysis of patients who have an indication oral anticoagulation which is not AF is undertaken, 10 years after the widespread uptake and use of NOACs, to assess their benefit regarding the reduction of ischaemic stroke and compare this with the harm from bleeding which can accompany this therapy. We also sought to assess the therapeutic safety advantage of both primary- and mini-doses of NOAC agents.
Methods
We conducted a systematic review and meta-analysis of prospective RCTs that compared an NOAC with placebo, aspirin, or dual antiplatelet therapy (DAPT) for a non-AF indication. This study was designed according to the PRISMA statement (Supplementary material online, Table SI).16
Search strategy
MEDLINE OVID, Cochrane, PubMed, and Embase databases were searched for RCTs published from 1 January 2010 until 30 April 2020. Studies were identified by one author (K.L.) with the assistance of a research librarian. The search strategy was formed using major medical subject headings combined with text and key words for pathologies with an indication for anticoagulation which was not AF. These pathologies were acute coronary syndrome (ACS), coronary artery disease (CAD), unstable angina, HF, venous thromboembolism (VTE), deep vein thrombosis, peripheral arterial disease (PAD), and ischaemic stroke/cerebrovascular accident, or synonyms of these. These were combined with synonyms of NOACs. The complete search strategy is listed in the Supplementary material online, Methods. An example of the search strategy in PubMed was [(‘anticoagulation’ OR ‘NOAC’ OR ‘apixaban’ OR ‘edoxaban’ OR ‘rivaroxaban’ OR ‘dabigatran’) AND (‘CAD’ OR ‘coronary artery disease’ OR ‘ACS’ OR ‘acute coronary syndrome’ OR ‘STEMI’ OR ‘NSTEMI’)] with such search strategy repeated for each pathology. The reference lists of all studies included in our meta-analysis were also reviewed to identify other acceptable publications.
Study selection
Studies were eligible if they met the following criteria: (i) RCT, (ii) written in English, (iii) non-AF indication for anticoagulation, (iv) compared an NOAC with placebo, aspirin, or DAPT, (v) minimum 3 months duration of anticoagulation, and (vi) ischaemic stroke as a predefined and separate outcome. The title and abstracts were reviewed by two authors (K.L. and P.T.) for suitability. When studies could not be excluded from the abstract, or the study was eligible for inclusion, the full text was reviewed. Disagreement was resolved by consensus or by a third author (S.N.). Special populations with extremely high rates of thrombotic events such as mechanical valve, antiphospholipid syndrome, and left ventricular assist device were excluded.
Quality assessment
The quality of the included studies was assessed on Jadad modified scale and was found high (Supplementary material online, Table SII). The risk of bias was assessed using revised Cochrane risk-of-bias tool for randomized trials.17 The risk of bias was assessed as low overall (Supplementary material online, Figure SI). One trial had ‘some concern’ of uneven distribution of comorbidities between the groups.18
Data extraction and outcomes
Data were extracted and corroborated by two investigators (K.L. and P.T.) using a standardized data extraction sheet. The primary efficacy outcome was the rate of ischaemic stroke. The primary safety outcome was the rates of major bleeding which also counted intracranial haemorrhage (ICH). When multiple definitions of major bleeding were described in a trial, the International Society of Thrombosis and Haemostasis (ISTH) definition was chosen preferentially as this provided the greatest degree of consistency among trials. Demographic data including age and gender, rates of existing CVD were extracted. The prevalence of pre-existing or new AF, if present, was accounted for.
Statistical analysis
The weighting factor reflects the relative impact in terms of death and disability of experiencing an ischaemic stroke while not receiving NOAC, vs. suffering a major bleed and ICH while receiving NOAC. We used the weighting scheme reported by Lip et al.,21 who assigned w1 = 1, w2 = 0.67, and w3 = 2.44 in decision-making around anticoagulation for AF. We also included a model that incorporated no weighting (weight of 1.0 for all events).
Results
A total of 2005 studies were identified. Of these, 13 RCTs12–15,18,22–30 were selected for inclusion in the meta-analysis (Supplementary material online, Figure SII). A brief summary of the included trials and their definitions for ischaemic stroke and bleeding is provided in Supplementary material online, Table SIV.
The included studies had a combined total of 89 383 patients (53 778 on NOAC, 35 605 on control drug). The mean age of participants was 65.5 ± 2.7 years. The CVD included CAD or ACS (78.3%), hypertension (81.5%), diabetes mellitus (35.1%), PAD (45.4%), HF (18.4%), renal disease (22.9%), and previous stroke (21.7%). A full breakdown of these demographic data is provided in Table 1.
