-
PDF
- Split View
-
Views
-
Cite
Cite
Jelena Pavlović, Philip Greenland, Jaap W Deckers, Maryam Kavousi, Albert Hofman, M Arfan Ikram, Oscar H Franco, Maarten JG Leening, Assessing gaps in cholesterol treatment guidelines for primary prevention of cardiovascular disease based on available randomised clinical trial evidence: The Rotterdam Study, European Journal of Preventive Cardiology, Volume 25, Issue 4, 1 March 2018, Pages 420–431, https://doi.org/10.1177/2047487317743352
Close - Share Icon Share
Abstract
The purpose of this study was to determine how American College of Cardiology/American Heart Association (ACC/AHA) 2013 and European Society of Cardiology 2016 guidelines for the primary prevention of atherosclerotic cardiovascular disease (CVD) compare in reflecting the totality of accrued randomised clinical trial evidence for statin treatment at population level.
From 1997–2008, 7279 participants aged 45–75 years, free of atherosclerotic cardiovascular disease, from the population-based Rotterdam Study were included. For each participant, we compared eligibility for each one of 11 randomised clinical trials on statin use in primary prevention of CVD, with recommendations on lipid-lowering therapy from the ACC/AHA and European Society of Cardiology (ESC) guidelines. Atherosclerotic cardiovascular disease incidence and cardiovascular disease mortality rates were calculated.
The proportion of participants eligible for each trial ranged from 0.4% for ALLHAT-LLT to 30.8% for MEGA. The likelihood of being recommended for lipid-lowering treatment was lowest for those eligible for low-to-intermediate risk RCTs (HOPE-3, MEGA, and JUPITER), and highest for high-risk individuals with diabetes (MRC/BHF HPS, CARDS, and ASPEN) or elderly PROSPER. Eligibility for an increasing number of randomised clinical trials correlated with a greater likelihood of being recommended lipid-lowering treatment by either guideline (p < 0.001 for both guidelines).
Compared to RCTs done in high risk populations, randomised clinical trials targeting low-to-intermediate risk populations are less well-reflected in the ACC/AHA, and even less so in the ESC guideline recommendations. Importantly, the low-to-intermediate risk population targeted by HOPE-3, the most recent randomised clinical trial in this field, is not well-captured by the current European prevention guidelines and should be specifically considered in future iterations of the guidelines.
Introduction
Clinical practice guidelines represent recommendations intended to facilitate evidence-based clinical decision-making. The foundations of treatment recommendations should be evidence, most importantly coming from double-blind placebo-controlled randomised clinical trials (RCTs). In comparison to other medical fields, cardiology practice guidelines are considered most likely to be derived from the evidence coming from RCTs.1
Guideline recommendations on statin use in primary prevention of atherosclerotic cardiovascular disease (CVD) are derived from a large number of RCTs conducted in the past 25 years (Table 1).2,3 However, extrapolating recommendations on statin use in the prevention of atherosclerotic CVD within the boundaries of available RCT data has its limits, as it is near impossible to conduct RCTs in unselected populations or to expect that multiple RCTs done in selected risk groups would ensure that everyone is represented.
