-
PDF
- Split View
-
Views
-
Cite
Cite
Kausik K Ray, Bart Molemans, W Marieke Schoonen, Periklis Giovas, Sarah Bray, Gaia Kiru, Jennifer Murphy, Maciej Banach, Stefano De Servi, Dan Gaita, Ioanna Gouni-Berthold, G Kees Hovingh, Jacek J Jozwiak, J Wouter Jukema, Robert Gabor Kiss, Serge Kownator, Helle K Iversen, Vincent Maher, Luis Masana, Alexander Parkhomenko, André Peeters, Piers Clifford, Katarina Raslova, Peter Siostrzonek, Stefano Romeo, Dimitrios Tousoulis, Charalambos Vlachopoulos, Michal Vrablik, Alberico L Catapano, Neil R Poulter, the DA VINCI study, EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study, European Journal of Preventive Cardiology, Volume 28, Issue 11, November 2021, Pages 1279–1289, https://doi.org/10.1093/eurjpc/zwaa047
Close - Share Icon Share
Abstract
To provide contemporary data on the implementation of European guideline recommendations for lipid-lowering therapies (LLTs) across different settings and populations and how this impacts low-density lipoprotein cholesterol (LDL-C) goal achievement.
An 18 country, cross-sectional, observational study of patients prescribed LLT for primary or secondary prevention in primary or secondary care across Europe. Between June 2017 and November 2018, data were collected at a single visit, including LLT in the preceding 12 months and most recent LDL-C. Primary outcome was the achievement of risk-based 2016 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) LDL-C goal while receiving stabilized LLT; 2019 goal achievement was also assessed. Overall, 5888 patients (3000 primary and 2888 secondary prevention patients) were enrolled; 54% [95% confidence interval (CI) 52–56] achieved their risk-based 2016 goal and 33% (95% CI 32–35) achieved their risk-based 2019 goal. High-intensity statin monotherapy was used in 20% and 38% of very high-risk primary and secondary prevention patients, respectively. Corresponding 2016 goal attainment was 22% and 45% (17% and 22% for 2019 goals) for very high-risk primary and secondary prevention patients, respectively. Use of moderate–high-intensity statins in combination with ezetimibe (9%), or any LLT with PCSK9 inhibitors (1%), was low; corresponding 2016 and 2019 goal attainment was 53% and 20% (ezetimibe combination), and 67% and 58% (PCSK9i combination).
Gaps between clinical guidelines and clinical practice for lipid management across Europe persist, which will be exacerbated by the 2019 guidelines. Even with optimized statins, greater utilization of non-statin LLT is likely needed to reduce these gaps for patients at highest risk.
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality and morbidity in Europe.1 Compelling evidence from clinical trials2 has led joint guidelines by the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS)3,4 to recommend statins as first-line pharmacotherapy to lower low-density lipoprotein cholesterol (LDL-C), and therefore reduce cardiovascular (CV) risk. Moreover, these guidelines recommend risk-based LDL-C goals (Supplementary material online, Table S1) and aim to optimize LDL-C reduction, with a focus on improving uptake of high-intensity statins (a proxy for achieving a 50% reduction in LDL-C).3 Hence, the 2016 and 2019 iterations recommend that both LDL-C goals and a 50% reduction in LDL-C should be achieved for those at high or very high risk.3,4
Recent surveys assessing use of lipid-lowering therapy (LLT) and attainment of 2016 LDL-C goals have focused on patients with coronary artery disease in secondary care settings. Overall goal attainment was found to be low.5 The 2019 ESC/EAS guideline update recommends even lower LDL-C goals for very high risk, high risk, and moderate risk categories. The attainment of these lower goals and whether optimizing statin alone is sufficient to achieve these lower goals are as yet unclear.4 The EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care (DA VINCI study) was designed to provide contemporary information regarding LDL-C goal attainment for patients across Europe in diverse healthcare settings and previously under-studied patient groups.
Methods
Study design
This cross-sectional study planned to enrol 6000 adults receiving LLT at primary and secondary care clinics across 18 European countries (Supplementary material online, Appendix S2 and Figure S1) between 21 June 2017 and 20 November 2018. The planned sample size was chosen to allow precise estimates of the primary outcome measure within each country (Supplementary material online, Appendix S4.1). There were no formal study visits and patients were approached for participation at their routine clinic visit (Supplementary material online, Figure S2a). The number of primary and secondary prevention patients enrolled at each site was capped to ensure an overall ratio of ∼1:1. Secondary care sites specializing in coronary, peripheral, and cerebral (arterial) disease were included, and patients with these disease manifestations enrolled in an overall ratio of 1:2:2, respectively. The study protocol was approved by the institutional review board or independent ethics committee from each site. The study was designed by the Academic Executive Committee in conjunction with the sponsor Amgen [full protocol available online (ENCePP; registration no. EU PAS 22075), Supplementary material online, Appendix S1 and Appendix S3].
Eligibility criteria
Full eligibility criteria are available in Supplementary material online, Appendix S4.2. Major inclusion criteria included: being aged ≥18 years; providing informed consent; being prescribed LLT at enrolment or within 12 months prior to enrolment; and having an LDL-C measurement recorded up to 14 months before enrolment (obtained independently of participation in a clinical trial). Major exclusion criteria included: a diagnosis of familial hypercholesterolaemia with a history of CV events; comorbidities or personal circumstances that could affect clinical decision-making; a positive human immunodeficiency virus (HIV) status; pregnancy or breastfeeding; participating in an interventional clinical trial within 6 months before the enrolment date; and a life expectancy of <1 year at enrolment.
Data extraction
The following data were collected from medical records at a single (enrolment) visit using a standardized electronic case report form (e-CRF; InForm v6·0): demographics, relevant past medical history, height, weight, and blood pressure; most recent lipid value recorded within 14 months prior to (and including) the enrolment visit; LLT at the enrolment visit and in the preceding 12 months; history of intolerance to any statin at any dose; reason for LLT prescription in patients without previous atherosclerotic CVD (ASCVD) events; and concomitant medications.