| Trial Name . | Total population . | Mean follow-up . | Mean age . | Sex (male) . | Hypertension . | T2DM . | Heart failure a . | ACS . | CAD . | PAD . | Strokeb . | Renal impairment . | Aspirin monotherapy . | P2Y12 inhibitor monotherapy . | DAPT . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO AC, n . | Con trol, n . | Months . | NO AC, n . | Con trol, n . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | |
| APPRAISE-2 | 3705 | 3687 | 8 | 67 | 67 | 2496 (76.4) | 2518 (68.3) | NA | NA | 1804 (48.7) | 1732 (47) | 1023 (27.6) | 1053 (28.6) | 3705 (100) | 3687 (100) | 3705 (100) | 3687 (100) | 664 (17.9) | 674 (18.3) | 378 (10.2) | 364 (9.9) | 1048 (28.3) | 1089 (29.5) | 593 (16) | 589 (16) | 0 | 0 | 3001 (81) | 2986 (81) |
| APPRAISE J | 99 | 52 | 6 | 65 | 63.9 | 89 (89.9) | 42 (80.8) | NA | NA | 34 (34.3) | 26 (50) | 10 (10) | 4 (7.7) | 99 (100) | 52 (100) | 99 (100) | 52 (100) | 1 (1.0) | 1 (1.9) | 6 (4) | 0 (0) | 60 (60.5) | 29 (55.8) | 3 (3) | 2 (4) | 0 | 0 | 97 (97) | 50 (96.2) |
| ATLAS ACS 2- TIMI 51 | 10 350 | 5176 | 13 | 61.9 | 61.5 | 7718 (74.6) | 3881 (75) | 6969 (67.3) | 3494 (67.5) | 3317 (32) | 1647 (31.8) | NA | NA | 10 350 (100) | 5176 (100) | 10 350 (100) | 5176 (100) | NA | NA | 275 (2.7) | 127 (2.5) | NA | NA | 600 (5.8) | 300 (5.8) | 0 | 0 | 9602 (92.8) | 4811 (92.9) |
| COMMANDER HF | 2507 | 2515 | 21.1 | 66.5 | 66.3 | 1956 (78) | 1916 (76.2) | 1897 (75.7) | 1886 (75) | 1024 (40.8) | 1028 (40.9) | 2507 (100) | 2515 (100) | 1911 (76.2) | 1892 (75.2) | 1911 (100) | 1892 (100) | NA | NA | 208 (8.3) | 245 (9.7) | 965 (38.5) | 980 (39) | 1421 (56.7) | 1506 (59.9) | 0 | 0 | 901 (36.2) | 839 (33.4) |
| COMPASS 2.5 mg | 8313 | 4130 | 23.7 | 69 | 69 | 6577 (79) | 3307 (80) | 6280 (76) | 3109 (75) | 3043 (37) | 1520 (37) | 1909 (23) | 976 (23) | 5654 (68) | 2860 (69) | 8131 (100) | 4130 (100) | 1656 (20) | 820 (20) | 279 (3) | 134 (3) | NA | NA | 8313 (100) | 4130 (100) | 0 | 0 | 0 | 0 |
| COMPASS 5 mg | 8250 | 4131 | 23.7 | 69 | 69 | 6600 (80) | 3308 (80) | 6214 (75) | 3109 (75) | 3015 (37) | 1250 (37) | 1893 (23) | 976 (23) | 5653 (69) | 2860 (69) | 8250 (100) | 4131 (100) | 1609 (20) | 821 (20) | 250 (3) | 134 (3) | NA | NA | 0 | 4131 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 2.5 mg | 2492 | 1252 | 21 | 67 | 67 | 1774 (71) | 893 (71) | 1966 (78.9) | 1008 (80.6) | 1100 (44.1) | 552 (44.1) | NA | NA | NA | NA | 1656 (66.5) | 820 (65.5) | 2492 (100) | 1252 (100) | 171 (6.9) | 77 (6.2) | 688 (27.6) | 353 (28.2) | 2492 (100) | 1252 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 5 mg | 2474 | 1252 | 21 | 67 | 67 | 1800 (73) | 894 (71) | 1939 (78.4) | 1009 (80.6) | 1083 (43.8) | 552 (44.1) | NA | NA | NA | NA | 1609 (65) | 820 (65.5) | 2472 (100) | 1252 (100) | 177 (7.2) | 77 (6.2) | 681 (27.5) | 353 (28.2) | 0 | 1252 (100) | 0 | 0 | 0 | 0 |
| EINSTEIN CHOICE | 2234 | 1131 | 11.5 | 59.5 | 60 | 1222 (54.8) | 643 (56.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 92 (4) | 64 (5.6) | 0 | 1131 (100) | 0 | 0 | 0 | 0 |
| GEMINI ACS 1 | 1519 | 1518 | 9.7 | 62 | 63 | 1134 (75) | 1141 (75) | 1085 (71) | 1139 (75) | 446 (29) | 469 (30) | 157 (10) | 153 (10) | 1519 (100) | 1518 (100) | 1519 (100) | 1518 (100) | 66 (4) | 74 (5) | NA | NA | NA | NA | 0 | 0 | 1519 (100) | 0 | 0 | 1518 (100) |
| MANAGE | 877 | 877 | 16 | 70 | 70 | 453 (52) | 443 (51) | 585 (67) | 587 (67) | 222 (25) | 243 (27) | NA | NA | 116 (13) | 110 (13) | NA | NA | 124 (14) | 128 (15) | 29 (3) | 42 (5) | NA | NA | 588 (67) | 561 (64) | 23 (3) | 41 (5) | 12 (1) | 21 (3) |
| NAVIGATE ESUS | 3609 | 3604 | 11 | 66.9 | 66.9 | 2232 (61) | 2204 (61) | 2782 (77) | 2803 (78) | 889 (25) | 917 (25) | NA | NA | NA | NA | NA | NA | NA | NA | 3609 (100) | 3604 (100) | NA | NA | 0 | 3604 (100) | 0 | 0 | 0 | 0 |
| RE-DEEM | 1490 | 371 | 5.4 | 61.5 | 61.5 | 1121 (75.4) | 290 (78.4) | 995 (66.9) | 243 (65.5) | 471 (31.7) | 109 (29.4) | 166 (11.2) | 50 (13.5) | 432 (29) | 106 (28.6) | NA | NA | 101 (6.8) | 19 (5.1) | NA | NA | 112 (7.5) | 28 (7.5) | 6 (0.4) | 2 (0.4) | 0 | 0 | 1478 (99.2) | 368 (99.2) |
| RESPECT ESUS | 2695 | 2695 | 19 | 64.5 | 63.9 | 1694 (62.9) | 1709 (63.4) | 1996 (74.1) | 1985 (73.7) | 585 (21.7) | 639 (23.7) | 117 (4.3) | 124 (4.6) | 168 (6.2) | 172 (6.4) | 301 (11.2) | 276 (10.2) | NA | NA | 2695 (100) | 2695 (100) | 227 (8.4) | 203 (7.5) | 72 (2.9) | 2695 (100) | 0 | 0 | 0 | 0 |
| VOYAGER | 3286 | 3278 | 28 | 67 | 67 | 2439 (74.2) | 2421 (73.9) | 2684 (81.7) | 2658 (81.1) | 1313 (40) | 1316 (40.1) | NA | NA | 365 (11.1) | 349 (10.6) | 1052 (32) | 1015 (31) | 3286 (100) | 3278 (100) | NA | NA | 661 (20.1) | 666 (20.3) | 1582 (48.6) | 1579 (48.6) | 0 | 0 | 1658 (50.5) | 1655 (50.5) |
| Totalc | 53 900 | 35 669 | 18.3 | 65.5 | 65.5 | 39 305 (72.9) | 25 610 (71.8) | 35 392 (79.4) | 23 031 (83.7) | 18 346 (35.5) | 12 000 (34.7) | 5375 (18.8) | 3436 (18.0) | 29 972 (75.3) | 18 782 (74.7) | 37 531 (81.6) | 22 502 (75.0) | 12 471 (42.7) | 8319 (29.2) | 8071 (17.8) | 7505 (25.6) | 3873 (21.9) | 3099 (23.9) | 15 665 (29) | 22 734 (63.7) | 1542 (2.8) |
| 16 755 (31.1) | 12 248 (34.3) |
| Trial Name . | Total population . | Mean follow-up . | Mean age . | Sex (male) . | Hypertension . | T2DM . | Heart failure a . | ACS . | CAD . | PAD . | Strokeb . | Renal impairment . | Aspirin monotherapy . | P2Y12 inhibitor monotherapy . | DAPT . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO AC, n . | Con trol, n . | Months . | NO AC, n . | Con trol, n . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | |
| APPRAISE-2 | 3705 | 3687 | 8 | 67 | 67 | 2496 (76.4) | 2518 (68.3) | NA | NA | 1804 (48.7) | 1732 (47) | 1023 (27.6) | 1053 (28.6) | 3705 (100) | 3687 (100) | 3705 (100) | 3687 (100) | 664 (17.9) | 674 (18.3) | 378 (10.2) | 364 (9.9) | 1048 (28.3) | 1089 (29.5) | 593 (16) | 589 (16) | 0 | 0 | 3001 (81) | 2986 (81) |
| APPRAISE J | 99 | 52 | 6 | 65 | 63.9 | 89 (89.9) | 42 (80.8) | NA | NA | 34 (34.3) | 26 (50) | 10 (10) | 4 (7.7) | 99 (100) | 52 (100) | 99 (100) | 52 (100) | 1 (1.0) | 1 (1.9) | 6 (4) | 0 (0) | 60 (60.5) | 29 (55.8) | 3 (3) | 2 (4) | 0 | 0 | 97 (97) | 50 (96.2) |
| ATLAS ACS 2- TIMI 51 | 10 350 | 5176 | 13 | 61.9 | 61.5 | 7718 (74.6) | 3881 (75) | 6969 (67.3) | 3494 (67.5) | 3317 (32) | 1647 (31.8) | NA | NA | 10 350 (100) | 5176 (100) | 10 350 (100) | 5176 (100) | NA | NA | 275 (2.7) | 127 (2.5) | NA | NA | 600 (5.8) | 300 (5.8) | 0 | 0 | 9602 (92.8) | 4811 (92.9) |
| COMMANDER HF | 2507 | 2515 | 21.1 | 66.5 | 66.3 | 1956 (78) | 1916 (76.2) | 1897 (75.7) | 1886 (75) | 1024 (40.8) | 1028 (40.9) | 2507 (100) | 2515 (100) | 1911 (76.2) | 1892 (75.2) | 1911 (100) | 1892 (100) | NA | NA | 208 (8.3) | 245 (9.7) | 965 (38.5) | 980 (39) | 1421 (56.7) | 1506 (59.9) | 0 | 0 | 901 (36.2) | 839 (33.4) |
| COMPASS 2.5 mg | 8313 | 4130 | 23.7 | 69 | 69 | 6577 (79) | 3307 (80) | 6280 (76) | 3109 (75) | 3043 (37) | 1520 (37) | 1909 (23) | 976 (23) | 5654 (68) | 2860 (69) | 8131 (100) | 4130 (100) | 1656 (20) | 820 (20) | 279 (3) | 134 (3) | NA | NA | 8313 (100) | 4130 (100) | 0 | 0 | 0 | 0 |
| COMPASS 5 mg | 8250 | 4131 | 23.7 | 69 | 69 | 6600 (80) | 3308 (80) | 6214 (75) | 3109 (75) | 3015 (37) | 1250 (37) | 1893 (23) | 976 (23) | 5653 (69) | 2860 (69) | 8250 (100) | 4131 (100) | 1609 (20) | 821 (20) | 250 (3) | 134 (3) | NA | NA | 0 | 4131 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 2.5 mg | 2492 | 1252 | 21 | 67 | 67 | 1774 (71) | 893 (71) | 1966 (78.9) | 1008 (80.6) | 1100 (44.1) | 552 (44.1) | NA | NA | NA | NA | 1656 (66.5) | 820 (65.5) | 2492 (100) | 1252 (100) | 171 (6.9) | 77 (6.2) | 688 (27.6) | 353 (28.2) | 2492 (100) | 1252 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 5 mg | 2474 | 1252 | 21 | 67 | 67 | 1800 (73) | 894 (71) | 1939 (78.4) | 1009 (80.6) | 1083 (43.8) | 552 (44.1) | NA | NA | NA | NA | 1609 (65) | 820 (65.5) | 2472 (100) | 1252 (100) | 177 (7.2) | 77 (6.2) | 681 (27.5) | 353 (28.2) | 0 | 1252 (100) | 0 | 0 | 0 | 0 |
| EINSTEIN CHOICE | 2234 | 1131 | 11.5 | 59.5 | 60 | 1222 (54.8) | 643 (56.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 92 (4) | 64 (5.6) | 0 | 1131 (100) | 0 | 0 | 0 | 0 |
| GEMINI ACS 1 | 1519 | 1518 | 9.7 | 62 | 63 | 1134 (75) | 1141 (75) | 1085 (71) | 1139 (75) | 446 (29) | 469 (30) | 157 (10) | 153 (10) | 1519 (100) | 1518 (100) | 1519 (100) | 1518 (100) | 66 (4) | 74 (5) | NA | NA | NA | NA | 0 | 0 | 1519 (100) | 0 | 0 | 1518 (100) |
| MANAGE | 877 | 877 | 16 | 70 | 70 | 453 (52) | 443 (51) | 585 (67) | 587 (67) | 222 (25) | 243 (27) | NA | NA | 116 (13) | 110 (13) | NA | NA | 124 (14) | 128 (15) | 29 (3) | 42 (5) | NA | NA | 588 (67) | 561 (64) | 23 (3) | 41 (5) | 12 (1) | 21 (3) |
| NAVIGATE ESUS | 3609 | 3604 | 11 | 66.9 | 66.9 | 2232 (61) | 2204 (61) | 2782 (77) | 2803 (78) | 889 (25) | 917 (25) | NA | NA | NA | NA | NA | NA | NA | NA | 3609 (100) | 3604 (100) | NA | NA | 0 | 3604 (100) | 0 | 0 | 0 | 0 |
| RE-DEEM | 1490 | 371 | 5.4 | 61.5 | 61.5 | 1121 (75.4) | 290 (78.4) | 995 (66.9) | 243 (65.5) | 471 (31.7) | 109 (29.4) | 166 (11.2) | 50 (13.5) | 432 (29) | 106 (28.6) | NA | NA | 101 (6.8) | 19 (5.1) | NA | NA | 112 (7.5) | 28 (7.5) | 6 (0.4) | 2 (0.4) | 0 | 0 | 1478 (99.2) | 368 (99.2) |
| RESPECT ESUS | 2695 | 2695 | 19 | 64.5 | 63.9 | 1694 (62.9) | 1709 (63.4) | 1996 (74.1) | 1985 (73.7) | 585 (21.7) | 639 (23.7) | 117 (4.3) | 124 (4.6) | 168 (6.2) | 172 (6.4) | 301 (11.2) | 276 (10.2) | NA | NA | 2695 (100) | 2695 (100) | 227 (8.4) | 203 (7.5) | 72 (2.9) | 2695 (100) | 0 | 0 | 0 | 0 |
| VOYAGER | 3286 | 3278 | 28 | 67 | 67 | 2439 (74.2) | 2421 (73.9) | 2684 (81.7) | 2658 (81.1) | 1313 (40) | 1316 (40.1) | NA | NA | 365 (11.1) | 349 (10.6) | 1052 (32) | 1015 (31) | 3286 (100) | 3278 (100) | NA | NA | 661 (20.1) | 666 (20.3) | 1582 (48.6) | 1579 (48.6) | 0 | 0 | 1658 (50.5) | 1655 (50.5) |
| Totalc | 53 900 | 35 669 | 18.3 | 65.5 | 65.5 | 39 305 (72.9) | 25 610 (71.8) | 35 392 (79.4) | 23 031 (83.7) | 18 346 (35.5) | 12 000 (34.7) | 5375 (18.8) | 3436 (18.0) | 29 972 (75.3) | 18 782 (74.7) | 37 531 (81.6) | 22 502 (75.0) | 12 471 (42.7) | 8319 (29.2) | 8071 (17.8) | 7505 (25.6) | 3873 (21.9) | 3099 (23.9) | 15 665 (29) | 22 734 (63.7) | 1542 (2.8) |
| 16 755 (31.1) | 12 248 (34.3) |
ACS, acute coronary syndrome; CAD, coronary artery disease; DAPT, dual antiplatelet (aspirin + P2Y12 inhibitor) therapy; ESUS, embolic stroke of undetermined source; NA, Not available; NOAC, non-vitamin K oral anticoagulant; PAD, peripheral artery disease; T2DM, Type 2 diabetes mellitus.