| Randomised clinical trial . | Sample size, n . | Age range, years . | Major entry criteriaa . | |
|---|---|---|---|---|
| Cholesterol, mg/dl . | Other . | |||
| Positive trials | ||||
| WOSCOPS, 1995 | 6595b | 45–64 | LDL 155–230 | Men only |
| AFCAPS/TexCAPS, 1998 | 6605 | 45–73 | Total 180–264; | Men ≥45 y, postmenopausal women ≥55 y |
| LDL 130–190; | ||||
| HDL ≤45 | ||||
| PROSPER, 2002 | 3239c | 70–82 | Total 155–350 | ≥1 additional risk factor |
| ASCOT-LLA, 2003 | 10,305b | 40–79 | Total ≤250 | Treated SBP >140 mm Hg, DBP >90 mm Hg; ≥3 additional risk factors |
| MRC/BHF HPS, 2003 | 2912c | 40–80 | Total ≥135 | DM type 2 |
| CARDS, 2004 | 2838 | 40–75 | LDL ≤160 | DM type 2, ≥1 additional risk factor |
| MEGA, 2006 | 7832 | 40–70 | Total 200–270 | Men and postmenopausal women |
| JUPITER, 2008 | 17,802 | 50–97 | LDL <130 | Men ≥50 y, women ≥60 y; CRP ≥2 mg/l |
| HOPE-3, 2016 | 12,705 | ≥55 | – | Men ≥55 y, women ≥65 y, ≥1 additional risk factor |
| Neutral trialsd | ||||
| ALLHAT-LLT, 2002 | 10,355b | 51–81 | LDL 120–189 | Treated SBP>160 mm Hg, DBP>100 mm Hg; ≥1 additional risk factor |
| ASPEN, 2006 | 1905c | 40–75 | LDL ≤160 | DM type 2 |
| Randomised clinical trial . | Sample size, n . | Age range, years . | Major entry criteriaa . | |
|---|---|---|---|---|
| Cholesterol, mg/dl . | Other . | |||
| Positive trials | ||||
| WOSCOPS, 1995 | 6595b | 45–64 | LDL 155–230 | Men only |
| AFCAPS/TexCAPS, 1998 | 6605 | 45–73 | Total 180–264; | Men ≥45 y, postmenopausal women ≥55 y |
| LDL 130–190; | ||||
| HDL ≤45 | ||||
| PROSPER, 2002 | 3239c | 70–82 | Total 155–350 | ≥1 additional risk factor |
| ASCOT-LLA, 2003 | 10,305b | 40–79 | Total ≤250 | Treated SBP >140 mm Hg, DBP >90 mm Hg; ≥3 additional risk factors |
| MRC/BHF HPS, 2003 | 2912c | 40–80 | Total ≥135 | DM type 2 |
| CARDS, 2004 | 2838 | 40–75 | LDL ≤160 | DM type 2, ≥1 additional risk factor |
| MEGA, 2006 | 7832 | 40–70 | Total 200–270 | Men and postmenopausal women |
| JUPITER, 2008 | 17,802 | 50–97 | LDL <130 | Men ≥50 y, women ≥60 y; CRP ≥2 mg/l |
| HOPE-3, 2016 | 12,705 | ≥55 | – | Men ≥55 y, women ≥65 y, ≥1 additional risk factor |
| Neutral trialsd | ||||
| ALLHAT-LLT, 2002 | 10,355b | 51–81 | LDL 120–189 | Treated SBP>160 mm Hg, DBP>100 mm Hg; ≥1 additional risk factor |
| ASPEN, 2006 | 1905c | 40–75 | LDL ≤160 | DM type 2 |
ALLHAT-LLT: Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT-LLA: Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial: The Lipid Lowering Arm; ASPEN: Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS: Collaborative Atorvastatin Diabetes Study; CI: confidence interval; CRP: C-reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; ESC: European Society of Cardiology; HDL: high-density lipoprotein; HOPE-3: Heart Outcomes Prevention Evaluation 3; JUPITER: Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL: low-density lipoprotein; MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; MRC/BHF HPS: Heart Protection Study of Cholesterol Lowering with Simvastatin; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; SBP: systolic blood pressure; WOSCOPS: West of Scotland Coronary Prevention Study.
Major criteria presented are simplified for presentation purposes. Details regarding all inclusion and exclusion criteria have been described previously 11 and are summarised in Table 1 in the Supplementary Material.
Up to 15% of the participants had a history of cardiovascular disease at baseline; no data presented on subgroup of participants free from cardiovascular disease.
Data from persons free from cardiovascular disease at baseline.