Aims and outcomes
Our aim was to comprehensively describe how LLT is used in Europe for primary and secondary prevention of ASCVD, in different healthcare settings and populations, in order to assess how current practice impacts LDL-C goal attainment.
The primary outcome was the proportion of patients achieving the risk-based LDL-C goals recommended by the 2016 ESC/EAS guidelines while receiving stabilized LLT. This was assessed at LDL-C measurement, with stabilized LLT defined as no change in dose or regimen for at least 28 days prior to LDL-C measurement. For individuals defined as primary prevention at LDL-C measurement, 10-year CV death risk was estimated using systematic coronary risk evaluation (SCORE) and they were categorized as low–moderate, high, or very high risk according to the ESC/EAS guidelines. Patients enrolled in the primary prevention cohort on the basis of conditions such as diabetes, familial hypercholesterolaemia, and reduced glomerular filtration rate were categorized as per ESC/EAS risk categories (low, moderate, high, very high). All patients defined as secondary prevention were categorized as very high risk. Estimated 10-year CV risk at LDL-C measurement in established ASCVD groups was estimated using REACH (Supplementary material online, Appendix S4.3).
Secondary outcomes included LLT use (type, dose, frequency; including combination therapy), assessed at the enrolment date. As the study was completed before publication of the updated 2019 ESC/EAS guidelines, a post hoc analysis of the proportion of patients achieving the LDL-C goals recommended in the 2019 guidelines was conducted for comparison.
Statistical analysis
All analyses were descriptive. Data were summarized overall, and separately for primary prevention and secondary prevention. Patients enrolled in the secondary prevention group were categorized as having coronary, peripheral, or cerebral disease based on management of the most recent manifestation of vascular disease at enrolment. Patients without a documented history of these disease manifestations were categorized as ‘other’. Continuous variables are reported as mean and standard deviation or standard error (SE) for normally distributed data, and as median and 25th and 75th percentiles (Q1 and Q3, respectively) for data with a skewed distribution. For categorical variables, the number and percentage of patients in each category are reported.
Results
Patient characteristics
In total, 5888 eligible patients were enrolled from 18 countries across 128 sites and included in the primary analysis set (Supplementary material online, Figure S2). Of these, 3000 were enrolled as primary prevention and 2888 as secondary prevention. The latter consisted of 2794 patients (97%) with established ASCVD, of whom 622 (22%) were being managed for coronary disease, 1136 (41%) for cerebral disease, and 1036 (37%) for peripheral disease; the remaining 94 patients (3%) had other evidence of atherosclerosis or other manifestation of vascular disease at enrolment. Demographic characteristics varied between primary and secondary prevention groups; the former had a greater proportion of women and a lower average age (Table 1).
| . | Overall (n = 5888) . | Primary prevention (n = 3000) . | Secondary prevention by ASCVD status . | ||||
|---|---|---|---|---|---|---|---|
| Established ASCVD total (n = 2794) . | ASCVD-coronary (n = 622) . | ASCVD-peripheral (n = 1036) . | ASCVD-cerebral (n = 1136) . | Other vascular secondary preventiona (n = 94) . | |||
| Female, n (%) | 2475 (42) | 1502 (50) | 939 (34) | 149 (24) | 323 (31) | 467 (41) | 34 (36) |
| Age (years), mean (SD) | 65 (12) | 63 (13) | 68 (10) | 67 (10) | 69 (9) | 67 (11) | 70 (10) |
| Ethnicity, white, n (%) | 5435 (92) | 2829 (94) | 2523 (90) | 550 (88) | 937 (90) | 1036 (91) | 83 (88) |
| Systolic blood pressure (mmHg), mean (SD) | 135 (17) | 134 (16) | 135 (18) | 133 (18) | 136 (19) | 136 (17.4) | 137 (23) |
| Diastolic blood pressure (mmHg), mean (SD) | 78 (11) | 79 (10) | 77 (11) | 77 (11) | 76 (11) | 78 (11) | 77 (13) |
| BMI, median (Q1–Q3) | 28 (25–32) | 28 (25–32) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) |
| Smoking history, n (%) | |||||||
| Non-smoker | 2854 (49) | 1732 (58) | 1089 (39) | 277 (45) | 242 (23) | 570 (50) | 33 (35) |
| Ex-smoker | 2059 (35) | 851 (28) | 1166 (42) | 267 (43) | 508 (49) | 391 (34) | 42 (45) |
| Light smoker | 313 (5) | 140 (5) | 167 (6) | 26 (4) | 76 (7) | 65 (6) | 6 (6) |
| Moderate smoker | 391 (7) | 161 (5) | 220 (8) | 30 (5) | 126 (12) | 64 (6) | 10 (11) |
| Heavy smoker | 253 (4) | 106 (4) | 144 (5) | 19 (3) | 84 (8) | 41 (4) | 3 (3) |
| Missing | 18 (<1) | 10 (<1) | 8 (<1) | 3 (<1) | 0 (0) | 5 (<1) | 0 (0) |
| Hypertension, n (%) | 4138 (70) | 1976 (66) | 2090 (75) | 455 (73) | 809 (78) | 826 (73) | 72 (77) |
