-
PDF
- Split View
-
Views
-
Cite
Cite
Linda E Scheffers, Linda E M vd Berg, Gamida Ismailova, Karolijn Dulfer, Johanna J M Takkenberg, Wim A Helbing, Physical exercise training in patients with a Fontan circulation: A systematic review, European Journal of Preventive Cardiology, Volume 28, Issue 11, November 2021, Pages 1269–1278, https://doi.org/10.1177/2047487320942869
Close - Share Icon Share
Abstract
Patients with a Fontan circulation have a reduced exercise capacity, which is an important prognostic predictor of morbidity and mortality. A way to increase exercise capacity in Fontan patients might be exercise training. This systematic review assesses the effects of exercise training investigated in Fontan patients in order to provide an overview of current insights.
Studies evaluating an exercise training intervention in Fontan patients published up to February 2020 were included in this systematic review.
From 3000 potential studies, 16 studies reported in 22 publications met the inclusion criteria. In total, 264 Fontan patients with mean age range 8.7–31 years, were included. Different training types including inspiratory muscle training, resistance training and aerobic training were investigated. Main outcome measures reported were peak oxygen uptake, cardiac function, lung function, physical activity levels and quality of life. Peak oxygen uptake increased significantly in 56% of the studies after training with an overall mean increase of +1.72 ml/kg/min (+6.3%). None of the studies reported negative outcome measures related to the exercise programme. In four studies an adverse event was reported, most likely unrelated to the training intervention.
Exercise training in Fontan patients is most likely safe and has positive effects on exercise capacity, cardiac function and quality of life. Therefore exercise training in Fontan patients should be encouraged. Further studies are required to assess the optimal training type, intensity, duration and long-term effects.
Introduction
The outcomes of patients treated with a Fontan procedure, a palliative surgical procedure used for univentricular heart defects, have improved remarkably in recent decades.1 Due to improvements in diagnosis and surgical strategy, patients with a Fontan circulation can now have a higher quality of life and increased life expectancy than before.2 Nevertheless, these patients continue to have impaired exercise capacity, which is an important prognostic predictor of morbidity and mortality.3–5
Patients after the Fontan procedure are less physically active compared to healthy peers.6 A lower level of physical activity might contribute to an impaired exercise capacity and is associated with an increased risk of numerous adverse health effects and even early death.6,7 Exercise training may increase both levels of physical activity and exercise capacity, and has shown promising results in patients with several types of congenital heart disease.8 However, patients with a Fontan circulation have a very different physiology compared to those with a normal (biventricular) circulation. In Fontan patients, the systemic venous return bypasses a sub-pulmonary ventricle and is directly connected to the pulmonary arteries.1 During exercise Fontan patients fail to adequately increase venous return and thereby pulmonary blood flow, leading to a reduced preload and an inability to increase stroke volume.1 A randomised controlled trial demonstrated that endurance training can improve exercise capacity in adolescents with Tetralogy of Fallot, but not in Fontan patients.9 This raises the question whether endurance training is the most effective way of training in Fontan patients, or whether Fontan patients should be trained differently.
Several observational studies and randomised controlled trials have investigated different types of exercise training with the aim to increase exercise capacity and quality of life, leading to varying results. This systematic review assesses the various types of exercise training investigated in Fontan patients, in order to provide an overview of current insight into the optimal kind of exercise training, intensity, duration and frequency.
Methods
Protocol registration and search strategy
This systematic review was conducted according to the PRISMA guidelines (Supplementary Material 1).10 Online databases were systematically searched on 19 February 2020 to identify all studies investigating the effects of training in Fontan patients. The online databases that were searched included: Embase, Medline Ovid, Web of Science, CINAHL EBSCOhost, Cochrane CENTRAL and Google Scholar. The search terms consisted of keywords related to Fontan circulation and training/exercise (Supplementary Material 2).
Inclusion criteria
All studies were screened on title and abstract by two independent reviewers (LES and GI) After this selection, the full text of these articles was studied. Inclusion criteria were: (a) studies should include patients with the diagnosis of Fontan circulation undergoing any type of exercise training, (b) studies had to be prospective intervention studies (c) studies had to be written in English and published after 1970. Exclusion criteria were: (a) articles including patients with various congenital heart diseases without describing patients with a Fontan circulation separately in the results section, (b) studies that did not provide baseline or post-intervention measurements, (c) results from conference papers and abstracts. In cases of disagreement about inclusion between the reviewers, a consensus was negotiated.
Data extraction
After full-text screening, the two reviewers (LES and GI) extracted all needed data from the eligible articles independently using Microsoft Office Excel 2016. After data extraction, each reviewer verified the other reviewer’s data entries. All study characteristics, patient characteristics and outcome measurements that were relevant to the training intervention were extracted (Supplementary Material 3).
Quality assessment appraisal
To assess the quality, the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) checklist was used for cohort studies, the CARE case report guidelines for case reports and the Cochrane Risk of Bias tool was used for randomised controlled trials.11,12
Statistical analyses
Given the heterogeneous nature of the training interventions and outcome measurements of the retrieved studies, a meta-analysis was not performed and the results are presented as descriptive data. Continuous variables are presented as mean ± standard deviation (SD) or, if not normally distributed, as median. Categorical variables are presented as counts and percentages.
Results
Included studies
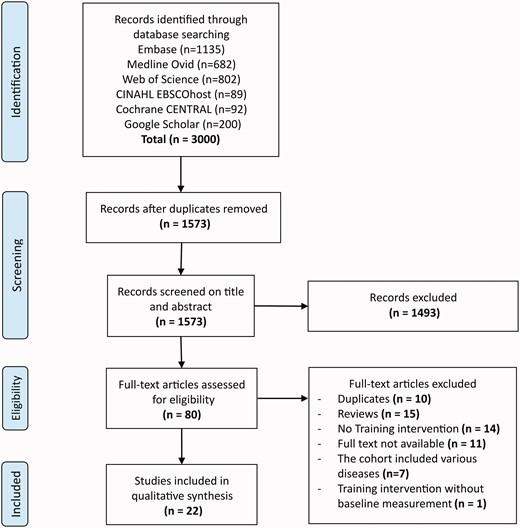
We identified a total of 3000 studies. Full-text eligibility was assessed for 80 articles (Figure 1). Finally, 22 articles were included. In these 22 articles, 16 individual cohorts were described. Another six articles described identical patient populations with different outcome measures.13–17
Quality assessment appraisal
No studies were excluded based on quality. All studies but two met over 75% of the STROBE checklist criteria (Supplementary Material 4).18,19 The single case report met 80% of the CARE checklist criteria (Supplementary Material 5).20 The randomised controlled trials had a low risk of bias according to the Cochrane Risk of Bias tool (Supplementary Material 6).9,21
Study characteristics and patient characteristics
The study designs of all included studies are described in Table 1, patient characteristics are summarised in Table 2. In total, a group of 264 Fontan patients participated in a training programme, 54 Fontan patients and 32 healthy participants served as controls. Seven studies included children only, four studies included adults only, five studies included a mix of children and adults. All studies, except the single case report, included both sexes, 47% of the participants were female. The mean age of the patients ranged from 8.7–31 years. In total, 66% of the participants had a dominant left ventricle (LV) and 53% of all patients underwent the extracardiac conduit (ECC) procedure.