ALLHAT-LLT and ASPEN trials are denoted as neutral, as no statistically significant effect of statin use on clinical cardiovascular end points was reported.
| Randomised clinical trial . | Sample size, n . | Age range, years . | Major entry criteriaa . | |
|---|---|---|---|---|
| Cholesterol, mg/dl . | Other . | |||
| Positive trials | ||||
| WOSCOPS, 1995 | 6595b | 45–64 | LDL 155–230 | Men only |
| AFCAPS/TexCAPS, 1998 | 6605 | 45–73 | Total 180–264; | Men ≥45 y, postmenopausal women ≥55 y |
| LDL 130–190; | ||||
| HDL ≤45 | ||||
| PROSPER, 2002 | 3239c | 70–82 | Total 155–350 | ≥1 additional risk factor |
| ASCOT-LLA, 2003 | 10,305b | 40–79 | Total ≤250 | Treated SBP >140 mm Hg, DBP >90 mm Hg; ≥3 additional risk factors |
| MRC/BHF HPS, 2003 | 2912c | 40–80 | Total ≥135 | DM type 2 |
| CARDS, 2004 | 2838 | 40–75 | LDL ≤160 | DM type 2, ≥1 additional risk factor |
| MEGA, 2006 | 7832 | 40–70 | Total 200–270 | Men and postmenopausal women |
| JUPITER, 2008 | 17,802 | 50–97 | LDL <130 | Men ≥50 y, women ≥60 y; CRP ≥2 mg/l |
| HOPE-3, 2016 | 12,705 | ≥55 | – | Men ≥55 y, women ≥65 y, ≥1 additional risk factor |
| Neutral trialsd | ||||
| ALLHAT-LLT, 2002 | 10,355b | 51–81 | LDL 120–189 | Treated SBP>160 mm Hg, DBP>100 mm Hg; ≥1 additional risk factor |
| ASPEN, 2006 | 1905c | 40–75 | LDL ≤160 | DM type 2 |
| Randomised clinical trial . | Sample size, n . | Age range, years . | Major entry criteriaa . | |
|---|---|---|---|---|
| Cholesterol, mg/dl . | Other . | |||
| Positive trials | ||||
| WOSCOPS, 1995 | 6595b | 45–64 | LDL 155–230 | Men only |
| AFCAPS/TexCAPS, 1998 | 6605 | 45–73 | Total 180–264; | Men ≥45 y, postmenopausal women ≥55 y |
| LDL 130–190; | ||||
| HDL ≤45 | ||||
| PROSPER, 2002 | 3239c | 70–82 | Total 155–350 | ≥1 additional risk factor |
| ASCOT-LLA, 2003 | 10,305b | 40–79 | Total ≤250 | Treated SBP >140 mm Hg, DBP >90 mm Hg; ≥3 additional risk factors |
| MRC/BHF HPS, 2003 | 2912c | 40–80 | Total ≥135 | DM type 2 |
| CARDS, 2004 | 2838 | 40–75 | LDL ≤160 | DM type 2, ≥1 additional risk factor |
| MEGA, 2006 | 7832 | 40–70 | Total 200–270 | Men and postmenopausal women |
| JUPITER, 2008 | 17,802 | 50–97 | LDL <130 | Men ≥50 y, women ≥60 y; CRP ≥2 mg/l |
| HOPE-3, 2016 | 12,705 | ≥55 | – | Men ≥55 y, women ≥65 y, ≥1 additional risk factor |
| Neutral trialsd | ||||
| ALLHAT-LLT, 2002 | 10,355b | 51–81 | LDL 120–189 | Treated SBP>160 mm Hg, DBP>100 mm Hg; ≥1 additional risk factor |
| ASPEN, 2006 | 1905c | 40–75 | LDL ≤160 | DM type 2 |
ALLHAT-LLT: Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT-LLA: Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial: The Lipid Lowering Arm; ASPEN: Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS: Collaborative Atorvastatin Diabetes Study; CI: confidence interval; CRP: C-reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; ESC: European Society of Cardiology; HDL: high-density lipoprotein; HOPE-3: Heart Outcomes Prevention Evaluation 3; JUPITER: Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL: low-density lipoprotein; MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; MRC/BHF HPS: Heart Protection Study of Cholesterol Lowering with Simvastatin; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; SBP: systolic blood pressure; WOSCOPS: West of Scotland Coronary Prevention Study.