Heart failure (left ventricular ejection fraction <40% or clinical heart failure).
Stroke as defined by the original trial.
After accounting for dropout patients, final combined total was 53 778 on NOAC and 35 605 on control drug.
| Trial Name . | Total population . | Mean follow-up . | Mean age . | Sex (male) . | Hypertension . | T2DM . | Heart failure a . | ACS . | CAD . | PAD . | Strokeb . | Renal impairment . | Aspirin monotherapy . | P2Y12 inhibitor monotherapy . | DAPT . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO AC, n . | Con trol, n . | Months . | NO AC, n . | Con trol, n . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | |
| APPRAISE-2 | 3705 | 3687 | 8 | 67 | 67 | 2496 (76.4) | 2518 (68.3) | NA | NA | 1804 (48.7) | 1732 (47) | 1023 (27.6) | 1053 (28.6) | 3705 (100) | 3687 (100) | 3705 (100) | 3687 (100) | 664 (17.9) | 674 (18.3) | 378 (10.2) | 364 (9.9) | 1048 (28.3) | 1089 (29.5) | 593 (16) | 589 (16) | 0 | 0 | 3001 (81) | 2986 (81) |
| APPRAISE J | 99 | 52 | 6 | 65 | 63.9 | 89 (89.9) | 42 (80.8) | NA | NA | 34 (34.3) | 26 (50) | 10 (10) | 4 (7.7) | 99 (100) | 52 (100) | 99 (100) | 52 (100) | 1 (1.0) | 1 (1.9) | 6 (4) | 0 (0) | 60 (60.5) | 29 (55.8) | 3 (3) | 2 (4) | 0 | 0 | 97 (97) | 50 (96.2) |
| ATLAS ACS 2- TIMI 51 | 10 350 | 5176 | 13 | 61.9 | 61.5 | 7718 (74.6) | 3881 (75) | 6969 (67.3) | 3494 (67.5) | 3317 (32) | 1647 (31.8) | NA | NA | 10 350 (100) | 5176 (100) | 10 350 (100) | 5176 (100) | NA | NA | 275 (2.7) | 127 (2.5) | NA | NA | 600 (5.8) | 300 (5.8) | 0 | 0 | 9602 (92.8) | 4811 (92.9) |
| COMMANDER HF | 2507 | 2515 | 21.1 | 66.5 | 66.3 | 1956 (78) | 1916 (76.2) | 1897 (75.7) | 1886 (75) | 1024 (40.8) | 1028 (40.9) | 2507 (100) | 2515 (100) | 1911 (76.2) | 1892 (75.2) | 1911 (100) | 1892 (100) | NA | NA | 208 (8.3) | 245 (9.7) | 965 (38.5) | 980 (39) | 1421 (56.7) | 1506 (59.9) | 0 | 0 | 901 (36.2) | 839 (33.4) |
| COMPASS 2.5 mg | 8313 | 4130 | 23.7 | 69 | 69 | 6577 (79) | 3307 (80) | 6280 (76) | 3109 (75) | 3043 (37) | 1520 (37) | 1909 (23) | 976 (23) | 5654 (68) | 2860 (69) | 8131 (100) | 4130 (100) | 1656 (20) | 820 (20) | 279 (3) | 134 (3) | NA | NA | 8313 (100) | 4130 (100) | 0 | 0 | 0 | 0 |
| COMPASS 5 mg | 8250 | 4131 | 23.7 | 69 | 69 | 6600 (80) | 3308 (80) | 6214 (75) | 3109 (75) | 3015 (37) | 1250 (37) | 1893 (23) | 976 (23) | 5653 (69) | 2860 (69) | 8250 (100) | 4131 (100) | 1609 (20) | 821 (20) | 250 (3) | 134 (3) | NA | NA | 0 | 4131 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 2.5 mg | 2492 | 1252 | 21 | 67 | 67 | 1774 (71) | 893 (71) | 1966 (78.9) | 1008 (80.6) | 1100 (44.1) | 552 (44.1) | NA | NA | NA | NA | 1656 (66.5) | 820 (65.5) | 2492 (100) | 1252 (100) | 171 (6.9) | 77 (6.2) | 688 (27.6) | 353 (28.2) | 2492 (100) | 1252 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 5 mg | 2474 | 1252 | 21 | 67 | 67 | 1800 (73) | 894 (71) | 1939 (78.4) | 1009 (80.6) | 1083 (43.8) | 552 (44.1) | NA | NA | NA | NA | 1609 (65) | 820 (65.5) | 2472 (100) | 1252 (100) | 177 (7.2) | 77 (6.2) | 681 (27.5) | 353 (28.2) | 0 | 1252 (100) | 0 | 0 | 0 | 0 |
| EINSTEIN CHOICE | 2234 | 1131 | 11.5 | 59.5 | 60 | 1222 (54.8) | 643 (56.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 92 (4) | 64 (5.6) | 0 | 1131 (100) | 0 | 0 | 0 | 0 |
| GEMINI ACS 1 | 1519 | 1518 | 9.7 | 62 | 63 | 1134 (75) | 1141 (75) | 1085 (71) | 1139 (75) | 446 (29) | 469 (30) | 157 (10) | 153 (10) | 1519 (100) | 1518 (100) | 1519 (100) | 1518 (100) | 66 (4) | 74 (5) | NA | NA | NA | NA | 0 | 0 | 1519 (100) | 0 | 0 | 1518 (100) |
| MANAGE | 877 | 877 | 16 | 70 | 70 | 453 (52) | 443 (51) | 585 (67) | 587 (67) | 222 (25) | 243 (27) | NA | NA | 116 (13) | 110 (13) | NA | NA | 124 (14) | 128 (15) | 29 (3) | 42 (5) | NA | NA | 588 (67) | 561 (64) | 23 (3) | 41 (5) | 12 (1) | 21 (3) |
| NAVIGATE ESUS | 3609 | 3604 | 11 | 66.9 | 66.9 | 2232 (61) | 2204 (61) | 2782 (77) | 2803 (78) | 889 (25) | 917 (25) | NA | NA | NA | NA | NA | NA | NA | NA | 3609 (100) | 3604 (100) | NA | NA | 0 | 3604 (100) | 0 | 0 | 0 | 0 |
| RE-DEEM | 1490 | 371 | 5.4 | 61.5 | 61.5 | 1121 (75.4) | 290 (78.4) | 995 (66.9) | 243 (65.5) | 471 (31.7) | 109 (29.4) | 166 (11.2) | 50 (13.5) | 432 (29) | 106 (28.6) | NA | NA | 101 (6.8) | 19 (5.1) | NA | NA | 112 (7.5) | 28 (7.5) | 6 (0.4) | 2 (0.4) | 0 | 0 | 1478 (99.2) | 368 (99.2) |
| RESPECT ESUS | 2695 | 2695 | 19 | 64.5 | 63.9 | 1694 (62.9) | 1709 (63.4) | 1996 (74.1) | 1985 (73.7) | 585 (21.7) | 639 (23.7) | 117 (4.3) | 124 (4.6) | 168 (6.2) | 172 (6.4) | 301 (11.2) | 276 (10.2) | NA | NA | 2695 (100) | 2695 (100) | 227 (8.4) | 203 (7.5) | 72 (2.9) | 2695 (100) | 0 | 0 | 0 | 0 |