| Diabetes, n (%) | 2293 (39) | 1169 (39) | 1082 (39) | 238 (38) | 473 (46) | 371 (33) | 42 (45) |
| Chronic kidney disease ≥ grade 3, n (%) | 432 (7) | 179 (6) | 242 (9) | 46 (7) | 124 (12) | 72 (6) | 11 (12) |
| Familial hypercholesterolaemia | 284 (5) | 284 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular bed involvement, n (%) | |||||||
| Coronary | 1007 (17) | 0 (0) | 985 (35) | 618 (99) | 271 (26) | 96 (9) | 22 (23) |
| Cerebral | 1296 (22) | 0 (0) | 1277 (46) | 31 (5) | 122 (12) | 1124 (99) | 19 (20) |
| Peripheral | 1125 (19) | 0 (0) | 1069 (38) | 24 (4) | 1014 (98) | 31 (3) | 56 (60) |
| . | Overall (n = 5888) . | Primary prevention (n = 3000) . | Secondary prevention by ASCVD status . | ||||
|---|---|---|---|---|---|---|---|
| Established ASCVD total (n = 2794) . | ASCVD-coronary (n = 622) . | ASCVD-peripheral (n = 1036) . | ASCVD-cerebral (n = 1136) . | Other vascular secondary preventiona (n = 94) . | |||
| Female, n (%) | 2475 (42) | 1502 (50) | 939 (34) | 149 (24) | 323 (31) | 467 (41) | 34 (36) |
| Age (years), mean (SD) | 65 (12) | 63 (13) | 68 (10) | 67 (10) | 69 (9) | 67 (11) | 70 (10) |
| Ethnicity, white, n (%) | 5435 (92) | 2829 (94) | 2523 (90) | 550 (88) | 937 (90) | 1036 (91) | 83 (88) |
| Systolic blood pressure (mmHg), mean (SD) | 135 (17) | 134 (16) | 135 (18) | 133 (18) | 136 (19) | 136 (17.4) | 137 (23) |
| Diastolic blood pressure (mmHg), mean (SD) | 78 (11) | 79 (10) | 77 (11) | 77 (11) | 76 (11) | 78 (11) | 77 (13) |
| BMI, median (Q1–Q3) | 28 (25–32) | 28 (25–32) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) |
| Smoking history, n (%) | |||||||
| Non-smoker | 2854 (49) | 1732 (58) | 1089 (39) | 277 (45) | 242 (23) | 570 (50) | 33 (35) |
| Ex-smoker | 2059 (35) | 851 (28) | 1166 (42) | 267 (43) | 508 (49) | 391 (34) | 42 (45) |
| Light smoker | 313 (5) | 140 (5) | 167 (6) | 26 (4) | 76 (7) | 65 (6) | 6 (6) |
| Moderate smoker | 391 (7) | 161 (5) | 220 (8) | 30 (5) | 126 (12) | 64 (6) | 10 (11) |
| Heavy smoker | 253 (4) | 106 (4) | 144 (5) | 19 (3) | 84 (8) | 41 (4) | 3 (3) |
| Missing | 18 (<1) | 10 (<1) | 8 (<1) | 3 (<1) | 0 (0) | 5 (<1) | 0 (0) |
| Hypertension, n (%) | 4138 (70) | 1976 (66) | 2090 (75) | 455 (73) | 809 (78) | 826 (73) | 72 (77) |
| Diabetes, n (%) | 2293 (39) | 1169 (39) | 1082 (39) | 238 (38) | 473 (46) | 371 (33) | 42 (45) |
| Chronic kidney disease ≥ grade 3, n (%) | 432 (7) | 179 (6) | 242 (9) | 46 (7) | 124 (12) | 72 (6) | 11 (12) |
| Familial hypercholesterolaemia | 284 (5) | 284 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular bed involvement, n (%) | |||||||
| Coronary | 1007 (17) | 0 (0) | 985 (35) | 618 (99) | 271 (26) | 96 (9) | 22 (23) |
| Cerebral | 1296 (22) | 0 (0) | 1277 (46) | 31 (5) | 122 (12) | 1124 (99) | 19 (20) |
| Peripheral | 1125 (19) | 0 (0) | 1069 (38) | 24 (4) | 1014 (98) | 31 (3) | 56 (60) |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; SD, standard deviation.
Patients with other evidence of atherosclerosis or other manifestation of vascular disease at enrolment.
| . | Overall (n = 5888) . | Primary prevention (n = 3000) . | Secondary prevention by ASCVD status . | ||||
|---|---|---|---|---|---|---|---|
| Established ASCVD total (n = 2794) . | ASCVD-coronary (n = 622) . | ASCVD-peripheral (n = 1036) . | ASCVD-cerebral (n = 1136) . | Other vascular secondary preventiona (n = 94) . | |||
| Female, n (%) | 2475 (42) | 1502 (50) | 939 (34) | 149 (24) | 323 (31) | 467 (41) | 34 (36) |
| Age (years), mean (SD) | 65 (12) | 63 (13) | 68 (10) | 67 (10) | 69 (9) | 67 (11) | 70 (10) |
| Ethnicity, white, n (%) | 5435 (92) | 2829 (94) | 2523 (90) | 550 (88) | 937 (90) | 1036 (91) | 83 (88) |
| Systolic blood pressure (mmHg), mean (SD) | 135 (17) | 134 (16) | 135 (18) | 133 (18) | 136 (19) | 136 (17.4) | 137 (23) |
| Diastolic blood pressure (mmHg), mean (SD) | 78 (11) | 79 (10) | 77 (11) | 77 (11) | 76 (11) | 78 (11) | 77 (13) |
| BMI, median (Q1–Q3) | 28 (25–32) | 28 (25–32) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) |
| Smoking history, n (%) | |||||||
| Non-smoker | 2854 (49) | 1732 (58) | 1089 (39) | 277 (45) | 242 (23) | 570 (50) | 33 (35) |
| Ex-smoker | 2059 (35) | 851 (28) | 1166 (42) | 267 (43) | 508 (49) | 391 (34) | 42 (45) |
| Light smoker | 313 (5) | 140 (5) | 167 (6) | 26 (4) | 76 (7) | 65 (6) | 6 (6) |
| Moderate smoker | 391 (7) | 161 (5) | 220 (8) | 30 (5) | 126 (12) | 64 (6) | 10 (11) |
| Heavy smoker | 253 (4) | 106 (4) | 144 (5) | 19 (3) | 84 (8) | 41 (4) | 3 (3) |
| Missing | 18 (<1) | 10 (<1) | 8 (<1) | 3 (<1) | 0 (0) | 5 (<1) | 0 (0) |
| Hypertension, n (%) | 4138 (70) | 1976 (66) | 2090 (75) | 455 (73) | 809 (78) | 826 (73) | 72 (77) |
| Diabetes, n (%) | 2293 (39) | 1169 (39) | 1082 (39) | 238 (38) | 473 (46) | 371 (33) | 42 (45) |