Major criteria presented are simplified for presentation purposes. Details regarding all inclusion and exclusion criteria have been described previously 11 and are summarised in Table 1 in the Supplementary Material.
Up to 15% of the participants had a history of cardiovascular disease at baseline; no data presented on subgroup of participants free from cardiovascular disease.
Data from persons free from cardiovascular disease at baseline.
ALLHAT-LLT and ASPEN trials are denoted as neutral, as no statistically significant effect of statin use on clinical cardiovascular end points was reported.
Two prevailing evidence-based guidelines for cholesterol-lowering recommendations in adults without established atherosclerotic CVD are formulated by the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC).4,5 Our study is the first to investigate to what extent these guidelines differ at population level in reflecting the total accrued RCT evidence for statin treatment in primary prevention of atherosclerotic CVD. We sought to assess whether the available RCT evidence on statins is fully incorporated in the ACC/AHA 2013 and ESC 2016 guideline recommendations.
Methods
Study population and setting
The Rotterdam Study is a prospective population-based cohort study, established in 1990 in the city of Rotterdam, the Netherlands. Up until 2008, a total of 14,926 participants, aged ≥45 years, were recruited in three phases. The participants were extensively examined at baseline and every 3–4 years. The Rotterdam Study rationale and design have been described in detail previously.6
Participants included in the analysis visited the research centre for the third examination of the original cohort (RS-I-3; aged ≥61 years; 1997–1999), the baseline examination of the second cohort (RS-II-1; aged ≥55 years; 2000–2001), and the baseline examination of the third cohort (RS-III-1; aged ≥45 years; 2006–2008). In these three visits combined, 10,522 participants were examined. For the purpose of investigating primary prevention strategies, we excluded participants with a history of atherosclerotic CVD, defined as any of the following: myocardial infarction, coronary or other arterial revascularization procedure, stroke, transient ischaemic attack, or repeated prescription of nitrates (as a proxy for individuals with angina pectoris).7–10 Next, we excluded participants older than 75 years of age, since current prevention guidelines do not provide clear guidance in the elderly. We thus included a total of 7279 participants aged 45–75 years.
The Rotterdam Study complies with the Declaration of Helsinki and has been approved by the medical ethics committee according to the Wet Bevolkingsonderzoek: ERGO (Population Screening Act: Rotterdam Study), executed by the Ministry of Health, Welfare and Sports of the Netherlands. All participants provided written informed consent for their participation in the study and to obtain information from their treating physicians.
Outcomes
The main outcomes were: (a) atherosclerotic CVD incidence rate, composed of fatal and non-fatal myocardial infarction, coronary revascularization, coronary heart disease mortality, and non-haemorrhagic stroke; and (b) CVD mortality rate. Details regarding the follow-up and adjudication of events have been described in detail previously.7,9 Events were adjudicated until 1 January 2012.
Co-variables
Assessment of cardiovascular risk factors, medication use, lifestyle factors and other co-variables measured at the research centre or during the home interview, have been described in detail previously.11
Trial selection and trial eligibility
To be considered for this study, RCTs were required to describe the effect of statin use compared to placebo on clinical atherosclerotic CVD incidence or all-cause mortality using a double-blind placebo-controlled design in a population, or substantial subset of the population, free of atherosclerotic CVD. We identified 11 primary prevention RCTs in total, 10 previously described in the meta-analysis by Brugts et al.,2 plus the recently published Heart Outcomes Prevention Evaluation 3 (HOPE-3) trial that was not considered by the current ACC/AHA and ESC guidelines (Table 1).3
Every participant was checked for trial eligibility for each of the 11 RCTs separately by utilising all published inclusion and exclusion criteria. These criteria varied among trials due to differences in their design and hypothesis (Table 1 in Supplementary Material).11 Some of the major inclusion criteria were age, sex, serum levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, C-reactive protein (CRP), history of diabetes mellitus, blood pressure levels, and postmenopausal status for women (Table 1).