| . | Year of publication . | Study design . | Training group n . | Control group n . | Kind of training . | Intensity of training . | Duration in weeks . | Training sessions per week n . | Time per session min . | Training implementationa . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies including children only: | ||||||||||
| Jacobsen17,23 | 2018 | Cohort | 13 | 0 | Aerobic + resistance exercises | – | 12 | 3–4 | 60 | Unsupervised, on television |
| Sutherland (home)25 | 2018 | Randomised trial | 11 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Unsupervised |
| Sutherland (hospital)25 | 2018 | Randomised trial | 6 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Supervised |
| Wittekind26 | 2018 | Cohort | 10 | 0 | Aerobic + resistance exercises | 70–80% of HRR | 12 | 2 | 60 | Supervised |
| Hedlund28,39 | 2017 | Cohort with controls | 30 | 25 | Aerobic training | – | 12 | 2 | 45 | Supervised |
| Laohachai30 | 2017 | Cohort | 23 | 0 | Inspiratory muscle training | 30% of MIP | 6 | 7 | 30 | Unsupervised |
| Longmuir24 | 2013 | Randomised trial | 30 | 0 | Aerobic + resistance exercises | – | 52 | 7 | 10–15 | Unsupervised |
| Opocher19 | 2005 | Cohort | 10 | 0 | Aerobic training | 50–70% of HR at peak VO2 | 32 | 2 | 30–45 | Unsupervised |
| Studies including adults only: | ||||||||||
| Fritz21 | 2019 | Randomised controlled trial | 42 | 22 | Inspiratory muscle training | Adjusted individually | 26 | 7 | 3 sets with 10–30 repetitions | Unsupervised |
| Wu31 | 2018 | Cohort | 12 | 0 | Inspiratory muscle training | 40% of MIP | 12 | 5 | 30 | Unsupervised |
| Cordina32 | 2012 | Cohort with controls | 6 | 5 | High-weight resistance training focusing on calf muscles. | – | 20 | 3 | 60 | Supervised |
| Lichtman20 | 2008 | Case study | 1 | 0 | Aerobic + resistance exercises | 19/20 on Borg scale | – | – | – | Supervised |
| Studies including children and adults: | ||||||||||
| Ait Ali18 | 2018 | Cohort with controls | 8 | 6 | Respiratory training | – | 12 | 1 | 120 | Supervised |
| Duppen9,13–16 | 2015 | Randomised controlled trail | 26 | 17 | Aerobic training | Rest HR plus 60–70% of HRR | 12 | 2 | 60 | Supervised |
| Brassard22 | 2005 | Cohort with controls | 5 | 9 | Aerobic + resistance exercises | 50–80% of HR at peak VO2 | 8 | 3 | 20–30 | Half of the patients were supervised, half unsupervised |
| Minamisawa29 | 2001 | Cohort | 11 | 0 | Aerobic training | 60–80% of peak HR | 8–12 | 2–3 | 30–40 | Unsupervised |
| Balfour27 | 1991 | Cohort | 1 | 0 | Aerobic training | >70% of peak HR | 12 | 3 | 30–40 | Supervised |
| . | Year of publication . | Study design . | Training group n . | Control group n . | Kind of training . | Intensity of training . | Duration in weeks . | Training sessions per week n . | Time per session min . | Training implementationa . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies including children only: | ||||||||||
| Jacobsen17,23 | 2018 | Cohort | 13 | 0 | Aerobic + resistance exercises | – | 12 | 3–4 | 60 | Unsupervised, on television |
| Sutherland (home)25 | 2018 | Randomised trial | 11 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Unsupervised |
| Sutherland (hospital)25 | 2018 | Randomised trial | 6 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Supervised |
| Wittekind26 | 2018 | Cohort | 10 | 0 | Aerobic + resistance exercises | 70–80% of HRR | 12 | 2 | 60 | Supervised |
| Hedlund28,39 | 2017 | Cohort with controls | 30 | 25 | Aerobic training | – | 12 | 2 | 45 | Supervised |
| Laohachai30 | 2017 | Cohort | 23 | 0 | Inspiratory muscle training | 30% of MIP | 6 | 7 | 30 | Unsupervised |
| Longmuir24 | 2013 | Randomised trial | 30 | 0 | Aerobic + resistance exercises | – | 52 | 7 | 10–15 | Unsupervised |
| Opocher19 | 2005 | Cohort | 10 | 0 | Aerobic training | 50–70% of HR at peak VO2 | 32 | 2 | 30–45 | Unsupervised |
| Studies including adults only: | ||||||||||
| Fritz21 | 2019 | Randomised controlled trial | 42 | 22 | Inspiratory muscle training | Adjusted individually | 26 | 7 | 3 sets with 10–30 repetitions | Unsupervised |
| Wu31 | 2018 | Cohort | 12 | 0 | Inspiratory muscle training | 40% of MIP | 12 | 5 | 30 | Unsupervised |
| Cordina32 | 2012 | Cohort with controls | 6 | 5 | High-weight resistance training focusing on calf muscles. | – | 20 | 3 | 60 | Supervised |
| Lichtman20 | 2008 | Case study | 1 | 0 | Aerobic + resistance exercises | 19/20 on Borg scale | – | – | – | Supervised |
| Studies including children and adults: | ||||||||||
| Ait Ali18 | 2018 | Cohort with controls | 8 | 6 | Respiratory training | – | 12 | 1 | 120 | Supervised |
| Duppen9,13–16 | 2015 | Randomised controlled trail | 26 | 17 | Aerobic training | Rest HR plus 60–70% of HRR | 12 | 2 | 60 | Supervised |
| Brassard22 | 2005 | Cohort with controls | 5 | 9 | Aerobic + resistance exercises | 50–80% of HR at peak VO2 | 8 | 3 | 20–30 | Half of the patients were supervised, half unsupervised |
| Minamisawa29 | 2001 | Cohort | 11 | 0 | Aerobic training | 60–80% of peak HR | 8–12 | 2–3 | 30–40 | Unsupervised |
| Balfour27 | 1991 | Cohort | 1 | 0 | Aerobic training | >70% of peak HR | 12 | 3 | 30–40 | Supervised |
HR: heart rate; HRR: heart rate reserve; min: minutes; MIP: maximal inspiratory pressure; n: number; peak HR: peak heart rate; VO2: oxygen uptake.