| VOYAGER | 3286 | 3278 | 28 | 67 | 67 | 2439 (74.2) | 2421 (73.9) | 2684 (81.7) | 2658 (81.1) | 1313 (40) | 1316 (40.1) | NA | NA | 365 (11.1) | 349 (10.6) | 1052 (32) | 1015 (31) | 3286 (100) | 3278 (100) | NA | NA | 661 (20.1) | 666 (20.3) | 1582 (48.6) | 1579 (48.6) | 0 | 0 | 1658 (50.5) | 1655 (50.5) |
| Totalc | 53 900 | 35 669 | 18.3 | 65.5 | 65.5 | 39 305 (72.9) | 25 610 (71.8) | 35 392 (79.4) | 23 031 (83.7) | 18 346 (35.5) | 12 000 (34.7) | 5375 (18.8) | 3436 (18.0) | 29 972 (75.3) | 18 782 (74.7) | 37 531 (81.6) | 22 502 (75.0) | 12 471 (42.7) | 8319 (29.2) | 8071 (17.8) | 7505 (25.6) | 3873 (21.9) | 3099 (23.9) | 15 665 (29) | 22 734 (63.7) | 1542 (2.8) |
| 16 755 (31.1) | 12 248 (34.3) |
| Trial Name . | Total population . | Mean follow-up . | Mean age . | Sex (male) . | Hypertension . | T2DM . | Heart failure a . | ACS . | CAD . | PAD . | Strokeb . | Renal impairment . | Aspirin monotherapy . | P2Y12 inhibitor monotherapy . | DAPT . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO AC, n . | Con trol, n . | Months . | NO AC, n . | Con trol, n . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | NO AC, n (%) . | Con trol, n (%) . | |
| APPRAISE-2 | 3705 | 3687 | 8 | 67 | 67 | 2496 (76.4) | 2518 (68.3) | NA | NA | 1804 (48.7) | 1732 (47) | 1023 (27.6) | 1053 (28.6) | 3705 (100) | 3687 (100) | 3705 (100) | 3687 (100) | 664 (17.9) | 674 (18.3) | 378 (10.2) | 364 (9.9) | 1048 (28.3) | 1089 (29.5) | 593 (16) | 589 (16) | 0 | 0 | 3001 (81) | 2986 (81) |
| APPRAISE J | 99 | 52 | 6 | 65 | 63.9 | 89 (89.9) | 42 (80.8) | NA | NA | 34 (34.3) | 26 (50) | 10 (10) | 4 (7.7) | 99 (100) | 52 (100) | 99 (100) | 52 (100) | 1 (1.0) | 1 (1.9) | 6 (4) | 0 (0) | 60 (60.5) | 29 (55.8) | 3 (3) | 2 (4) | 0 | 0 | 97 (97) | 50 (96.2) |
| ATLAS ACS 2- TIMI 51 | 10 350 | 5176 | 13 | 61.9 | 61.5 | 7718 (74.6) | 3881 (75) | 6969 (67.3) | 3494 (67.5) | 3317 (32) | 1647 (31.8) | NA | NA | 10 350 (100) | 5176 (100) | 10 350 (100) | 5176 (100) | NA | NA | 275 (2.7) | 127 (2.5) | NA | NA | 600 (5.8) | 300 (5.8) | 0 | 0 | 9602 (92.8) | 4811 (92.9) |
| COMMANDER HF | 2507 | 2515 | 21.1 | 66.5 | 66.3 | 1956 (78) | 1916 (76.2) | 1897 (75.7) | 1886 (75) | 1024 (40.8) | 1028 (40.9) | 2507 (100) | 2515 (100) | 1911 (76.2) | 1892 (75.2) | 1911 (100) | 1892 (100) | NA | NA | 208 (8.3) | 245 (9.7) | 965 (38.5) | 980 (39) | 1421 (56.7) | 1506 (59.9) | 0 | 0 | 901 (36.2) | 839 (33.4) |
| COMPASS 2.5 mg | 8313 | 4130 | 23.7 | 69 | 69 | 6577 (79) | 3307 (80) | 6280 (76) | 3109 (75) | 3043 (37) | 1520 (37) | 1909 (23) | 976 (23) | 5654 (68) | 2860 (69) | 8131 (100) | 4130 (100) | 1656 (20) | 820 (20) | 279 (3) | 134 (3) | NA | NA | 8313 (100) | 4130 (100) | 0 | 0 | 0 | 0 |
| COMPASS 5 mg | 8250 | 4131 | 23.7 | 69 | 69 | 6600 (80) | 3308 (80) | 6214 (75) | 3109 (75) | 3015 (37) | 1250 (37) | 1893 (23) | 976 (23) | 5653 (69) | 2860 (69) | 8250 (100) | 4131 (100) | 1609 (20) | 821 (20) | 250 (3) | 134 (3) | NA | NA | 0 | 4131 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 2.5 mg | 2492 | 1252 | 21 | 67 | 67 | 1774 (71) | 893 (71) | 1966 (78.9) | 1008 (80.6) | 1100 (44.1) | 552 (44.1) | NA | NA | NA | NA | 1656 (66.5) | 820 (65.5) | 2492 (100) | 1252 (100) | 171 (6.9) | 77 (6.2) | 688 (27.6) | 353 (28.2) | 2492 (100) | 1252 (100) | 0 | 0 | 0 | 0 |
| COMPASS-PAD 5 mg | 2474 | 1252 | 21 | 67 | 67 | 1800 (73) | 894 (71) | 1939 (78.4) | 1009 (80.6) | 1083 (43.8) | 552 (44.1) | NA | NA | NA | NA | 1609 (65) | 820 (65.5) | 2472 (100) | 1252 (100) | 177 (7.2) | 77 (6.2) | 681 (27.5) | 353 (28.2) | 0 | 1252 (100) | 0 | 0 | 0 | 0 |
| EINSTEIN CHOICE | 2234 | 1131 | 11.5 | 59.5 | 60 | 1222 (54.8) | 643 (56.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 92 (4) | 64 (5.6) | 0 | 1131 (100) | 0 | 0 | 0 | 0 |
| GEMINI ACS 1 | 1519 | 1518 | 9.7 | 62 | 63 | 1134 (75) | 1141 (75) | 1085 (71) | 1139 (75) | 446 (29) | 469 (30) | 157 (10) | 153 (10) | 1519 (100) | 1518 (100) | 1519 (100) | 1518 (100) | 66 (4) | 74 (5) | NA | NA | NA | NA | 0 | 0 | 1519 (100) | 0 | 0 | 1518 (100) |
| MANAGE | 877 | 877 | 16 | 70 | 70 | 453 (52) | 443 (51) | 585 (67) | 587 (67) | 222 (25) | 243 (27) | NA | NA | 116 (13) | 110 (13) | NA | NA | 124 (14) | 128 (15) | 29 (3) | 42 (5) | NA | NA | 588 (67) | 561 (64) | 23 (3) | 41 (5) | 12 (1) | 21 (3) |