Guideline recommendations
First, for every participant, we calculated the estimated 10-year risk of hard atherosclerotic CVD using the recommended sex-specific Pooled Cohort equations (PCE) for white individuals as published12 and 10-year risk of fatal CVD using the sex-specific Systematic COronary Risk Evaluation (SCORE) equations for low-risk countries as published.13
In this study, positive treatment recommendations were defined based on the definite treatment recommendations for both ACC/AHA and ESC guidelines. For the main analyses, we did not incorporate recommendations on consider treatment. In additional sensitivity analyses we combined definite treatment with consider treatment recommendations into a single overall positive treatment recommendation.
A positive definite recommendation for lipid-lowering treatment as advocated by the ACC/AHA 2013 guidelines was defined as either having: a 10-year hard atherosclerotic CVD risk ≥7.5% and an LDL cholesterol level ≥70 mg/dl; or an LDL cholesterol level ≥190 mg/dl; or diabetes mellitus and an LDL cholesterol level ≥70 mg/dl.14
A positive definite recommendation for lipid-lowering treatment as advocated by the ESC 2016 guidelines was defined as either having: an LDL cholesterol level ≥100 mg/dl (≥2.5 mmol/l) combined with a 10-year CVD mortality risk of 5–10% or a high-risk equivalent (diabetes mellitus, total cholesterol >310 mg/dl (>8.0 mmol/l), systolic blood pressure/diastolic blood pressure ≥180/110 mm Hg, or estimated glomerular filtration rate 30–60 ml/min/1.73 m2); or an LDL cholesterol level ≥70 mg/dl (≥1.8 mmol/l) combined with a 10-year CVD mortality risk ≥10% or estimated glomerular filtration rate <30 ml/min/1.73 m2.4
Statistical analysis
We determined the proportion of the study population that would have been eligible for each of the 11 RCTs and the overlap in eligibility between these trials. Observed atherosclerotic CVD incidence rates and CVD mortality rates were determined for individuals eligible for each of the 11 trials based on up to 10 years of follow-up. Next, for each of the 11 trial eligible subsets of the population, we determined the proportion that would be recommended to initiate lipid-lowering treatment following the ACC/AHA 2013 and ESC 2016 guidelines. In addition, we determined the probability of being recommended for lipid-lowering treatment by the ACC/AHA 2013 and ESC 2016 guidelines, in relation to the number of trials an individual would be eligible for. The value of p-for-trend on this association was estimated using linear regression weighted for the number of atherosclerotic CVD events during follow-up.
Cardiovascular events are deemed to have a more pronounced impact in younger and middle-aged individuals. We therefore looked into participants aged 46–65 years who died from CVD during follow-up in relation to their estimated risk cardiovascular risk. Within these individuals we compared the probabilities of being recommended lipid-lowering therapy based on the guidelines and the probability of being eligible for one or more RCTs on statin therapy.
Lastly, in order to study the relation between the amount of evidence and likelihood of positive guideline recommendations, we created categories based on the total number of RCTs that a participant was eligible for (0, 1, 2, 3 or ≥4 RCTs). We then determined the probability of having a definite ACC/AHA or ESC guideline recommendation on lipid-lowering therapy. This was done in the overall study population and within clinically relevant subgroups.
Missing values were present for up to 4.7% of traditional cardiovascular risk factors and for up to 7.1% of other co-variables, and were handled by single imputation using expectation-maximization algorithm.15
Out of 7279 participants included in the analysis, we had follow-up data available for 7251 individuals with a total of 52,200 person-years of observed follow-up out of a potential 55,589 person-years (implying 93.9% completeness of follow-up).16 Incidence rates are reported with 95% confidence intervals (CIs) based on a Poisson distribution. We considered p-values <0.05 statistically significant. We used IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, New York, USA), R version 3.2.3 and its library ‘epitools’ for all analyses.