The sign ‘–' indicates not mentioned in the article.
aA study was referred to as supervised when all training sessions were instructor-led.
| . | Year of publication . | Study design . | Training group n . | Control group n . | Kind of training . | Intensity of training . | Duration in weeks . | Training sessions per week n . | Time per session min . | Training implementationa . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies including children only: | ||||||||||
| Jacobsen17,23 | 2018 | Cohort | 13 | 0 | Aerobic + resistance exercises | – | 12 | 3–4 | 60 | Unsupervised, on television |
| Sutherland (home)25 | 2018 | Randomised trial | 11 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Unsupervised |
| Sutherland (hospital)25 | 2018 | Randomised trial | 6 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Supervised |
| Wittekind26 | 2018 | Cohort | 10 | 0 | Aerobic + resistance exercises | 70–80% of HRR | 12 | 2 | 60 | Supervised |
| Hedlund28,39 | 2017 | Cohort with controls | 30 | 25 | Aerobic training | – | 12 | 2 | 45 | Supervised |
| Laohachai30 | 2017 | Cohort | 23 | 0 | Inspiratory muscle training | 30% of MIP | 6 | 7 | 30 | Unsupervised |
| Longmuir24 | 2013 | Randomised trial | 30 | 0 | Aerobic + resistance exercises | – | 52 | 7 | 10–15 | Unsupervised |
| Opocher19 | 2005 | Cohort | 10 | 0 | Aerobic training | 50–70% of HR at peak VO2 | 32 | 2 | 30–45 | Unsupervised |
| Studies including adults only: | ||||||||||
| Fritz21 | 2019 | Randomised controlled trial | 42 | 22 | Inspiratory muscle training | Adjusted individually | 26 | 7 | 3 sets with 10–30 repetitions | Unsupervised |
| Wu31 | 2018 | Cohort | 12 | 0 | Inspiratory muscle training | 40% of MIP | 12 | 5 | 30 | Unsupervised |
| Cordina32 | 2012 | Cohort with controls | 6 | 5 | High-weight resistance training focusing on calf muscles. | – | 20 | 3 | 60 | Supervised |
| Lichtman20 | 2008 | Case study | 1 | 0 | Aerobic + resistance exercises | 19/20 on Borg scale | – | – | – | Supervised |
| Studies including children and adults: | ||||||||||
| Ait Ali18 | 2018 | Cohort with controls | 8 | 6 | Respiratory training | – | 12 | 1 | 120 | Supervised |
| Duppen9,13–16 | 2015 | Randomised controlled trail | 26 | 17 | Aerobic training | Rest HR plus 60–70% of HRR | 12 | 2 | 60 | Supervised |
| Brassard22 | 2005 | Cohort with controls | 5 | 9 | Aerobic + resistance exercises | 50–80% of HR at peak VO2 | 8 | 3 | 20–30 | Half of the patients were supervised, half unsupervised |
| Minamisawa29 | 2001 | Cohort | 11 | 0 | Aerobic training | 60–80% of peak HR | 8–12 | 2–3 | 30–40 | Unsupervised |
| Balfour27 | 1991 | Cohort | 1 | 0 | Aerobic training | >70% of peak HR | 12 | 3 | 30–40 | Supervised |
| . | Year of publication . | Study design . | Training group n . | Control group n . | Kind of training . | Intensity of training . | Duration in weeks . | Training sessions per week n . | Time per session min . | Training implementationa . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies including children only: | ||||||||||
| Jacobsen17,23 | 2018 | Cohort | 13 | 0 | Aerobic + resistance exercises | – | 12 | 3–4 | 60 | Unsupervised, on television |
| Sutherland (home)25 | 2018 | Randomised trial | 11 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Unsupervised |
| Sutherland (hospital)25 | 2018 | Randomised trial | 6 | 0 | Aerobic + resistance exercises | 65–85% of HR at peak VO2 | 8 | 2 | 60 | Supervised |
| Wittekind26 | 2018 | Cohort | 10 | 0 | Aerobic + resistance exercises | 70–80% of HRR | 12 | 2 | 60 | Supervised |
| Hedlund28,39 | 2017 | Cohort with controls | 30 | 25 | Aerobic training | – | 12 | 2 | 45 | Supervised |
| Laohachai30 | 2017 | Cohort | 23 | 0 | Inspiratory muscle training | 30% of MIP | 6 | 7 | 30 | Unsupervised |
| Longmuir24 | 2013 | Randomised trial | 30 | 0 | Aerobic + resistance exercises | – | 52 | 7 | 10–15 | Unsupervised |
| Opocher19 | 2005 | Cohort | 10 | 0 | Aerobic training | 50–70% of HR at peak VO2 | 32 | 2 | 30–45 | Unsupervised |
| Studies including adults only: | ||||||||||
| Fritz21 | 2019 | Randomised controlled trial | 42 | 22 | Inspiratory muscle training | Adjusted individually | 26 | 7 | 3 sets with 10–30 repetitions | Unsupervised |
| Wu31 | 2018 | Cohort | 12 | 0 | Inspiratory muscle training | 40% of MIP | 12 | 5 | 30 | Unsupervised |
| Cordina32 | 2012 | Cohort with controls | 6 | 5 | High-weight resistance training focusing on calf muscles. | – | 20 | 3 | 60 | Supervised |
| Lichtman20 | 2008 | Case study | 1 | 0 | Aerobic + resistance exercises | 19/20 on Borg scale | – | – | – | Supervised |
| Studies including children and adults: | ||||||||||
| Ait Ali18 | 2018 | Cohort with controls | 8 | 6 | Respiratory training | – | 12 | 1 | 120 | Supervised |
| Duppen9,13–16 | 2015 | Randomised controlled trail | 26 | 17 | Aerobic training | Rest HR plus 60–70% of HRR | 12 | 2 | 60 | Supervised |
| Brassard22 | 2005 | Cohort with controls | 5 | 9 | Aerobic + resistance exercises | 50–80% of HR at peak VO2 | 8 | 3 | 20–30 | Half of the patients were supervised, half unsupervised |
| Minamisawa29 | 2001 | Cohort | 11 | 0 | Aerobic training | 60–80% of peak HR | 8–12 | 2–3 | 30–40 | Unsupervised |
| Balfour27 | 1991 | Cohort | 1 | 0 | Aerobic training | >70% of peak HR | 12 | 3 | 30–40 | Supervised |
HR: heart rate; HRR: heart rate reserve; min: minutes; MIP: maximal inspiratory pressure; n: number; peak HR: peak heart rate; VO2: oxygen uptake.
