-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis D Laoutaris, The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’, European Journal of Preventive Cardiology, Volume 25, Issue 12, 1 August 2018, Pages 1257–1262, https://doi.org/10.1177/2047487318776097
Close - Share Icon Share
Abstract
Evidence from large multicentre exercise intervention trials in heart failure patients, investigating both moderate continuous aerobic training and high intensity interval training, indicates that the ‘crème de la crème’ exercise programme for this population remains to be found. The ‘aerobic/resistance/inspiratory (ARIS) muscle training hypothesis in heart failure’ is introduced, suggesting that combined ARIS muscle training may result in maximal exercise pathophysiological and functional benefits in heart failure patients. The hypothesis is based on the decoding of the ‘skeletal muscle hypothesis in heart failure’ and on revision of experimental evidence to date showing that exercise and functional intolerance in heart failure patients are associated not only with reduced muscle endurance, indication for aerobic training (AT), but also with reduced muscle strength and decreased inspiratory muscle function contributing to weakness, dyspnoea, fatigue and low aerobic capacity, forming the grounds for the addition of both resistance training (RT) and inspiratory muscle training (IMT) to AT. The hypothesis will be tested by comparing all potential exercise combinations, ARIS, AT/RT, AT/IMT, AT, evaluating both functional and cardiac indices in a large sample of heart failure patients of New York Heart Association class II–III and left ventricular ejection fraction ≤35% ad hoc by the multicentre randomized clinical trial, Aerobic Resistance, InSpiratory Training OutcomeS in Heart Failure (ARISTOS-HF trial).
Introduction
The European Society of Cardiology firmly recommends regular aerobic training (AT) in patients with heart failure to improve functional capacity and symptoms (class I indication, level of evidence A).1 The benefits of AT entail both central and peripheral adaptations which are clinically translated into improvement of endothelial function,2 neuro-hormonal profile,3,4 exercise capacity and quality of life (QoL),5 anti-remodelling5 and anti-inflammatory effects6 as well as reduced morbidity and mortality.7 However, the largest exercise intervention trial to date, the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), in 2331 patients with left ventricular ejection fraction (LVEF) ≤ 35% and New York Heart Association (NYHA) functional class II to IV (mostly II–III), demonstrated that moderate continuous AT (MCAT) resulted in a statistically significant modest improvement of self-reported health status and exercise capacity compared with usual care.8,9 Subgroup analysis did not show a significant change in plasma biomarkers, although in patients for whom N-terminal pro-brain natriuretic peptide (NT-proBNP) levels decreased after training, there was a concomitant increase in peak oxygen consumption (peakVO2).10 A modest significant reduction of both all-cause mortality or hospitalization and cardiovascular mortality or heart failure hospitalization was found only after adjusting for highly prognostic baseline characteristics.9 Thus, improvement of the outcomes tested was considered to be lower than anticipated and was partially attributed to the low adherence to training.9
Consequently, an on-going discussion on how to further improve exercise efficiency started. Lines of evidence demonstrated that high intensity interval training (HIIT) was more effective in reversing left ventricular remodelling, improving cardiac output, endothelial function, peakVO2 and QoL compared with MCAT, while the improvement in aerobic capacity was mainly attributed to a cardiac rather than a peripheral effect.11,12 Thus, the multicentre randomized Study of Myocardial Recovery After Exercise Training in Heart Failure (SMARTEX-HF) suggested that HIIT may be superior to MCAT in improving left ventricular dimensions and aerobic capacity in patients with heart failure. Nevertheless, both exercise training programmes showed similar and smaller benefits than expected, while no significant benefit in QoL was shown for either of the programmes in 261 patients with LVEF ≤35% and NYHA II–III.13 These findings were confirmed by a recent study which also showed that a HIIT benefit in submaximal exercise capacity was due to a peripheral rather than a central contribution.14,15
Thus, raised expectations were lowered and the idea of an ‘optimum’ exercise programme for heart failure patients started to fade away, moving towards the concept of tailoring an exercise programme according to patient specificity.16,17 The European Association of Preventive Cardiology Exercise Prescription in Everyday Practice and Rehabilitative Training (EXPERT) tool has been recently introduced, representing a digital training and decision support system, using algorithms including a number of variables, to enable an optimized exercise prescription for each individual.17 Although application of such a tool requires a notorious amount of evidence, the design is both rational and promising, awaiting its evaluation in clinical practice. Meanwhile, there could still be remaining options for a single, effective exercise programme for heart failure patients which could facilitate the widespread implementation of cardiac rehabilitation exercise training programmes. The present revision faces the challenge as to whether such an exercise programme is still possible.