Results
The study population included 3041 men and 4238 women, aged 45–75 years, free from atherosclerotic CVD at baseline. Mean age was 61.1 (standard deviation (SD) 6.9) years, 8.4% had diabetes mellitus, 24.7% were current smokers and 24% were using blood pressure-lowering medication (Table 2).
| . | Total . | Men . | Women . |
|---|---|---|---|
| . | (n = 7279) . | (n = 3041) . | (n = 4238) . |
| Age, mean (SD), years | 61.1 (6.9) | 61.0 (6.9) | 61.1 (6.9) |
| White, n (%) | 6920 (95.1) | 2913 (95.8) | 4007 (94.5) |
| Systolic blood pressure, mean (SD), mm Hg | 137 (20) | 140 (20) | 136 (21) |
| Diastolic blood pressure, mean (SD), mmHg | 80 (11) | 82 (11) | 79 (11) |
| Body mass index, mean (SD), kg/m2 | 27.3 (4.3) | 27.2 (3.6) | 27.4 (4.7) |
| Waist-hip ratio, mean (SD) | 0.89 (0.09) | 0.95 (0.07) | 0.85 (0.08) |
| Total cholesterol, mean (SD), mg/dl | 223 (38) | 216 (38) | 229 (38) |
| LDL cholesterol, mean (SD), mg/dl | 142 (35) | 139 (35) | 144 (36) |
| HDL cholesterol, mean (SD), mg/dl | 55 (16) | 48 (13) | 60 (16) |
| Triglycerides, mean (SD), mg/dl | 135 (75) | 145 (87) | 127 (63) |
| C-reactive protein,a mg/l | 1.4 (0.6–3.2) | 1.3 (0.5–2.8) | 1.5 (0.6–3.4) |
| eGFR,a ml/min/1.73 m2 | 83 (74–93) | 85 (76–95) | 81 (73–92) |
| Current smoking, n (%) | 1798 (24.7) | 864 (28.4) | 934 (22.0) |
| Former smoking, n (%) | 3254 (44.7) | 1615 (53.1) | 1639 (38.7) |
| Use of blood pressure-lowering medication, n (%) | 1746 (24.0) | 702 (23.1) | 1044 (24.6) |
| Use of statins, n (%) | 674 (9.3) | 274 (9.0) | 400 (9.4) |
| History of heart failure, n (%) | 42 (0.6) | 20 (0.7) | 22 (0.5) |
| History of atrial fibrillation, n (%) | 178 (2.4) | 94 (3.1) | 84 (2.0) |
| History of type 2 diabetes mellitus, n (%) | 609 (8.4) | 296 (9.7) | 313 (7.4) |
| Postmenopausal, n (%) | – | – | 3758 (88.7) |
| . | Total . | Men . | Women . |
|---|---|---|---|
| . | (n = 7279) . | (n = 3041) . | (n = 4238) . |
| Age, mean (SD), years | 61.1 (6.9) | 61.0 (6.9) | 61.1 (6.9) |
| White, n (%) | 6920 (95.1) | 2913 (95.8) | 4007 (94.5) |
| Systolic blood pressure, mean (SD), mm Hg | 137 (20) | 140 (20) | 136 (21) |
| Diastolic blood pressure, mean (SD), mmHg | 80 (11) | 82 (11) | 79 (11) |
| Body mass index, mean (SD), kg/m2 | 27.3 (4.3) | 27.2 (3.6) | 27.4 (4.7) |
| Waist-hip ratio, mean (SD) | 0.89 (0.09) | 0.95 (0.07) | 0.85 (0.08) |
| Total cholesterol, mean (SD), mg/dl | 223 (38) | 216 (38) | 229 (38) |
| LDL cholesterol, mean (SD), mg/dl | 142 (35) | 139 (35) | 144 (36) |
| HDL cholesterol, mean (SD), mg/dl | 55 (16) | 48 (13) | 60 (16) |
| Triglycerides, mean (SD), mg/dl | 135 (75) | 145 (87) | 127 (63) |
| C-reactive protein,a mg/l | 1.4 (0.6–3.2) | 1.3 (0.5–2.8) | 1.5 (0.6–3.4) |
| eGFR,a ml/min/1.73 m2 | 83 (74–93) | 85 (76–95) | 81 (73–92) |
| Current smoking, n (%) | 1798 (24.7) | 864 (28.4) | 934 (22.0) |
| Former smoking, n (%) | 3254 (44.7) | 1615 (53.1) | 1639 (38.7) |
| Use of blood pressure-lowering medication, n (%) | 1746 (24.0) | 702 (23.1) | 1044 (24.6) |
| Use of statins, n (%) | 674 (9.3) | 274 (9.0) | 400 (9.4) |
| History of heart failure, n (%) | 42 (0.6) | 20 (0.7) | 22 (0.5) |
| History of atrial fibrillation, n (%) | 178 (2.4) | 94 (3.1) | 84 (2.0) |
| History of type 2 diabetes mellitus, n (%) | 609 (8.4) | 296 (9.7) | 313 (7.4) |
| Postmenopausal, n (%) | – | – | 3758 (88.7) |
eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SD: standard deviation.