The sign ‘–' indicates not mentioned in the article.
aA study was referred to as supervised when all training sessions were instructor-led.
| . | Age in years, mean (range)/ mean ± SD . | Sex, % female . | Ventricle type, % left . | Age in years at Fontan completion, mean (range)/ mean ± SD . | Fontan type ECC/ICC/ICT/ KW/ LT/AP/AVA/O in % . | Open fenestration in % . |
|---|---|---|---|---|---|---|
| Studies including children only: | ||||||
| Jacobsen17,23 | 10.5 (9–12) | 55 | 43 | 3.1 | 93 ECC, 7 LT | 45% |
| Sutherland (home)25 | 15 ± 2.7 | 45 | – | – | 100 ECC | – |
| Sutherland (hospital)25 | 16 ± 2.5 | 33 | – | – | 67 ECC, 17 LT, 17 AP | – |
| Wittekind26 | 12 ± 2.8 | 60 | 50 | 3.5 | 50 ECC, 50 LT | – |
| Hedlund28,39 | 14.2 ± 3.2 | 47 | – | 2.4 (1.1–6.4) | – | 0% |
| Laohachai30 | 16 ± 2 | 48 | 57 | 5 ± 2 (3–9) | – | 0% |
| Longmuir24 | 9.1 | 41 | 33 | 2.9 | 87 ECC, 13 LT | – |
| Opocher19 | 8.7 ± 0.6 | 10 | 70 | 1.7 (1–3) | – | – |
| Studies including adults only: | ||||||
| Fritz21 | 28.6 (24.7–36.5) | 50 | 86 | 6.3 (4.0–9.9) | 9 AP, 8 AVA, 25 TCPCu | – |
| Wu31 | 28.8 (25.7–45.5) | 45 | 73 | 7.8 (3.9–16.5) | 9 ECC, 64 LT, 27 AP | 18% |
| Cordina32 | 31 ± 4 | 18 | 82 | 11 ± 4 | 18 ECC, 45 ICC, 36 AP | 9% |
| Lichtman20 | 28 | 100 | 100 | 26 | 100 AP | – |
| Studies including children and adults: | ||||||
| Ait Ali18 | 17.5 (10.4–22.8) | 13 | 43 | 3.96 (0.8–7.4) | 71 ECC, 7 ICC, 14 ICT, 7 KW | – |
| Duppen9,13–16 | 15 ± 3 | 60 | 69 | 3 (2.5–4) | 42 ECC, 54 ILT, 4 O | – |
| Brassard22 | 16 ± 5 | 43 | 100 | – | – | – |
| Minamisawa29 | 19 ± 4 | 55 | 82 | 14 ± 6 | 100 AP | – |
| Balfour27 | 19.8 | NP | 100 | – | – | – |
| . | Age in years, mean (range)/ mean ± SD . | Sex, % female . | Ventricle type, % left . | Age in years at Fontan completion, mean (range)/ mean ± SD . | Fontan type ECC/ICC/ICT/ KW/ LT/AP/AVA/O in % . | Open fenestration in % . |
|---|---|---|---|---|---|---|
| Studies including children only: | ||||||
| Jacobsen17,23 | 10.5 (9–12) | 55 | 43 | 3.1 | 93 ECC, 7 LT | 45% |
| Sutherland (home)25 | 15 ± 2.7 | 45 | – | – | 100 ECC | – |
| Sutherland (hospital)25 | 16 ± 2.5 | 33 | – | – | 67 ECC, 17 LT, 17 AP | – |
| Wittekind26 | 12 ± 2.8 | 60 | 50 | 3.5 | 50 ECC, 50 LT | – |
| Hedlund28,39 | 14.2 ± 3.2 | 47 | – | 2.4 (1.1–6.4) | – | 0% |
| Laohachai30 | 16 ± 2 | 48 | 57 | 5 ± 2 (3–9) | – | 0% |
| Longmuir24 | 9.1 | 41 | 33 | 2.9 | 87 ECC, 13 LT | – |
| Opocher19 | 8.7 ± 0.6 | 10 | 70 | 1.7 (1–3) | – | – |
| Studies including adults only: | ||||||
| Fritz21 | 28.6 (24.7–36.5) | 50 | 86 | 6.3 (4.0–9.9) | 9 AP, 8 AVA, 25 TCPCu | – |
| Wu31 | 28.8 (25.7–45.5) | 45 | 73 | 7.8 (3.9–16.5) | 9 ECC, 64 LT, 27 AP | 18% |
| Cordina32 | 31 ± 4 | 18 | 82 | 11 ± 4 | 18 ECC, 45 ICC, 36 AP | 9% |
| Lichtman20 | 28 | 100 | 100 | 26 | 100 AP | – |
| Studies including children and adults: | ||||||
| Ait Ali18 | 17.5 (10.4–22.8) | 13 | 43 | 3.96 (0.8–7.4) | 71 ECC, 7 ICC, 14 ICT, 7 KW | – |
| Duppen9,13–16 | 15 ± 3 | 60 | 69 | 3 (2.5–4) | 42 ECC, 54 ILT, 4 O | – |
| Brassard22 | 16 ± 5 | 43 | 100 | – | – | – |
| Minamisawa29 | 19 ± 4 | 55 | 82 | 14 ± 6 | 100 AP | – |
| Balfour27 | 19.8 | NP | 100 | – | – | – |
AP: atriopulmonary connection; AVA: atrioventricular anastomosis; ECC: extracardiac conduit; ICC: intracardiac conduit; ICT: intracardiac tunnel; KW: Kawashima Fontan; LT: lateral tunnel Fontan; O: other; SD: standard deviation; TCPCu: Total cavopulmonary connection undefined.
The symbol ‘-' indicates not mentioned in the article. Duppen et al. studies included Fontan patients and tetralogy of Fallot patients. The mean age and percentage of female patients is a mean of both patient groups together. All characteristics are extracted from the articles. Some articles included the dropouts in the patient characteristics, and some did not.