Where, whither, whence: skeletal muscle dysfunction and training in heart failure
Decoding the ‘skeletal muscle hypothesis in heart failure’
To solve the equation, we may need to look back in time, at the foundations of exercise training in heart failure, established by the ‘skeletal muscle hypothesis in heart failure’.18 The revised schema of this hypothesis by Piepoli and Coats19 resembles ‘a fascinating painting’ waiting for ‘hidden codes’ to be broken. It clearly states that reduced cardiac output/tissue hypoxia, inflammation, systemic catabolism and immobilization induce skeletal muscle metabolic, structural, autonomic and functional changes. These include protein degradation,20 increased level of inflammatory cytokines,21,22 a muscle fibre type shift from slow to fast twitch, reduced number of mitochondria, impaired oxidative metabolism and early acidosis, resulting in reduced muscle endurance, increased activatory reflexes (chemoreflex and ergoreflex) and sympathetic drive as well as over-ventilation, contributing to fatigue, low aerobic capacity and dyspnoea.23–25 These skeletal muscle derangements form the bedrock and emphasize the above-all evidenced AT in heart failure.
But, there maybe more to it. The hypothesis also refers to peripheral muscle fibre atrophy and reduced muscle bulk, contributing to a well-documented reduced muscle strength26 associated with impaired daily activity requiring strength and formulating the pathophysiological and functional basis for the application of strength or resistance training (RT). A potential respiratory muscle myopathy despite its constant activation was also suggested as part of the hypothesis. It is now known that although the increased ventilatory demands result in a chronic increase in diaphragmatic load and an opposite diaphragmatic muscle fibre type shift from fast to slow twitch, systemic myopathy associated with protein degradation, over-expression of tumour necrosis factor-alpha, altered intracellular calcium regulation, fibre atrophy and impaired mitochondrial and oxidative enzyme capacity27,28 result in a decrease in both inspiratory muscle strength (PImax) and endurance, associated with increased dyspnoea.29,30 Inspiratory muscle work capacity (SustainedPImax), an inspiratory endurance index, was found to be depressed even in patients with near normal PImax,30 while unloading respiratory muscle work with a ventilator during exercise resulted in an increase in limb blood flow.31 An increased diaphragmatic metaboreflex activity associated with respiratory muscle fatigue has been proposed in patients with heart failure, resulting in increased sympathetic drive and limb vasoconstriction and contributing to peripheral fatigue and reduced exercise capacity. Thus, molecular, cellular and functional impairments in the heart failure diaphragm formulate the basis for inspiratory muscle training (IMT).
Selective and additional benefits of RT and IMT in patients with heart failure
It appears that not only low aerobic capacity, but both reduced peripheral muscle strength and inspiratory muscle function are associated with exercise intolerance, disease severity and poor prognosis in the heart failure population,32,33 while an increasing number of studies confirm the benefits of RT and IMT in patients with heart failure.