| Chronic kidney disease ≥ grade 3, n (%) | 432 (7) | 179 (6) | 242 (9) | 46 (7) | 124 (12) | 72 (6) | 11 (12) |
| Familial hypercholesterolaemia | 284 (5) | 284 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular bed involvement, n (%) | |||||||
| Coronary | 1007 (17) | 0 (0) | 985 (35) | 618 (99) | 271 (26) | 96 (9) | 22 (23) |
| Cerebral | 1296 (22) | 0 (0) | 1277 (46) | 31 (5) | 122 (12) | 1124 (99) | 19 (20) |
| Peripheral | 1125 (19) | 0 (0) | 1069 (38) | 24 (4) | 1014 (98) | 31 (3) | 56 (60) |
| . | Overall (n = 5888) . | Primary prevention (n = 3000) . | Secondary prevention by ASCVD status . | ||||
|---|---|---|---|---|---|---|---|
| Established ASCVD total (n = 2794) . | ASCVD-coronary (n = 622) . | ASCVD-peripheral (n = 1036) . | ASCVD-cerebral (n = 1136) . | Other vascular secondary preventiona (n = 94) . | |||
| Female, n (%) | 2475 (42) | 1502 (50) | 939 (34) | 149 (24) | 323 (31) | 467 (41) | 34 (36) |
| Age (years), mean (SD) | 65 (12) | 63 (13) | 68 (10) | 67 (10) | 69 (9) | 67 (11) | 70 (10) |
| Ethnicity, white, n (%) | 5435 (92) | 2829 (94) | 2523 (90) | 550 (88) | 937 (90) | 1036 (91) | 83 (88) |
| Systolic blood pressure (mmHg), mean (SD) | 135 (17) | 134 (16) | 135 (18) | 133 (18) | 136 (19) | 136 (17.4) | 137 (23) |
| Diastolic blood pressure (mmHg), mean (SD) | 78 (11) | 79 (10) | 77 (11) | 77 (11) | 76 (11) | 78 (11) | 77 (13) |
| BMI, median (Q1–Q3) | 28 (25–32) | 28 (25–32) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) |
| Smoking history, n (%) | |||||||
| Non-smoker | 2854 (49) | 1732 (58) | 1089 (39) | 277 (45) | 242 (23) | 570 (50) | 33 (35) |
| Ex-smoker | 2059 (35) | 851 (28) | 1166 (42) | 267 (43) | 508 (49) | 391 (34) | 42 (45) |
| Light smoker | 313 (5) | 140 (5) | 167 (6) | 26 (4) | 76 (7) | 65 (6) | 6 (6) |
| Moderate smoker | 391 (7) | 161 (5) | 220 (8) | 30 (5) | 126 (12) | 64 (6) | 10 (11) |
| Heavy smoker | 253 (4) | 106 (4) | 144 (5) | 19 (3) | 84 (8) | 41 (4) | 3 (3) |
| Missing | 18 (<1) | 10 (<1) | 8 (<1) | 3 (<1) | 0 (0) | 5 (<1) | 0 (0) |
| Hypertension, n (%) | 4138 (70) | 1976 (66) | 2090 (75) | 455 (73) | 809 (78) | 826 (73) | 72 (77) |
| Diabetes, n (%) | 2293 (39) | 1169 (39) | 1082 (39) | 238 (38) | 473 (46) | 371 (33) | 42 (45) |
| Chronic kidney disease ≥ grade 3, n (%) | 432 (7) | 179 (6) | 242 (9) | 46 (7) | 124 (12) | 72 (6) | 11 (12) |
| Familial hypercholesterolaemia | 284 (5) | 284 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular bed involvement, n (%) | |||||||
| Coronary | 1007 (17) | 0 (0) | 985 (35) | 618 (99) | 271 (26) | 96 (9) | 22 (23) |
| Cerebral | 1296 (22) | 0 (0) | 1277 (46) | 31 (5) | 122 (12) | 1124 (99) | 19 (20) |
| Peripheral | 1125 (19) | 0 (0) | 1069 (38) | 24 (4) | 1014 (98) | 31 (3) | 56 (60) |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; SD, standard deviation.
Patients with other evidence of atherosclerosis or other manifestation of vascular disease at enrolment.
Cardiovascular risk profile
The distribution of CV risk factors was generally similar across the three ASCVD subgroups, although those managed for peripheral arterial disease at enrolment were more likely to have a prior history of vascular disease in other vascular beds (Table 1). The estimated 10-year risk of CV death was calculated for primary prevention patients using SCORE (Figure 1A). Within those also evaluable for LDL-C goal attainment, the majority (67%) were low–moderate risk (1391/2073), 29% were high risk (593/2073), and few (4%) were very high risk (89/2073). Estimated REACH score could be calculated for 2659 patients with established ASCVD (Figure 1B); 82% (2188/2659) had a predicted 10-year risk of fatal and non-fatal CV events >20% and 31% (820/2659) had a risk >40%.
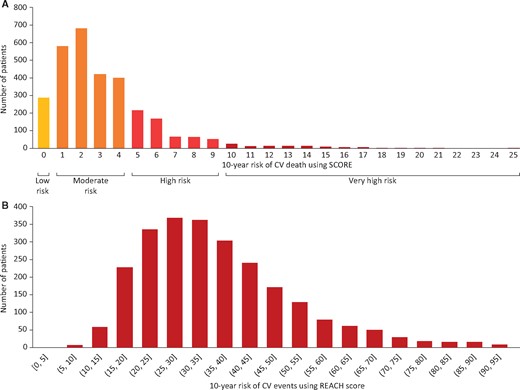
Estimated 10-year cardiovascular risk at low-density lipoprotein cholesterol measurement in primary prevention groupa (A) and estimated 10-year risk of fatal and non-fatal cardiovascular events at low-density lipoprotein cholesterol measurement in established atherosclerotic cardiovascular disease groupb (B). aData shown are for all patients considered primary prevention at low-density lipoprotein cholesterol measurement (n = 3142); of these, 2073 were on stabilized lipid-lowering therapy at low-density lipoprotein cholesterol measurement and had data available to calculate systematic coronary risk evaluation and glomerular filtration rate risk. bData shown are for all patients considered having established atherosclerotic cardiovascular disease at low-density lipoprotein cholesterol measurement (n = 2659); of these, 2039 were on stabilized lipid-lowering therapy at low-density lipoprotein cholesterol measurement. CV, cardiovascular; REACH, Reduction of Atherothrombosis for Continued Health; SCORE, systematic coronary risk evaluation.