| . | Age in years, mean (range)/ mean ± SD . | Sex, % female . | Ventricle type, % left . | Age in years at Fontan completion, mean (range)/ mean ± SD . | Fontan type ECC/ICC/ICT/ KW/ LT/AP/AVA/O in % . | Open fenestration in % . |
|---|---|---|---|---|---|---|
| Studies including children only: | ||||||
| Jacobsen17,23 | 10.5 (9–12) | 55 | 43 | 3.1 | 93 ECC, 7 LT | 45% |
| Sutherland (home)25 | 15 ± 2.7 | 45 | – | – | 100 ECC | – |
| Sutherland (hospital)25 | 16 ± 2.5 | 33 | – | – | 67 ECC, 17 LT, 17 AP | – |
| Wittekind26 | 12 ± 2.8 | 60 | 50 | 3.5 | 50 ECC, 50 LT | – |
| Hedlund28,39 | 14.2 ± 3.2 | 47 | – | 2.4 (1.1–6.4) | – | 0% |
| Laohachai30 | 16 ± 2 | 48 | 57 | 5 ± 2 (3–9) | – | 0% |
| Longmuir24 | 9.1 | 41 | 33 | 2.9 | 87 ECC, 13 LT | – |
| Opocher19 | 8.7 ± 0.6 | 10 | 70 | 1.7 (1–3) | – | – |
| Studies including adults only: | ||||||
| Fritz21 | 28.6 (24.7–36.5) | 50 | 86 | 6.3 (4.0–9.9) | 9 AP, 8 AVA, 25 TCPCu | – |
| Wu31 | 28.8 (25.7–45.5) | 45 | 73 | 7.8 (3.9–16.5) | 9 ECC, 64 LT, 27 AP | 18% |
| Cordina32 | 31 ± 4 | 18 | 82 | 11 ± 4 | 18 ECC, 45 ICC, 36 AP | 9% |
| Lichtman20 | 28 | 100 | 100 | 26 | 100 AP | – |
| Studies including children and adults: | ||||||
| Ait Ali18 | 17.5 (10.4–22.8) | 13 | 43 | 3.96 (0.8–7.4) | 71 ECC, 7 ICC, 14 ICT, 7 KW | – |
| Duppen9,13–16 | 15 ± 3 | 60 | 69 | 3 (2.5–4) | 42 ECC, 54 ILT, 4 O | – |
| Brassard22 | 16 ± 5 | 43 | 100 | – | – | – |
| Minamisawa29 | 19 ± 4 | 55 | 82 | 14 ± 6 | 100 AP | – |
| Balfour27 | 19.8 | NP | 100 | – | – | – |
| . | Age in years, mean (range)/ mean ± SD . | Sex, % female . | Ventricle type, % left . | Age in years at Fontan completion, mean (range)/ mean ± SD . | Fontan type ECC/ICC/ICT/ KW/ LT/AP/AVA/O in % . | Open fenestration in % . |
|---|---|---|---|---|---|---|
| Studies including children only: | ||||||
| Jacobsen17,23 | 10.5 (9–12) | 55 | 43 | 3.1 | 93 ECC, 7 LT | 45% |
| Sutherland (home)25 | 15 ± 2.7 | 45 | – | – | 100 ECC | – |
| Sutherland (hospital)25 | 16 ± 2.5 | 33 | – | – | 67 ECC, 17 LT, 17 AP | – |
| Wittekind26 | 12 ± 2.8 | 60 | 50 | 3.5 | 50 ECC, 50 LT | – |
| Hedlund28,39 | 14.2 ± 3.2 | 47 | – | 2.4 (1.1–6.4) | – | 0% |
| Laohachai30 | 16 ± 2 | 48 | 57 | 5 ± 2 (3–9) | – | 0% |
| Longmuir24 | 9.1 | 41 | 33 | 2.9 | 87 ECC, 13 LT | – |
| Opocher19 | 8.7 ± 0.6 | 10 | 70 | 1.7 (1–3) | – | – |
| Studies including adults only: | ||||||
| Fritz21 | 28.6 (24.7–36.5) | 50 | 86 | 6.3 (4.0–9.9) | 9 AP, 8 AVA, 25 TCPCu | – |
| Wu31 | 28.8 (25.7–45.5) | 45 | 73 | 7.8 (3.9–16.5) | 9 ECC, 64 LT, 27 AP | 18% |
| Cordina32 | 31 ± 4 | 18 | 82 | 11 ± 4 | 18 ECC, 45 ICC, 36 AP | 9% |
| Lichtman20 | 28 | 100 | 100 | 26 | 100 AP | – |
| Studies including children and adults: | ||||||
| Ait Ali18 | 17.5 (10.4–22.8) | 13 | 43 | 3.96 (0.8–7.4) | 71 ECC, 7 ICC, 14 ICT, 7 KW | – |
| Duppen9,13–16 | 15 ± 3 | 60 | 69 | 3 (2.5–4) | 42 ECC, 54 ILT, 4 O | – |
| Brassard22 | 16 ± 5 | 43 | 100 | – | – | – |
| Minamisawa29 | 19 ± 4 | 55 | 82 | 14 ± 6 | 100 AP | – |
| Balfour27 | 19.8 | NP | 100 | – | – | – |
AP: atriopulmonary connection; AVA: atrioventricular anastomosis; ECC: extracardiac conduit; ICC: intracardiac conduit; ICT: intracardiac tunnel; KW: Kawashima Fontan; LT: lateral tunnel Fontan; O: other; SD: standard deviation; TCPCu: Total cavopulmonary connection undefined.
The symbol ‘-' indicates not mentioned in the article. Duppen et al. studies included Fontan patients and tetralogy of Fallot patients. The mean age and percentage of female patients is a mean of both patient groups together. All characteristics are extracted from the articles. Some articles included the dropouts in the patient characteristics, and some did not.
Training programme
Information about the different types of training, training duration, training intensity, follow-up and supervision can be found in Table 2.
Types of training
Studies provided different types of exercise training. Six studies investigated the effects of a combination of aerobic and resistance exercises.20,22–26 Five studies investigated the effects of aerobic training.9,19,27–29 One of these also added stress management counselling, dietary counselling and education about the heart.27
Ait Ali et al. investigated the effects of respiratory training by using a combination of education about the respiratory system and breathing exercises.18 Three studies provided inspiratory muscle training using a threshold inspiratory muscle training device.21,30,31
The study of Cordina et al. is the only one that investigated effects of high-weight resistance training, focusing on the lower limbs.32
Training duration
Most training programs lasted 12 weeks. All studies had a follow-up immediately after training. Four studies had long-term follow-ups, after 6 months or after 1 year.23,24,28,32 The amount of training sessions ranged between 1–7 times a week, with a mean of three times a week. The average training session lasted 50 min, ranging from 10–120 min.
Training supervision
An instructor supervised all training sessions in nine studies. In two studies a supervised training programme was compared to an unsupervised training programme.25,27 All studies using unsupervised training started with at least one supervised session. Researchers had frequent contact with patients to assure compliance in all studies.
Recruitment, compliance and drop-outs
Four studies reported recruitment data, in these studies a mean percentage of 46% refused to participate.9,24,25,32 All studies but three reported attendance rate for the training sessions.21,28,29 The mean percentage of overall attendance was 79%. All studies reported drop-out rates, and overall 39 patients (15%) dropped out of the studies. Most patients dropped-out during training due to personal reasons, five patients dropped-out during long-term follow-up, three patients did not reach maximal effort during cardiopulmonary exercise testing (CPET) and six patients were excluded due to low attendance rates.