Low to moderate intensity RT, as a selective training modality34 or as an adjunct to AT,35 was shown to be safe without evidence of adverse remodelling in patients with heart failure. Selective RT was capable of increasing muscle strength and walking distance,36 limb blood flow,37 mitochondrial ATP production rate,38 peakVO2 and daily life activities requiring force, improving QoL.36–40 Combined AT/RT resulted in an additional improvement in peripheral muscle strength, submaximal exercise capacity and QoL compared with AT.40–45 Although an additional benefit of combined AT/RT in peakVO2 compared with AT alone has not been confirmed,40 there are findings to show additional benefits of RT in flow-mediated vasodilation46 and ventilatory and metabolic efficiency.47
IMT was also shown to be safe and appears to be mostly effective if used at high training intensities targeting both PImax and SustainedPImax30,48–50 or at lower intensities in selected patients with inspiratory muscle weakness.51 Selective IMT improved inspiratory muscle function and exertional dyspnoea,48–50,52–54 limb blood flow55,56 and soluble tumour necrosis factor receptor I,49 resulting in an improvement in the submaximal exercise capacity, peakVO2 and QoL of heart failure patients.48–50,57,58 More recently, IMT improved intercostal and forearm muscle oxygen saturation during respiratory fatigue in patients with heart failure, providing evidence for a potential IMT induced attenuation of respiratory muscle metaboreflex activity, presenting as a possible mechanism besides reduction of dyspnoea, for the increase in peakVO2 observed after IMT.59 Combined AT/IMT resulted in additional benefits in inspiratory muscle function, dyspnoea, peakVO2, QoL, NT-proBNP and C-reactive protein levels, over AT.60,61 Furthermore, AT/RT combined with non-invasive ventilatory support was shown to further improve dyspnoea and QoL compared with AT/RT.62
Thus, combined training programmes using AT, RT and IMT appear to gain increased recognition with RT as the prominent intervention to reverse or attenuate the loss of muscle mass and improve muscle strength and IMT to improve respiratory muscle function and dyspnoea, while both RT and IMT offer concomitant benefits, through different mechanisms, in exercise and functional capacity and QoL in patients with heart failure. The combination of AT with other training modalities such as functional electrical stimulation (FES) did not show additional benefits in exercise capacity compared with AT alone,63 although FES could be more advantageous for less active patients with severe heart failure.64 Ultimately, findings from our lab showed for the first time that a triple exercise programme of combined moderate continuous AT with low to moderate RT and with high intensity IMT (Aerobic/Resistance/InSpiratory: ARIS) was safe, produced positive changes in left ventricular structure and function and resulted in additional benefits in both peripheral and diaphragmatic muscle function, dyspnoea, cardiopulmonary exercise parameters and QoL compared with AT alone.65
Thus, decoding the resilient ‘skeletal muscle hypothesis in heart failure’, together with revision of the experimental evidence to date, formulates the basis for the conception and introduction of the ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’.
The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’
The ‘ARIS muscle training hypothesis in heart failure’ states that exercise and functional intolerance in patients with heart failure are associated not only with reduced muscle endurance but also with reduced muscle strength and decreased inspiratory muscle function, contributing to weakness, dyspnoea, fatigue and low aerobic capacity, and that combined aerobic/resistance/inspiratory muscle training may result in maximal exercise pathophysiological and functional benefits in heart failure patients (Figure 1). The hypothesis will be tested by comparing all potential exercise combinations such as ARIS, AT/RT, AT/IMT, and AT, evaluating both functional and cardiac indices in a large sample of heart failure patients NYHA II–III, LVEF ≤35% ad hoc by the multicentre randomized clinical trial Aerobic Resistance, InSpiratory Training OutcomeS in Heart Failure (ARISTOS-HF trial (‘optimum’ = ‘άριστος’, Greek)) in order to provide the evidence for the ‘crème de la crème’ exercise programme for heart failure patients.66
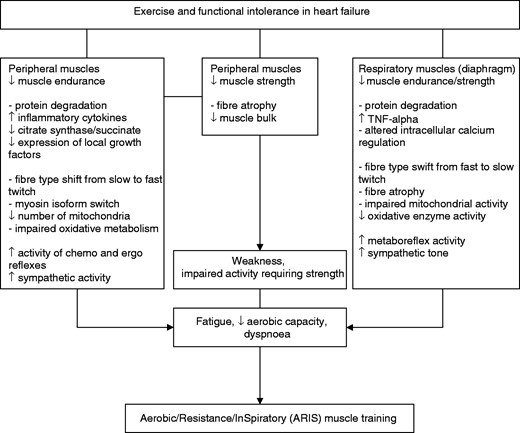
The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’: exercise and functional intolerance are associated not only with reduced muscle endurance, but also with reduced muscle strength and decreased inspiratory muscle function, contributing to weakness, dyspnoea, fatigue and low aerobic capacity. Combined aerobic/resistance/inspiratory muscle training may result in maximal exercise pathophysiological and functional benefits in heart failure patients.
TNF: tumour necrosis factor
Declaration of conflicting interests
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.




Comments