Use of lipid-lowering therapy
Use of LLT at enrolment and at the time of LDL-C measurement are summarized in Supplementary material online, Table S3a/Figure S3 and Table S3b/Figure S4, respectively. There was very little change in the pattern of LLT regimen between the time of LDL-C measurement and enrolment (Supplementary material online, Table S3). Among patients receiving stabilized LLT and in whom LDL-C goal could be assessed, 94% of primary prevention patients (1944/2073) and 94% (1912/2039) of those with established ASCVD were receiving a statin. In primary prevention, use of high-intensity statins was 22% (448/2073). High-intensity statins were more often used in established ASCVD [42% (858/2039)], with proportionally higher use among those being managed for coronary disease [51% (240/470)] than for peripheral [39% (320/818)] or cerebral [40% (298/751)] disease. Across all risk categories, moderate-intensity statins as monotherapy was the most frequently used regimen (Figure 2). Ezetimibe was used in combination with moderate- or high-intensity statins in 9% of patients (380/4122) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors used in combination with statins and/or ezetimibe in 1% of patients (49/4122) (Figure 2A).
European Society of Cardiology/European Atherosclerosis Society 2016 and 2019 risk-based low-density lipoprotein cholesterol goal attainment among patients stabilized on lipid-lowering regimens summarized by level of risk and statin regimen. (A) The overall group summarized by level of risk and statin regimen (B) The Primary prevention group summarized by level of risk and statin regimen. (C) The established atherosclerotic cardiovascular disease groupb summarized by level of risk and statin regimen. aGoal attainment for low risk is the same for 2016 and 2019 (<3.0 mmol/L) so only one bar is displayed. bIndividuals with established atherosclerotic cardiovascular disease are, by definition, very high cardiovascular risk. N is the number of patients in the category with non-missing low-density lipoprotein cholesterol goal data. Patients enrolled as secondary prevention whose first atherosclerotic cardiovascular disease event occurred after the date low-density lipoprotein cholesterol levels were stabilized are included in the primary prevention group. Among patients enrolled as secondary prevention, 142 had their first cardiovascular event recorded after their most recent low-density lipoprotein cholesterol measurement, hence they were analysed as primary prevention patients for outcomes assessed at low-density lipoprotein cholesterol measurement, such as goal attainment. For outcomes assessed at enrolment, these 142 patients were analysed as secondary prevention. Definitions of lipid-lowering therapy categories are described in Supplementary material online, Table S2. LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor.
Attainment of 2016 European Society of Cardiology/European Atherosclerosis Society guideline recommended low-density lipoprotein cholesterol goals
Overall risk-based 2016 LDL-C goal attainment was observed in 54% [95% confidence interval (CI) 52–56] of patients. Stratifying patients by risk, LDL-C goal attainment among those at low, moderate, high, and very high risk was 63% (95% CI 56–70), 75% (95% CI 73–78), 63% (95% CI 59–67), and 39% (95% CI 37–41), respectively (Figure 2A). The LDL-C goal of <3.0 mmol/L recommended for low–moderate risk categories was achieved in the majority of patients, irrespective of statin monotherapy regimen. Differences in goal attainment with different statin monotherapy regimens were most apparent in very high-risk patients where an LDL-C goal <1.8 mmol/L is recommended: low-intensity, 20% (95% CI 11–33); moderate-intensity, 34% (95% CI 31–37); high-intensity, 45% (95% CI 41–48).
For the primary prevention group, most patients were at low–moderate risk, and their LDL-C goal of <3.0 mmol/L was achieved in the majority irrespective of statin dose (66–80% achievement). Goal attainment using low-intensity statin monotherapy was higher in moderate-risk patients at 67% (95% CI 55–78) compared with 25% (95% CI 5–70) in very high-risk patients (Figure 2B). Few patients at very high risk attained LDL-C goals of <1.8 mmol/L regardless of statin regimen: low-intensity, 25% (95% CI 5–70); moderate-intensity, 23% (95% CI 14–35); high-intensity, 22% (95% CI 9–45). Among the very high-risk group, only two patients received potent statin and ezetimibe combinations and neither achieved their LDL-C goal.
Among patients with established ASCVD, 39% (95% CI 37–41) achieved the very high-risk goal of LDL-C <1.8 mmol/L (Figure 2C) ranging from 36% (95% CI 32–39) for cerebral disease to 44% (95% CI 40–49) for coronary disease (Supplementary material online, Figure S5). Goal attainment was observed in less than half of patients irrespective of statin intensity when used as monotherapy, but was more likely with the use of high-intensity statin [45% (95% CI 42–49)]. Goal attainment was also more likely in patients treated with statin in combination with ezetimibe or PCSK9 inhibitor therapies; 54% (95% CI 47–61) and 67% (95% CI 47–82), respectively (Figure 2C).
2016 European Society of Cardiology/European Atherosclerosis Society guideline low-density lipoprotein cholesterol goal attainment by country and healthcare setting
Overall risk-based goal attainment by country is shown in Supplementary material online, Figure S6, which ranged between 21% (CI 16.8%–26.6%) in Ukraine and 73.3% (CI 64.8%–80.4%) in Italy. In most countries, risk-based goal attainment was similar to the risk-based goal attainment in the overall study population. Overall risk-based goal attainment was similar for patients enrolled in primary and secondary care settings (Supplementary material online, Figure S7).
Attainment of 2019 European Society of Cardiology/European Atherosclerosis Society guideline low-density lipoprotein cholesterol goals
Overall, fewer patients attained the 2019 LDL-C goals than the 2016 goals [33% (95% CI 32–35) vs. 54% (95% CI 52–56)], with a lower likelihood of goal attainment with increasing risk, i.e. lower LDL-C goal (Figure 2A). Only 18% (95% CI 17–20) of very high-risk patients achieved LDL-C goals of <1.4 mmol/L. Among very high-risk patients receiving statin monotherapy, goal attainment was 14% (95% CI 7–26), 16% (95% CI 13–18), and 22% (95% CI 19–25) in those receiving low-, moderate-, and high-intensity statins, respectively. Overall, 37% (95% CI 32–42) of patients receiving ezetimibe combination therapy and 57% (95% CI 43–70%) of those receiving PCSK9 inhibitor combination therapy achieved their risk-based LDL-C goal.