To convert total, LDL, and HDL cholesterol from mg/dl to SI (in mmol/l) multiply by 0.0259; and for triglycerides by 0.0113.
Median (25th–75th percentiles) because of skewed distribution.
| . | Total . | Men . | Women . |
|---|---|---|---|
| . | (n = 7279) . | (n = 3041) . | (n = 4238) . |
| Age, mean (SD), years | 61.1 (6.9) | 61.0 (6.9) | 61.1 (6.9) |
| White, n (%) | 6920 (95.1) | 2913 (95.8) | 4007 (94.5) |
| Systolic blood pressure, mean (SD), mm Hg | 137 (20) | 140 (20) | 136 (21) |
| Diastolic blood pressure, mean (SD), mmHg | 80 (11) | 82 (11) | 79 (11) |
| Body mass index, mean (SD), kg/m2 | 27.3 (4.3) | 27.2 (3.6) | 27.4 (4.7) |
| Waist-hip ratio, mean (SD) | 0.89 (0.09) | 0.95 (0.07) | 0.85 (0.08) |
| Total cholesterol, mean (SD), mg/dl | 223 (38) | 216 (38) | 229 (38) |
| LDL cholesterol, mean (SD), mg/dl | 142 (35) | 139 (35) | 144 (36) |
| HDL cholesterol, mean (SD), mg/dl | 55 (16) | 48 (13) | 60 (16) |
| Triglycerides, mean (SD), mg/dl | 135 (75) | 145 (87) | 127 (63) |
| C-reactive protein,a mg/l | 1.4 (0.6–3.2) | 1.3 (0.5–2.8) | 1.5 (0.6–3.4) |
| eGFR,a ml/min/1.73 m2 | 83 (74–93) | 85 (76–95) | 81 (73–92) |
| Current smoking, n (%) | 1798 (24.7) | 864 (28.4) | 934 (22.0) |
| Former smoking, n (%) | 3254 (44.7) | 1615 (53.1) | 1639 (38.7) |
| Use of blood pressure-lowering medication, n (%) | 1746 (24.0) | 702 (23.1) | 1044 (24.6) |
| Use of statins, n (%) | 674 (9.3) | 274 (9.0) | 400 (9.4) |
| History of heart failure, n (%) | 42 (0.6) | 20 (0.7) | 22 (0.5) |
| History of atrial fibrillation, n (%) | 178 (2.4) | 94 (3.1) | 84 (2.0) |
| History of type 2 diabetes mellitus, n (%) | 609 (8.4) | 296 (9.7) | 313 (7.4) |
| Postmenopausal, n (%) | – | – | 3758 (88.7) |
| . | Total . | Men . | Women . |
|---|---|---|---|
| . | (n = 7279) . | (n = 3041) . | (n = 4238) . |
| Age, mean (SD), years | 61.1 (6.9) | 61.0 (6.9) | 61.1 (6.9) |
| White, n (%) | 6920 (95.1) | 2913 (95.8) | 4007 (94.5) |
| Systolic blood pressure, mean (SD), mm Hg | 137 (20) | 140 (20) | 136 (21) |
| Diastolic blood pressure, mean (SD), mmHg | 80 (11) | 82 (11) | 79 (11) |
| Body mass index, mean (SD), kg/m2 | 27.3 (4.3) | 27.2 (3.6) | 27.4 (4.7) |
| Waist-hip ratio, mean (SD) | 0.89 (0.09) | 0.95 (0.07) | 0.85 (0.08) |
| Total cholesterol, mean (SD), mg/dl | 223 (38) | 216 (38) | 229 (38) |
| LDL cholesterol, mean (SD), mg/dl | 142 (35) | 139 (35) | 144 (36) |
| HDL cholesterol, mean (SD), mg/dl | 55 (16) | 48 (13) | 60 (16) |
| Triglycerides, mean (SD), mg/dl | 135 (75) | 145 (87) | 127 (63) |
| C-reactive protein,a mg/l | 1.4 (0.6–3.2) | 1.3 (0.5–2.8) | 1.5 (0.6–3.4) |