| NAVIGATE ESUS | 3609 | 3604 | 11 | 66.9 | 66.9 | 2232 (61) | 2204 (61) | 2782 (77) | 2803 (78) | 889 (25) | 917 (25) | NA | NA | NA | NA | NA | NA | NA | NA | 3609 (100) | 3604 (100) | NA | NA | 0 | 3604 (100) | 0 | 0 | 0 | 0 |
| RE-DEEM | 1490 | 371 | 5.4 | 61.5 | 61.5 | 1121 (75.4) | 290 (78.4) | 995 (66.9) | 243 (65.5) | 471 (31.7) | 109 (29.4) | 166 (11.2) | 50 (13.5) | 432 (29) | 106 (28.6) | NA | NA | 101 (6.8) | 19 (5.1) | NA | NA | 112 (7.5) | 28 (7.5) | 6 (0.4) | 2 (0.4) | 0 | 0 | 1478 (99.2) | 368 (99.2) |
| RESPECT ESUS | 2695 | 2695 | 19 | 64.5 | 63.9 | 1694 (62.9) | 1709 (63.4) | 1996 (74.1) | 1985 (73.7) | 585 (21.7) | 639 (23.7) | 117 (4.3) | 124 (4.6) | 168 (6.2) | 172 (6.4) | 301 (11.2) | 276 (10.2) | NA | NA | 2695 (100) | 2695 (100) | 227 (8.4) | 203 (7.5) | 72 (2.9) | 2695 (100) | 0 | 0 | 0 | 0 |
| VOYAGER | 3286 | 3278 | 28 | 67 | 67 | 2439 (74.2) | 2421 (73.9) | 2684 (81.7) | 2658 (81.1) | 1313 (40) | 1316 (40.1) | NA | NA | 365 (11.1) | 349 (10.6) | 1052 (32) | 1015 (31) | 3286 (100) | 3278 (100) | NA | NA | 661 (20.1) | 666 (20.3) | 1582 (48.6) | 1579 (48.6) | 0 | 0 | 1658 (50.5) | 1655 (50.5) |
| Totalc | 53 900 | 35 669 | 18.3 | 65.5 | 65.5 | 39 305 (72.9) | 25 610 (71.8) | 35 392 (79.4) | 23 031 (83.7) | 18 346 (35.5) | 12 000 (34.7) | 5375 (18.8) | 3436 (18.0) | 29 972 (75.3) | 18 782 (74.7) | 37 531 (81.6) | 22 502 (75.0) | 12 471 (42.7) | 8319 (29.2) | 8071 (17.8) | 7505 (25.6) | 3873 (21.9) | 3099 (23.9) | 15 665 (29) | 22 734 (63.7) | 1542 (2.8) |
| 16 755 (31.1) | 12 248 (34.3) |
ACS, acute coronary syndrome; CAD, coronary artery disease; DAPT, dual antiplatelet (aspirin + P2Y12 inhibitor) therapy; ESUS, embolic stroke of undetermined source; NA, Not available; NOAC, non-vitamin K oral anticoagulant; PAD, peripheral artery disease; T2DM, Type 2 diabetes mellitus.
Heart failure (left ventricular ejection fraction <40% or clinical heart failure).
Stroke as defined by the original trial.
After accounting for dropout patients, final combined total was 53 778 on NOAC and 35 605 on control drug.
Atrial fibrillation
Most trials had actively excluded patients with a history of AF or pre-existing indication for NOAC (Supplementary material online, Table SV). During follow-up, the incidence of new AF was also extremely low, with the highest rate of 3% observed during follow-up in the NAVIGATE ESUS trial.15
Past stroke
While the two ESUS trials exclusively enrolled patients with previous ESUS; in other eleven trials, only 4.9 ± 3.1% patients had a previous history of stroke or transient ischaemic attack.
Heterogeneity
The heterogeneity between studies was found to be moderate with overall I2 of 46%, and range of 39–57% within various grouped analyses.
Stroke reduction
Over a mean follow-up of 18.3 months, 1429 (1.6%) ischaemic strokes occurred. The primary efficacy outcomes are summarized in Figure 1. The rate of ischaemic stroke was significantly reduced using NOAC (OR 0.74, CI 0.62–0.87, P = 0.0005; 1.1 vs. 1.8 events per 100 person-years), with number needed to treat of 153 patients to prevent one stroke. The benefit of NOAC was seen when compared with placebo (OR 0.71, 95% CI 0.52–0.96, P = 0.02) and aspirin (OR 0.76, CI 0.60–0.95, P = 0.02), though not compared with DAPT.
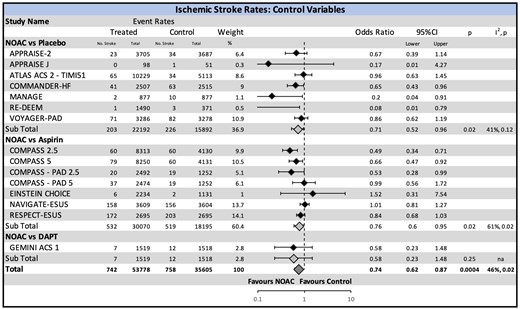
Ischaemic stroke risk with non-vitamin K oral anticoagulant stratified by the comparison drug. There was an overall significant reduction in ischaemic stroke with the use of non-vitamin K oral anticoagulant. A significant reduction is also seen in the sub-analysis of non-vitamin K oral anticoagulant vs. placebo and non-vitamin K oral anticoagulant vs. aspirin. CI, confidence interval; NOAC, non-vitamin K oral anticoagulant.
Additional grouped analyses by strength of the NOAC dose (primary- vs. mini-doses), indication for anticoagulation, and the presence or absence of concomitant DAPT or P2Y12 inhibitor in the NOAC arm are shown in Figure 2 and Supplementary material online, Figure SIII and SIV, respectively. An interesting outcome was the significant reduction in ischaemic stroke in the patients treated with mini-doses of NOAC (OR 0.70, CI 0.58–0.84, P = 0.0001) which was not seen in the primary-dose group (OR 0.79, CI 0.59–1.06, P = 0.09). The interaction between primary- and mini-doses of NOAC was, however, insignificant (P = 0.5). A clear benefit was seen in those with left ventricular dysfunction (OR 0.65, CI 0.43–0.96, P = 0.03) and stable CAD or PAD (OR 0.68, CI 0.53–0.88, P = 0.003).