In the primary prevention group, three-quarters of moderate-risk patients achieved the 2016 goals (<3.0 mmol/L) compared with 60% attainment for the 2019 goals (<2.6 mmol/L), and approximately half to two-thirds achieved 2019 goals with statin monotherapy irrespective of potency (Figure 2B). Among individuals at high and very high risk, 2019 goal attainment was approximately half that of 2016 [25% (95% CI 22–29) vs. 63% and 11% (95% CI 6–20) vs. 21%, respectively] (Figure 2B). In high-risk patients receiving statin monotherapy, LDL-C goal attainment (<1.8 mmol/L) ranged from 7% (95% CI 2–21) in those receiving low-intensity statins to 29% (95% CI 21–38) for high-intensity statins. Among those receiving combination therapy with ezetimibe or PCSK9 inhibitors, goal attainment in high-risk patients was 21% (95% CI 9–41) and 33% (95% CI 6–79), respectively.
Among the established ASCVD group, 2019 goal attainment was approximately half that of 2016 [18% (95% CI 17–20) and 39%, respectively] (Figure 2C and Supplementary material online, Figure S8). Even with high-intensity statins, only 22% (95% CI 19–25) attained 2019 goals. Among patients using ezetimibe in combination with potent statins and PCSK9 inhibitors in combination with any LLT, goal attainment was 21% (95% CI 15–27) and 58% (95% CI 39–76), respectively.
Lipid-lowering therapy-stabilized low-density lipoprotein cholesterol levels
Among patients receiving stabilized LLT at the time of LDL-C measurement, mean LDL-C levels were 2.54 mmol/L (SE 0.02) for primary prevention patients (n = 2558); and 2.02 mmol/L (SE 0.04), 2.20 mmol/L (SE 0.03), and 2.19 mmol/L (SE 0.03), respectively, for patients being managed for coronary, peripheral, and cerebral disease (established ASCVD, n = 2039) (Figure 3). Among all patients receiving high- or moderate-intensity statins as monotherapy, mean LDL-C levels were 2.18 mmol/L (SE 0.03) and 2.31 mmol/L (SE 0.02), respectively.
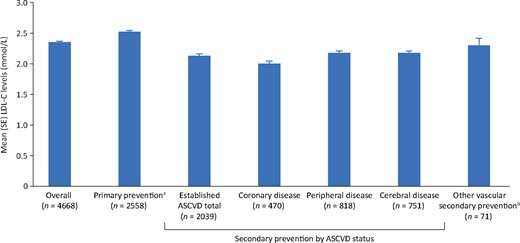
Mean low-density lipoprotein cholesterol levels in patients with stabilized lipid-lowering therapy. n, the number of patients in the category on stabilized lipid-lowering therapy and with non-missing low-density lipoprotein cholesterol goal data. aPrimary prevention: at low-density lipoprotein cholesterol measurement, 142 patients enrolled as secondary prevention, whose first atherosclerotic cardiovascular disease event occurred after the date low-density lipoprotein cholesterol levels were stabilized, are included in the primary prevention group. bPatients with other evidence of atherosclerosis or other manifestation of vascular disease at enrolment. ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; SE, standard error.
Discussion
This cross-sectional European study, conducted in the 2 years after the 2016 iteration of the ESC/EAS dyslipidaemia guidelines, shows that, overall, only 54% of patients achieved their risk-based 2016 LDL-C goal, with goal attainment higher among individuals at lower CV risk and lower among those at higher risk. Since the first registry studies were conducted over 20 years ago,6 it is apparent that average LDL-C levels have decreased with the use of LLT, however, goal attainment remains low among those at higher risk. Although the ESC/EAS LDL-C goals for very high-risk patients do not distinguish between primary and secondary prevention, greater use of high-intensity statins was observed in secondary prevention patients. Among patients receiving high-intensity statins as monotherapy, LDL-C goals were achieved in 22% of very high-risk primary prevention patients and 45% of patients with established ASCVD. Among patients with ASCVD, use of high-intensity statins was greater among those being managed for coronary disease than for peripheral or cerebral disease.
Following the completion of our study, updated 2019 ESC/EAS guidelines were published. These guidelines advocate for a ≥50% reduction in LDL-C from the untreated state in addition to lower, more stringent LDL-C goals (<1.8 and <1.4 mmol/L for those at high and very high risk, respectively, and <2.6 mmol/L for those at moderate risk). The DA VINCI study provides an opportunity to assess the impact of these guideline updates on future clinical practice. For example, after the National Institute for Health and Care Excellence (NICE) lipid guidelines were updated in 2014, placing a greater emphasis on use of more potent statin regimens, it was estimated that, of 3.3 million individuals with ASCVD in the UK, 2.4 million would require statin up-titration to achieve full concordance with the updated guidelines. Furthermore, of 3.5 million high-risk primary prevention patients, 1.6 million would require statin up-titration.7
Among primary prevention patients, the changes in ESC/EAS recommendations between 2016 and 2019 had little impact on patients in the moderate-risk group. While overall goal attainment in DA VINCI fell by 15% as goals moved to below 2.6 mmol/L, the 2019 goal was largely attained with moderate- or high-intensity statin monotherapy. However, goal attainment in high and very high-risk patients fell by approximately half to only 25% and 11%, respectively, reflecting the difficulty of achieving even more stringent goals. Moderate-intensity statins on average achieve ∼30–50% reduction in LDL-C;4 therefore, to achieve the recommended at least 50% reduction, three-quarters of high and very high-risk primary prevention patients and half of secondary prevention patients on moderate-intensity statins would need to at least double their dose. However, doubling doses of statins on average achieves only an additional 6% lowering in LDL-C.8 As stabilized LDL-C levels were 2.36 mmol/L for those receiving moderate-intensity statins as monotherapy, it is unlikely that the 67% of patients currently failing to achieve 2019 goals would successfully attain goals through increasing statin dosing alone. Those not attaining goals more likely require add-on non-statin therapies, such as ezetimibe or PCSK9 inhibitors, which could reduce LDL-C by a further 20–25% and 50–60%, respectively.9 In the present study, while the use of ezetimibe and PCSK9 inhibitors in combination with statins was low (9% and 1%, respectively), compared with statin monotherapy, a higher proportion of patients receiving these combination therapies achieved the lower 2019 LDL-C goals (37% and 57%, respectively). Extrapolating our findings to the wider European population of ∼454 million, of whom an estimated 22.3 million patients have ASCVD, as many as 18 million ASCVD patients across Europe are likely to require add-on non-statin LLT to achieve 2019 goals.