Study outcomes
Table 3 shows summarised study outcomes, Supplementary Material 7 shows detailed information about follow-up moments, test methods and all significant study outcomes.
| . | Peak VO2 . | Cardiac output (MR or echo) . | Adverse events . | Distance walked (6MWT) . | Activity levels . | Quality of life patient report . | Quality of life parent report . |
|---|---|---|---|---|---|---|---|
| Studies including children only: | |||||||
| Jacobsen17,23 | ↑ | – | No | – | = | ↓ | ↑ |
| Sutherland (home/hospital)25 | = | – | Yes | ↑ | – | ↑ | ↑ |
| Wittekind26 | ↑ | = | Yes | – | – | – | – |
| Hedlund28,39 | = | – | – | ↑ | ↑ | ↑ | ↑ |
| Laohachai30 | = | ↑ | No | – | – | – | – |
| Longmuir24 | ↑ | – | – | – | ↑ | – | – |
| Opocher19 | ↑ | – | No | – | – | – | – |
| Studies including adults only: | |||||||
| Fritz21 | = | – | Yes | – | – | – | – |
| Wu31 | = | – | No | – | – | = | – |
| Cordina32 | ↑ | ↑ | Yes | – | – | – | – |
| Lichtman20 | ↑ | – | – | – | – | ↑ | – |
| Studies including children and adults: | |||||||
| Ait Ali18 | ↑ | – | – | – | – | – | – |
| Duppen9,13–16 | = | = | – | – | = | ↑ | ↑ |
| Brassard22 | = | – | – | – | – | – | – |
| Minamisawa29 | ↑ | – | No | – | – | – | – |
| Balfour27 | ↑ | – | No | – | – | – | – |
| . | Peak VO2 . | Cardiac output (MR or echo) . | Adverse events . | Distance walked (6MWT) . | Activity levels . | Quality of life patient report . | Quality of life parent report . |
|---|---|---|---|---|---|---|---|
| Studies including children only: | |||||||
| Jacobsen17,23 | ↑ | – | No | – | = | ↓ | ↑ |
| Sutherland (home/hospital)25 | = | – | Yes | ↑ | – | ↑ | ↑ |
| Wittekind26 | ↑ | = | Yes | – | – | – | – |
| Hedlund28,39 | = | – | – | ↑ | ↑ | ↑ | ↑ |
| Laohachai30 | = | ↑ | No | – | – | – | – |
| Longmuir24 | ↑ | – | – | – | ↑ | – | – |
| Opocher19 | ↑ | – | No | – | – | – | – |
| Studies including adults only: | |||||||
| Fritz21 | = | – | Yes | – | – | – | – |
| Wu31 | = | – | No | – | – | = | – |
| Cordina32 | ↑ | ↑ | Yes | – | – | – | – |
| Lichtman20 | ↑ | – | – | – | – | ↑ | – |
| Studies including children and adults: | |||||||
| Ait Ali18 | ↑ | – | – | – | – | – | – |
| Duppen9,13–16 | = | = | – | – | = | ↑ | ↑ |
| Brassard22 | = | – | – | – | – | – | – |
| Minamisawa29 | ↑ | – | No | – | – | – | – |
| Balfour27 | ↑ | – | No | – | – | – | – |
6MWT: six-minute walk test; MR: magnetic resonance; VO2: oxygen uptake.
Symbol ↑ indicates a significant increase; = indicates no significant change; ↓ indicates significant decrease; – indicates not provided.
| . | Peak VO2 . | Cardiac output (MR or echo) . | Adverse events . | Distance walked (6MWT) . | Activity levels . | Quality of life patient report . | Quality of life parent report . |
|---|---|---|---|---|---|---|---|
| Studies including children only: | |||||||
| Jacobsen17,23 | ↑ | – | No | – | = | ↓ | ↑ |
| Sutherland (home/hospital)25 | = | – | Yes | ↑ | – | ↑ | ↑ |
| Wittekind26 | ↑ | = | Yes | – | – | – | – |
| Hedlund28,39 | = | – | – | ↑ | ↑ | ↑ | ↑ |
| Laohachai30 | = | ↑ | No | – | – | – | – |
| Longmuir24 | ↑ | – | – | – | ↑ | – | – |
| Opocher19 | ↑ | – | No | – | – | – | – |
| Studies including adults only: | |||||||
| Fritz21 | = | – | Yes | – | – | – | – |
| Wu31 | = | – | No | – | – | = | – |
| Cordina32 | ↑ | ↑ | Yes | – | – | – | – |
| Lichtman20 | ↑ | – | – | – | – | ↑ | – |
| Studies including children and adults: | |||||||
| Ait Ali18 | ↑ | – | – | – | – | – | – |
| Duppen9,13–16 | = | = | – | – | = | ↑ | ↑ |
| Brassard22 | = | – | – | – | – | – | – |
| Minamisawa29 | ↑ | – | No | – | – | – | – |
| Balfour27 | ↑ | – | No | – | – | – | – |
| . | Peak VO2 . | Cardiac output (MR or echo) . | Adverse events . | Distance walked (6MWT) . | Activity levels . | Quality of life patient report . | Quality of life parent report . |
|---|---|---|---|---|---|---|---|
| Studies including children only: | |||||||
| Jacobsen17,23 | ↑ | – | No | – | = | ↓ | ↑ |
| Sutherland (home/hospital)25 | = | – | Yes | ↑ | – | ↑ | ↑ |
| Wittekind26 | ↑ | = | Yes | – | – | – | – |
| Hedlund28,39 | = | – | – | ↑ | ↑ | ↑ | ↑ |
| Laohachai30 | = | ↑ | No | – | – | – | – |
| Longmuir24 | ↑ | – | – | – | ↑ | – | – |
| Opocher19 | ↑ | – | No | – | – | – | – |
| Studies including adults only: | |||||||
| Fritz21 | = | – | Yes | – | – | – | – |
| Wu31 | = | – | No | – | – | = | – |
| Cordina32 | ↑ | ↑ | Yes | – | – | – | – |
| Lichtman20 | ↑ | – | – | – | – | ↑ | – |
| Studies including children and adults: | |||||||
| Ait Ali18 | ↑ | – | – | – | – | – | – |
| Duppen9,13–16 | = | = | – | – | = | ↑ | ↑ |
| Brassard22 | = | – | – | – | – | – | – |
| Minamisawa29 | ↑ | – | No | – | – | – | – |
| Balfour27 | ↑ | – | No | – | – | – | – |
6MWT: six-minute walk test; MR: magnetic resonance; VO2: oxygen uptake.
Symbol ↑ indicates a significant increase; = indicates no significant change; ↓ indicates significant decrease; – indicates not provided.