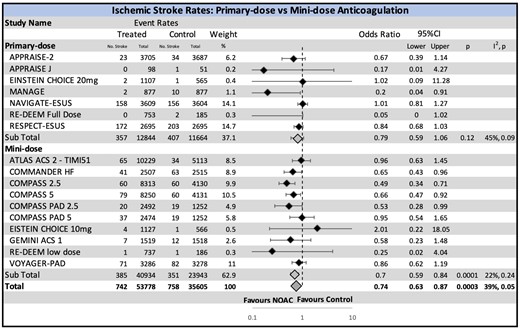
Ischaemic stroke risk with non-vitamin K oral anticoagulant stratified by the intensity of non-vitamin K oral anticoagulant dose. While the overall ischaemic stroke rate is reduced, an unexpected paradoxical effect is appreciated in the mini-dose (significant reduction) and primary-dose (non-significant reduction) anticoagulation subgroups. CI, confidence interval; NOAC, non-vitamin K oral anticoagulant.
Major bleeding risk
The most common reporting definition was the ISTH (11 trials). The primary safety outcome stratified by the type of comparator arm (placebo, aspirin, or DAPT) is summarized in Figure 3. Major bleeding was significantly increased by the use of NOAC (OR 1.74, CI 1.44–2.09, P < 0.00001; 2.1 vs. 1.0 bleeding events per 100 person-years), with number needed to harm of 89. This was most pronounced when NOAC was compared with the placebo (OR 1.85, CI 1.26–2.72, P = 0.002).
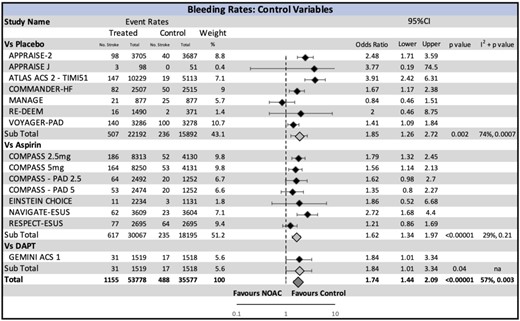
Major bleeding risk with non-vitamin K oral anticoagulant stratified by the comparison drug. A significant increase in bleeding with non-vitamin K oral anticoagulant is seen in the overall effect, and across all subgroups. CI, confidence interval; NOAC, non-vitamin K oral anticoagulant.
Other grouped analyses according to the strength of NOAC dose, choice of major bleeding definition, and the presence or absence of concomitant DAPT or P2Y12 in the NOAC arm also showed harm due to bleeding (Figure 4 and Supplementary material online, Figure SV and SVI, respectively). Both primary- and mini-doses of NOAC resulted in increased risk of major bleeding (OR 1.71, CI 1.10–2.65, P = 0.007 and OR 1.74, CI 1.44–2.10, P < 0.00001, respectively).
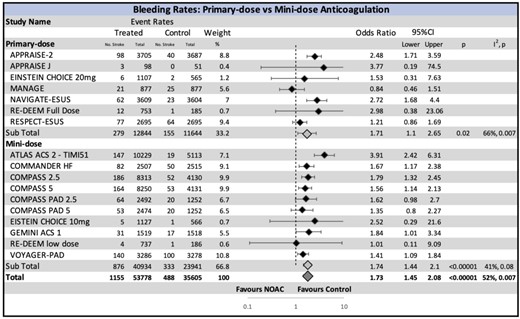
Major bleeding risk with non-vitamin K oral anticoagulant stratified by the intensity of non-vitamin K oral anticoagulant dose. A significant increase in bleeding is seen in both the low-dose and full-dose non-vitamin K oral anticoagulant groups. CI, confidence interval; NOAC, non-vitamin K oral anticoagulant.
Net clinical benefit
The weighted NCB (wNCB) combining ischaemic stroke and bleeding was calculated for the total population, for each comparison drug type, the strength of NOAC dose, and the presence or absence of concomitant DAPT or P2Y12 in the NOAC arm (Figure 5 and Supplementary material online, Table SVI). The positive advantage of ischaemic stroke prevention was most pronounced with mini-dose NOAC where stroke benefit just equalized with major bleeding (wNCB −0.06, 95% CI −0.45 to 0.33). Otherwise, the wNCB showed a trend towards harm, more so in studies that compared NOAC with aspirin in which there was clear harm with NOAC due to increased bleeding (wNCB −0.77, 95% CI −1.31 to −0.22). The unweighted NCB also showed similar range of values irrespective of the comparison drug or NOAC dose used (Supplementary material online, Figure SVII and Table SVI).
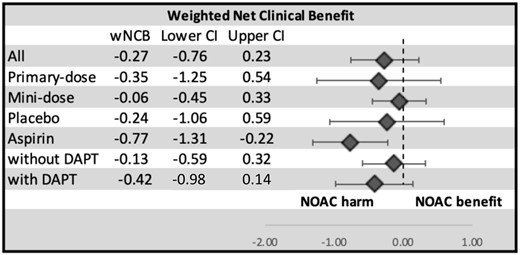
Weighted net clinical benefit of non-vitamin K oral anticoagulant. The weighted net clinical benefit combines the findings into a meaningful measure of benefit and harm (see text for calculation). Overall, there is a trend towards harm with the use of non-vitamin K oral anticoagulants. CI, confidence interval; DAPT, dual antiplatelet therapy; NOAC, non-vitamin K oral anticoagulant; wNCB, weighted net clinical benefit.
Exclusion of embolic stroke of undetermined source secondary prophylaxis trials
After exclusion of the two ESUS secondary prophylaxis trials, the ultimate results did not alter, and the wNCB with NOAC use remained poor (Supplementary material online, Results).
Sensitivity analysis for multiarmed trials
In the main analysis, multiarmed trials (COMPASS, COMPASS-PAD, EINSTEIN CHOICE, RE-DEEM) were dealt with pre-specified rules (Supplementary material online, Methods), whereby the comparator arm was halved to avoid double counting of the comparator group. To test those rules, we repeated the analysis of each NOAC-arm against the undivided comparator arm, which did not substantially change the final results (Supplementary material Figures SVIII and SIX).
Discussion
This meta-analysis of 13 RCTs, of 89 383 patients, with a 130 477 person-years follow-up provides a contemporary evaluation of the benefit and harm of anticoagulation with NOACs for the reduction of ischaemic stroke in patients with CVD in sinus rhythm. There is a significant reduction in ischaemic stroke seen in the entire population, as well as the subgroups of mini-dose anticoagulation and in stable CAD, PAD and left ventricular dysfunction. This is, however, counterbalanced by the key evidence of harm from major bleeding in all subgroups. Although the wNCB of mini-dose anticoagulation approximated zero (−0.06) with a narrow 95% CI (−0.47 to 0.35), overall, there was a consistent concerning trend towards harm. Even though the theoretical basis for incorporating NOACs as part of stroke reduction strategy in sinus rhythm is strong, the findings of this meta-analysis do not support their general use. NOAC agents warrant further evaluation in CVD focused on those with demonstrable severe atrial cardiopathy in sinus rhythm if they were to hold their same promise as in AF.