Every 1 mmol/L absolute reduction in LDL-C achieved with statins reduces all-cause mortality by ∼10% and major vascular events by 22%.10 Hence, guidelines, including those from ESC/EAS, have moved towards recommending at least a 50% reduction in LDL-C, in addition to achieving specific LDL-C goals for those at highest risk. This is intended to discourage use of less potent statin regimens in patients at high or very high risk whose LDL-C levels are close to goal, and who, using a goal-based approach alone, could potentially receive suboptimal LDL-C reduction, despite apparently being at goal. In this regard, our study offers insights into clinical practice. Among primary prevention patients, who were at high and very high risk, moderate-intensity statin therapy was the most commonly used regimen (64% and 69%, respectively). Although the 2016 goal of <2.6 mmol/L for high-risk patients was attained in 47–67% of those using statins of varying intensity as monotherapy, attainment of the very high-risk goal of <1.8 mmol/L was only attained by 22–25% of patients at very high risk with low to high-intensity statin monotherapy. These data highlight the opportunity to optimize LDL-C treatment in clinical practice. Namely, the need to first optimize statin dosing, thus maximizing LDL-C reduction, while recognizing that lower LDL-C goals may not be achievable with monotherapy, and the potential need for additional non-statin LLT. This is particularly relevant, for instance, for the 33% of patients in the present study with a 10-year estimated SCORE risk of CV death ≥5% (high and very high risk).
Although high-intensity statins were more frequently used in patients with ASCVD compared with very high-risk primary prevention patients, only 22% of individuals with ASCVD receiving high-intensity statin monotherapy achieved the 2019 LDL-C goal of below 1.4 mmol/L, compared with 45% achieving the 2016 LDL-C goal of below 1.8 mmol/L. Among patients with ASCVD receiving moderate-intensity statin monotherapy, only 16% achieved the 2019 LDL-C goal (vs. 35% achieving the 2016 goal). As average LDL-C among ASCVD patients in our study was higher than 2 mmol/L, increasing statin intensity from moderate to high, for instance from atorvastatin 10–20 mg or rosuvastatin 5–10 mg to atorvastatin 40–80 mg or rosuvastatin 20–40 mg, respectively, would only offer a further 6–12% LDL-C reduction. Therefore, for most patients, an LDL-C goal of <1.4 mmol/L would be unattainable with monotherapy. In this regard, our data offer further insights into potential benefits from combination therapy. Although 54% of ASCVD patients receiving moderate to high-intensity statin regimens with ezetimibe achieved LDL-C levels below 1.8 mmol/L, only 21% achieved levels below 1.4 mmol/L. By comparison, in the small number of ASCVD patients who received PCSK9 inhibitors in combination with other LLT, 67% and 58% of ASCVD patients attained LDL-C levels below 1.8 and 1.4 mmol/L, respectively.
Goal attainment alone, without consideration of baseline event rates, provides limited information to guide future public health policies to allocate resources to areas of unmet need. Using the REACH score to estimate risk of CV events suggested that more than 80% of ASCVD patients in the current study had a 10-year residual risk >20%, and one-third had a >40% residual risk. Reducing LDL-C from above 2 mmol/L to below 1.4 mmol/L could offer an ∼11% relative reduction in CV events and a 5% relative reduction in mortality,10 and thus offers considerable benefit for population health in very high-risk patients across Europe.
The clinical relevance of these findings should be considered. The failure to achieve ESC/EAS guideline recommended LDL-C values across 18 European countries may indicate systematic problems in the health care system such as physicians’ lack of familiarity with the ESC/EAS guideline recommendations, the high costs of medications such as PCSK9 inhibitors, the reluctance of patients to accept high-intensity LLT, or the concern regarding adverse events associated with statins. The acceptance of high-cost medications within health care systems and increased awareness of ESC/EAS guideline recommended LDL-C levels may depend on the perceived clinical value of these therapies and targets, which will vary. Some clinicians may place greater value on prevention of outcomes such as total mortality while others will place greater value on the prevention of important non-fatal events. Of note, the high 10 year predicted risk in DA VINCI underscores the importance of the control of all risk factors, including lipids, as a means to improve population health.
The strengths and limitations of our study merit consideration. Our study builds on earlier registries5,11 by including several previously unstudied countries’ data from primary care and specialist secondary care settings managing less well-studied groups, such as peripheral and cerebral disease, as well as coronary disease, in a systematic manner during the same time frame. Furthermore, we were able to quantify the large change in clinical practice that will be required across Europe to meet the latest 2019 guidelines. As untreated lipid levels were not available, we cannot quantify to what extent the ≥50% LDL-C reduction from baseline, in recommendations, was achieved, and therefore used high-intensity statin use as proxy. Moreover, physician biases in choice of LLT, pre-treatment LDL-C levels, as well as local prescribing restrictions could have influenced our observations about goal attainment. That said, our findings are consistent with results from randomized clinical trials on the comparative efficacy of different LLT regimens.9 Additionally, longitudinal data including clinical outcomes would have strengthened our findings. Finally, as with all registries, the sites who agreed to participate may be early adopters or those with a keen interest in LLTs and as such, the present findings may reflect a ‘best-case’ scenario than what may exist among sites who did not participate.