Exercise capacity
The main outcome parameter was peak oxygen uptake (VO2). All studies but one performed a CPET to measure peak VO2, in one study, peak VO2 was calculated from the shuttle run test.23 The changes in peak VO2 are summarised in Figure 2. Peak VO2 increased significantly in nine out of 16 studies after training (56%), in seven studies no significant change was observed in any direction. Overall the mean increase in peak VO2 was +1.72 ml/kg/min (+6.3%). The biggest increase (+15%) was noted in the study by Ait Ali et al.18 Two studies performed the six-minute walk test (6MWT), both studies reported an significant increase in distance walked during the test.25,28
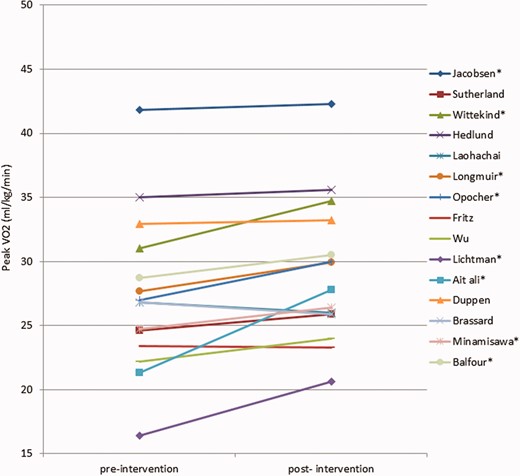
Peak oxygen uptake (VO2) changes before and after training. Cordina et al. is not shown, the study only mentioned improvement of peak VO2 in ml/min and percentage of predicted. *Significant increase.
Cardiac imaging
Four studies used cardiac imaging to assess training effects.9,26,30,32 Two studies showed no significant changes in cardiac function after training using stress echocardiography, dobutamine stress cardiac magnetic resonance imaging (CMR) and echocardiography.9,26 One study, using exercise CMR, showed a significant improvement in cardiac output of 0.3 l/min (p = 0.03) at rest but not during exercise, after 12 weeks of inspiratory muscle training.30 Cordina et al. performed an exercise CMR in trained state and following a 12-month detrain period. Patients showed a significant decrease in stroke volume at rest and at 0.75 W/kg exercise, also a significant decrease in mean aortic flow and mean IVC flow was seen after detraining.32
Adverse events
Six studies did not report on the occurrence of adverse events.9,18,20,22,24,28 Six studies reported no adverse events.19,23,27,29–31 Cordina et al. reported a patient having a transient ischaemic episode three days after his last training session.32 In two studies an adverse event occurred during the baseline CPET.25,26 One patient suffered from palpitations and premature ventricular complexes (PVCs), the other patient developed an arrhythmia.
Fritz et al. included three patients with diagnosed atrial flutter, which appeared during the training.21 In two of these patients atrial flutter could be terminated by electrical cardioversion, in one patient a cardiovascular ablation was performed resulting in a sinus arrest and an uneventful pacemaker implantation. Due to the patient's previous medical history implicating up to four cardioversions a year, these events were unlikely to have been attributable to the inspiratory muscle training.
Lung function
Five studies tested training effects on lung function.18,21,22,30,31 Three of these studies used inspiratory muscle training as exercise intervention, in one study maximal inspiratory pressure improved with 61% (p < 0.01).31 In all other studies lung function parameters remained unchanged after training.
Activity levels
The amount of time spend on physical activity was measured with an accelerometer in three studies.9,23,24 In all studies the amount of time spent on moderate-to-vigorous physical activity did not change directly after training. However, the only study with a long-term follow-up showed an increase in physical activity with 36 ± 31 min/week one year after training.24 Three studies investigated self-reported time spent on physical activity by interview, outcomes remained unchanged after training in two studies.9,23 In one study self-reported time spent on physical activity increased after training, and decreased again at the one year follow-up.28
Quality of life and psychosocial outcomes
Training effects on quality of life were measured in six studies.9,17,20,25,28,31 Four studies assessed both patient-reported and parent-reported quality of life.17,25,28 In two studies, both patients and parents reported a significantly better quality of life after training, in one study, this improvement was sustained at the one-year follow-up.25,28 Jacobsen et al. reported a decline in patient-reported quality of life at 6 months follow-up compared to baseline, whereas parents reported an increase in quality of life at 3 months and at 6 months follow-up in their child compared to baseline.17 In the study of Duppen et al., patient-reported and parent-reported quality of life improved on some quality of life domains in the child compared to controls.9 The patients in the study of Lichtman et al. reported a 70.5% improvement in quality of life after training, while patients in the study of Wu et al. reported no change.20,31
Duppen et al. investigated the effect of training on anxiety for exercise. Adolescents in the training group reported less anxiety for exercise on the anxiety thermometer after training compared to controls.9,14
Discussion
The current review assessed the various types of exercise training investigated in Fontan patients, in order to provide an overview regarding the most optimal kind of exercise training, intensity, duration or frequency. Although training programmes differed in type, duration, frequency, type of subject supervision and outcome measurements, the overall effects of training in Fontan patients were positive, including improved exercise capacity, cardiac function and quality of life.
Study population
Patients included in the studies were mostly children. Sixty-six percent of patients in the included studies had a dominant left ventricle.1 This may not be representative of the univentriculair heart population.1 Most likely this difference in incidence is due to the fact that patients with a dominant right ventricle may have worse outcomes and therefore did not meet the inclusion criteria of some studies.1 Most Fontan patients included in the studies used in this review had been operated on using the ECC technique to complete the total cavopulmonary connection (TCPC). This is in agreement with current surgical preferences in many centres.33
Exercise capacity
Peak VO2 increased significantly in approximately 60% of the studies. The overall mean increase in peak VO2 was +1.73 ml/kg/min (+6.3%). The largest increase in peak VO2 of 6.5 ml/kg/min was found in the study of Ait Ali et al. (+15%), investigating the effects of respiratory training.18 The high increase in peak VO2 can most likely also be explained by the fact that the patients started at a very low baseline peak VO2 before training compared to other studies, and therefore could increase the most. This is supported by the study of Lichtman et al. in which patients also had the lowest baseline peak VO2 and showed the second best increase in peak VO2.20
Most studies investigated the effects of a combination of resistance exercises and aerobic training. Fifty per cent of these studies found a significant increase in peak VO2.
Five studies used aerobic training, 60% of them found a significant increase in peak VO2. However, the randomised controlled trial included in this review did not show any improvement in exercise capacity or in cardiac function after aerobic training compared to controls.9 The lack of improvement in these Fontan patients can be explained by the high baseline peak VO2 (33 ml/kg/min). In the same study dobutamine stress magnetic resonance was used which demonstrated a lack of increase in cardiac output after the training program. This suggests that aerobic training might fail to increase the pulmonary blood flow, leading to unchanged cardiac output and peak VO2.