Stroke reduction in sinus rhythm
Patients with CVD are at an increased of ischaemic stroke. Abundant cerebrovascular pathology and atrial cardiopathy are common in patients with CAD, PAD, HF, or previous stroke, therefore predisposition to atherothrombotic, atheroembolic, and cardioembolic stroke events exists even in sinus rhythm.7–9 In fact, the CHA2DS2-VASc scheme that includes established CVD and various CV risk factors, provides prognostic information on future thromboembolic events even without documented AF.9 For comparison, patients in sinus rhythm with a score of 1 have 0.62% annual embolism risk, but those with a score of ≥5 have almost three-fold increased risk to 1.79%.31
Several old RCTs showed nearly 50% reduction in ischaemic stroke using vitamin K antagonists,10,11,32 however, this benefit was overwhelmed by two- to eight-fold increase in the major bleeding events. In contrast to vitamin K antagonists, NOAC agents have unequivocal safety with proven 30% reduction in major bleeding and almost 50% reduction in ICH.4 This, in addition to their ease of administration, has propelled investigations of their role as a dominant antithrombotic agent in the entire CVD spectrum. Most recently, the COMMANDER HF2,27 trial showed a substantial reduction in the rates of ischaemic stroke [0.9 vs. 1.3 events per 100 patient-years, hazard ratio (HR) 0.64, 95% CI 0.43–0.95]. Major ISTH bleeding was significantly increased in the main trial (HR 1.68, 95% CI 1.18–2.39) but the calculated wNCB was 0.17, modestly in favour of NOAC. Increase in bleeding could be attributed to concomitant use of aspirin and DAPT in 93% and 36% of COMMANDER HF patients, respectively. Nevertheless, in contrast to the broad expectations, our meta-analysis showed modest negative wNCB for all comparisons of NOAC, indicating NOAC agents may not really be beneficial in sinus rhythm. More robust data are necessary before we anticoagulate patients with CVD in sinus rhythm. Studies are ongoing to identify specific imaging, electrocardiographic and serum markers of severe atrial cardiopathy in patients with previous stroke in sinus rhythm.33 A large atrial cardiopathy trial is also ongoing to answer the role of full dose of NOAC after a cryptogenic stroke.34
Primary- vs. mini-dose non-vitamin K oral anticoagulant
Unlike vitamin K antagonists, the dose effects of NOACs are known to be predictable and linear.26,35–37 Expectedly, therefore, most studies have shown reduced ischaemic events but with increased bleeding as the NOAC doses are escalated.3–6,38 However, ATLAS ACS TIMI 46 found similar reduction in ischaemic outcomes across rivaroxaban 5, 10, and 20 mg total daily dose regimes, with considerably lower bleeding with smaller doses.36
In this analysis, we also found an unexpected result in which mini-dose anticoagulation with NOACs showed a reduction in ischaemic stroke rates, while the primary-dose anticoagulation did not (Figure 2), although this dose-based interaction was not significant. There could be two statistical reasons for this paradoxical finding. First, more than half (51%) of the primary-dose anticoagulation population was made up of patients from the ESUS secondary prophylaxis trials. Patients in both arms of these trials had a previous stroke and therefore had a much higher rate of future stroke than the other trials included (annualized stroke rate of 4–5% for ESUS trials compared with <1.5% for all other trials). As these trials were surprisingly negative for their primary outcome of recurrent stroke, their inclusion in our meta-analysis dominated the outcomes with primary-dose NOAC. But removing ESUS trials did not alter the ultimate conclusion of meta-analysis as presented in the Supplementary material online, Results. Another bias that may have accounted for this observation was the smaller size of the trials in the primary-dose group than the mini-dose group (24 508 vs. 65 875 patients). As stroke is an uncommon event, the power to detect differences in stroke was more likely in the analysis of mini-dose group.
Strengths and limitations
A major strength of this meta-analysis is enormous size and high quality of the included RCTs, minimum deviations from the intended interventions and accurate reporting of ischaemic and bleeding outcomes in the trials. The heterogeneity among RCTs was found moderate, mainly due to variability in their primary CVD indication, NOAC types and dosages, lack of uniformity of the comparator arm, and bleeding definition. This may have confounded our overall risk estimates for stroke and bleeding with NOAC use in sinus rhythm. To overcome this, we presented results of homogenous grouped analysis for each individual CVD, NOAC doses, and the presence or absence of concomitant DAPT or P2Y12 in the NOAC treatment arm. These will serve as a guide to which CVD and NOAC dose be most appropriate for future investigations in sinus rhythm where a favourable NCB may be likely. As individual patient-level data on stroke risk factors was not available, it reduced the opportunity to calculate NOAC benefit in high CHA2DS2VAS patients who arguably have the highest untreated stroke risk, and in whom NCB of NOAC may have been favourable.21,39 Our meta-analysis included patients who were on average 5 years younger than AF patients included in the NOAC vs. warfarin trials.3–5 Therefore, baseline ischaemic stroke risk was likely low in our studied population, hence may have underestimated ischaemic stroke benefit with NOAC. As an example, MANAGE trial, which randomized patients with mean age of 70 years (vs. mean 66), showed a marked reduction in ischaemic stroke rate and similar bleeding compared to placebo with a favourable wNCB of 0.71. On the contrary, EINSTEIN CHOICE trial, which randomized the youngest cohort of patients (mean age 59 years) with VTE had no reduction in stroke and more bleeding, with an overall wNCB of −0.26 suggesting harm. The concomitant use of aspirin or DAPT in the NOAC arm of many of the trials may have spuriously conferred major bleeding risk to NOAC use, although this can be contentious as the results of ESUS trials (that compared NOAC alone to aspirin alone) did not show a positive advantage with NOAC as well. Furthermore, our sub-analysis found no significant improvement in the wNCB even after excluding the seven trials which enrolled >30% patients on concomitant DAPT or P2Y12 inhibitor in the NOAC arm. In this meta-analysis, rivaroxaban was overrepresented (8 of 13 trials) while edoxaban was not represented at all. Therefore, the findings may be more applicable to rivaroxaban specifically rather than a class effect of all NOACs. This meta-analysis did not address mortality benefit with NOAC use in sinus rhythm; including all-cause mortality in the wNCB may have swayed the results.40 However, we anticipate doubling in major haemorrhage associated with NOAC would have still neutralized any implausible reduction in mortality. Arguably, the rates of ICH with NOAC are extremely low,4 and a higher weight to ICH may have negated therapeutic advantage of NOAC. But we found similar results with the unweighted NCB as well.
Conclusions
In patients with mixed CVD in sinus rhythm included in the meta-analysis, NOACs effectively reduced ischaemic stroke risk, though their benefit was outweighed by increased bleeding, with an overall trend towards harm. Only low-dose NOACs demonstrated some degree of clinical equivalency with an wNCB close to zero. On the whole, NOACs are not ready for primary or secondary prophylaxis against stroke in sinus rhythm. Further research is warranted selectively in those who may have highest untreated stroke risk in sinus rhythm, preferably with a low-dose NOAC without concomitant antiplatelet therapy.
Acknowledgements
We thank Dr Andrew Ha, University Health Network, Canada for his assistance in preparation of the manuscript.
Conflict of interest: No conflicts of interest for any of the authors.
References
NCT03830983. Atrial Cardiomyopathy in Patients with Stroke of Undetected Mechanism. https://clinicaltrials.gov/ct2/show/NCT03830983 (5 February 2019).




Comments