In conclusion, the DA VINCI study demonstrates that among patients receiving LLT fewer than half of high/very high-risk primary and secondary prevention patients achieved 2016 LDL-C goals, with approximately one-fifth achieving the lower 2019 goals. Even with optimized statin usage, the prevalent gap between guideline recommended LDL-C goals and their implementation in clinical care will require greater utilization of non-statin LLT in combination with statins for patients at highest risk.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/
Acknowledgements
The authors would like to thank Sinéad Flannery, PhD, of PharmaGenesis London, London, UK and Claire Desborough of Amgen Ltd, UK for medical writing and editorial support. K.K.R. and N.R.P. acknowledge their support by the NIHR Imperial Biomedical Research Centre and K.K.R. for additional support from the NIHR ARC for Northwest London. This manuscript is supported by the National Institute for Health Research Applied Research Collaboration Northwest London. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Funding
This work was supported by Amgen (Europe) GmbH.
Conflict of interest: K.K.R. reports grants from Amgen, during the conduct of the study; and personal fees from Abbvie, Aegerion, Akcea, Algorithm, AstraZeneca, Bayer, Boehringer Ingelheim, Cerenis, Cipla, Dr Reddys, Esperion, Kowa, Lilly, The Medicines Company, Novartis, Silence Therapeutics, Takeda, and Zuellig Pharma, and grants and personal fees from Amgen, Daiichi Sankyo, MSD, Pfizer, and Sanofi/Regeneron, outside the submitted work. B.M. is an employee of Amgen Inc. W.M.S. is an employee of Amgen Ltd. P.G. is an employee of Amgen Hellas, Greece. S.B. is an employee of Amgen Ltd. M.B. is on the speakers bureau for Akcea, Amgen, Daiichi Sankyo, KRKA, MSD, Mylan, Polpharma, Sanofi-Aventis, Servier, and Valeant; is a consultant to Abbott Vascular, Akcea, Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, MSD, Polfarmex, Resverlogix, and Sanofi-Aventis; and reports grants from Sanofi, Mylan, and Valeant. D.G. reports fees for educational activities from Amgen, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier. I.G.-B. reports personal fees and non-financial support from Akcea, Amgen, and Sanofi, and personal fees from Aegerion, Daiichi Sankyo, and Regeneron, outside the submitted work. G.K.H. reports research grants from the Netherlands Organization for Scientific Research (VIDI grant), Klinkerpad Fonds, and the European Union; institutional research support from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Ionis, Kowa, Pfizer, Regeneron, Roche, Sanofi, and The Medicines Company; speaker and consulting fees from Aegerion, Amgen, Regeneron, and Sanofi; and part-time employment at Novo Nordisk outside the submitted work. J.J.J. reports a research grant/support from Valeant and has served as a consultant or speaker for ALAB Laboratories, Amgen, Bioton, Boehringer Ingelheim, Celgene, Microlife, Servier, Teva, and Valeant. J.W.J./his department reports research grants from and/or was a speaker (with or without lecture fees) on a.o. (CME accredited) meetings sponsored by Amgen, AstraZeneca, Athera, Biotronik, Boston Scientific, Daiichi Sankyo, DalCor, Lilly, Medtronic, Merck-Schering-Plough, Pfizer, Roche, Sanofi-Aventis, The Medicines Company, the Netherlands Heart Foundation, CardioVascular Research the Netherlands (CVON), the Netherlands Heart Institute, and the European Community Framework KP7 Programme. S.K. reports grant/research support from Amgen, AstraZeneca, and Bayer, and consulting fees/honoraria from Amgen, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Mesi, MSD, Pfizer, Philips Healthcare, Sanofi, and Servier. R.G.K. reports fees as a member of the speakers bureau at Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Pfizer, and Sanofi-Aventis. H.K.I. reports fees for contribution to advisory boards or oral presentations from Allergan, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Pfizer, St Jude, and has no ownership interest and does not own stock in any pharmaceutical company. L.M. reports fees for lectures and/or advisory work from Amarin, Amgen, Amryt, Mylan, Novartis, Sanofi Regeneron, and Servier. A.Parkhomenko reports research grants and personal fees from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Aventis. K.R., clinical trials, consultant, presentations: Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Merck, Mylan, Novo Nordisk, Pfizer, Sanofi, and Zentiva. P.S. reports fees as a speaker and advisory board member from Amgen, AstraZeneca, MSD, and Sanofi. S.R. reports fees as a speaker and advisory board member from Akcea, Amgen, AstraZeneca, and Sanofi, and grants from Amgen, AstraZeneca, and Sanofi. C.V. reports research grant(s)/support and fees from Amgen, ELPEN, MSD, Sanofi, and Vianex. M.V. reports grants, personal fees, or non-financial support from Abbott Laboratories, Amgen, AstraZeneca, Boehringer Ingelheim, KRKA, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis, Servier, and Zentiva outside of the submitted work. A.L.C. reports research grant(s)/support from Amgen, Eli Lilly, Menarini, Mylan, Sanofi, and Sanofi Regeneron, and has served as a consultant for or received fees from Aegerion, Akcea, Amgen, Amryt, AstraZeneca, Daiichi Sankyo, Esperion, Genzyme, Ionis Pharmaceuticals, Kowa, Medco, Menarini, MSD, Mylan, Novartis, Recordati, Regeneron, and Sanofi. N.R.P. reports financial support from several pharmaceutical companies that manufacture lipid-lowering agents for consultancy fees (Amgen and Pfizer), research projects and staff (Amgen and Pfizer), and for arranging and speaking at educational meetings (Amgen, MSD, and Pfizer). He holds no stocks and shares in any such companies, and is supported by the National Institute for Health Research Senior Investigator Awards, Biomedical Research Centre funding, and the British Heart Foundation Research Centre Excellence Award. All other authors declared no conflict of interest.
Declaration of Helsinki
This study complies with the Declaration of Helsinki. The locally appointed ethics committee in each country approved the research protocol, and informed consent has been obtained from the patients.
References
European Cardiovascular Diseases Statistics 2017. http://www.ehnheart.org/cvd-statistics.html (22 November
Author notes
† The complete list of investigators and collaborators are listed in Supplementary material online, Appendix S1.







Comments