A way to increase pulmonary blood flow and thus preload during exercise in Fontan patients is by contraction of the leg muscles.34 Cordina et al. was the only group reporting the effects of high-weight resistance training focusing on the lower limbs, and showed promising results.32 After training, participants had more calf muscle mass and an increased exercise capacity. In addition, this study was the only study showing an increase in cardiac output during rest and during exercise compared to controls. These results are in accordance with the findings of Smith et al. as they showed a positive association between strength and exercise capacity.35 Despite the promising results, it is important to note that the study of Cordina et al. included only six patients.
Three more recent studies investigated the effects of inspiratory muscle training, in pulmonary blood flow and thereby preload.30,31 Inspiratory muscle training did not show any improvement in peak VO2. However, the study of Laohachai et al. did show that patients had an increased cardiac output during rest after inspiratory muscle training, but not during exercise.30 This corresponds to the findings of Hjortdal et al., as they showed that pulmonary blood flow in Fontan patients is highly dependent on inspiration during rest, but less during exercise.34
In total, seven studies included children only, peak VO2 increased in four of these studies. Four studies included adults only, and peak VO2 increased in two of these studies. However, the two studies in which exercise capacity did not increase, were both inspiratory muscle training studies. Inspiratory muscle training did not improve exercise capacity in children either. All other studies included a combination of children and adults, and in 42% of these studies peak VO2 increased significantly. Due to the small number of studies in each age category, mixed cohorts and the incomparable nature of some training programmes, drawing firm conclusions about potential age-related outcome differences was not possible.
Training duration, supervision and intensity
There seems to be a consensus about training duration as most training programmes lasted 8–12 weeks (63%). Instructor-led training sessions were most common. The percentage of studies that reached a significant increase in peak VO2 outcomes was higher for the supervised training groups (75%) versus the unsupervised groups (38%). Two studies compared home and hospital-based (instructor-led) training.22,25 Remarkably, no important outcome differences were found between the home and hospital-trained groups. However, patient numbers were small which limited the chances of finding a possible difference between the groups. Training intensity was mostly based on heart rate (HR) at peak VO2 or maximal inspiratory pressure.
Physical activity and quality of life
An important outcome measure could be time spent on physical activity. Physical activity levels are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health.6 Only one out of three studies that measured time spent on physical activity showed an increase in physical activity at 2 years follow-up. Unfortunately this study did not have controls, which makes it uncertain whether the increase in physical activity was due to the training programme.
Quality of life was measured in six studies.9,17,20,25,28,31 Overall, training improved patient-reported and parent-reported quality of life. The quality of life outcomes of some studies depended on the type of informant used. One study found that parents reported improvements in quality of life in their child after training, whereas the child reported a decrease.23 Another study found improvements on different domains of quality of life as reported by the parent versus the child.9 This disagreement is called the proxy problem, however both child reports and parent reports are considered complementary sources of information.36 One study found that adolescents in the training group reported less anxiety regarding exercise on the anxiety thermometer after training compared with controls.9,14
Safety
All studies concluded that exercise training is safe in children and adults with a Fontan circulation. Three adverse events occurred during the exercise intervention, probably unrelated to the training programme. However, it is important to note that most studies only included the less severely affected Fontan patients as their exclusion criteria were; frequent symptomatic arrhythmias, heart failure, exercise restrictions and very low exercise capacities. Therefore we cannot draw any firm conclusions whether training is safe for more severely affected Fontan patients.
Limitations of included studies
Several limitations of our review and the studies included have to be discussed. Selection bias is likely because unpublished data and abstracts were not included in this research. None of the studies reported negative results, reflecting potential publishing bias. The sample sizes of some studies was very small. In addition, Fontan patients were used as controls in four studies only, raising the question whether an increase in exercise capacity or 6MWT distance in patients without comparing to controls reflects a learning effect. Five studies included cohorts that were not uniform; including both adults and children. In addition, some patient cohorts were not described in enough detail, as information regarding ventricle type, Fontan type and whether patients had an open fenestration was not always present. Righini et al. showed the influence of pulmonary hypertension (PH) in CHD patients on exercise capacity, pulmonary pressure and pulmonary flow. Information about PH in our patient population is missing.37 The Fontan patients included in the studies may not have been representative of the complete Fontan patient population, particularly since patients with worse physical condition were excluded from training programmes. In addition, it is likely that patients interested in sports are more willing to participate in training programmes, which may be another reason that inclusion bias towards fitter patients might be present. Many training programmes were not described in detail, or training programmes were instructor-dependent, which makes external validation, repeatability and reproducibility, difficult. Some important predictive outcomes from the CPET, as OEUS, were not given in most studies.38 The drop-out rate was 15%, most studies did not describe the exact reasons for patients who discontinued participating in the training programme, possibly these patients experienced negative effects of the training. Another important question that remains unanswered is what the effect of training is on the long-term. Unfortunately, none of the studies had a follow-up later than 1 year after training.
Future
Currently the only known non-invasive way to improve exercise capacity in Fontan patients is training. More controlled research is needed to determine the added value of the different training types, and to design a standardised training programme. Based on the reviewed studies, resistance training focusing on the lower legs seems to be most promising.
Most studies included mixed cohorts of children and adults, or children only. It will be of great interest if future cohorts are as uniform as possible and also if there is a focus on training effects in adult patients.
At this time safety of training and training effects in more severely affected Fontan patients remain unknown. Future research should include more severely affected Fontan patients, as these patients might benefit the most from exercise training. Long-term effects of training on exercise capacity remain unknown, and future research should add long-term follow-up to investigate training effects.
Conclusion
In conclusion, exercise training seems safe and has positive effects on exercise capacity, cardiac function and quality of life in patients with a Fontan circulation. Considering these observations, exercise training in Fontan patients should be encouraged. Despite major differences in training programmes, a 2-weekly supervised training session consisting of a combination of aerobic and resistance training is a safe and effective way to increase peak VO2 and quality of life. Further studies are required to assess the optimal training type, intensity, duration and long-term effects to be recommended as standard of care in Fontan patients to increase exercise capacity, physical activity and, thereby, quality of life and potential life expectancy.
Supplemental Material
Supplementary material is available at European Journal of Preventive Cardiology online.
Author contribution
LES had the main role in research protocol design. LES and GI did the literature search, performed title and abstract screening and data extraction. LES drafted the manuscript. LES, LEMvB, KD and WAH contributed to interpretation. All authors (LEMvB, LES, KD, GI, JJMT and WAH) critically revised the manuscript and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: LES was supported by a grant from the non-profit organization Vrienden van Sophia. The funding source had no role in the collection, analysis and interpretation of the data, writing of the report or in the decision to submit the article for publication.
Acknowledgements
The authors wish to thank Wichor Bramer (Erasmus University Medical Center) for his assistance with the literature search.




Comments