| eGFR,a ml/min/1.73 m2 | 83 (74–93) | 85 (76–95) | 81 (73–92) |
| Current smoking, n (%) | 1798 (24.7) | 864 (28.4) | 934 (22.0) |
| Former smoking, n (%) | 3254 (44.7) | 1615 (53.1) | 1639 (38.7) |
| Use of blood pressure-lowering medication, n (%) | 1746 (24.0) | 702 (23.1) | 1044 (24.6) |
| Use of statins, n (%) | 674 (9.3) | 274 (9.0) | 400 (9.4) |
| History of heart failure, n (%) | 42 (0.6) | 20 (0.7) | 22 (0.5) |
| History of atrial fibrillation, n (%) | 178 (2.4) | 94 (3.1) | 84 (2.0) |
| History of type 2 diabetes mellitus, n (%) | 609 (8.4) | 296 (9.7) | 313 (7.4) |
| Postmenopausal, n (%) | – | – | 3758 (88.7) |
eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SD: standard deviation.
To convert total, LDL, and HDL cholesterol from mg/dl to SI (in mmol/l) multiply by 0.0259; and for triglycerides by 0.0113.
Median (25th–75th percentiles) because of skewed distribution.
Overall 2180 men (71.7%) and 2196 women (51.8%) were eligible for one or more RCTs. The proportion of the population eligible for each trial varied among 11 RCTs, starting at lowest with 31 participants (0.4%) eligible for Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT), to 2240 participants (30.8%) eligible for Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group (MEGA) (Table 2 in Supplementary Material, Table 3). For nine out of 11 RCTs, overall proportions of trial eligible population were <10% (Table 2 in Supplementary Material, Table 3). Overlap between eligibility for 11 trials was minimal with the exception of (a) trials done in individuals with diabetes (Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN), Collaborative Atorvastatin Diabetes Study (CARDS), and Heart Protection Study of Cholesterol Lowering with Simvastatin (MRC/BHF HPS)), and (b) dyslipidaemia trials from the 1990s (West of Scotland Coronary Prevention Study (WOSCOPS) and Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS)) where the majority of trial eligible individuals were also eligible for MEGA (Table 3 in Supplementary Material). Overall, 14 out of 31 persons (45.2%) eligible for ALLHAT-LLT, and 161 out of 185 persons (87.0%) eligible for ASPEN would qualify for at the least one out of the other nine RCTs with positive results.
Persons eligible for each randomised clinical trial with their corresponding event rates, and American College of Cardiology/American Heart Association (ACC/AHA) 2013 and European Society of Cardiology (ESC) 2016 definite treatment recommendations.
| . | . | Event rates per 1000 person-years . | ACC/AHA 2013 . | ESC 2016 . | |
|---|---|---|---|---|---|
| Randomised clinical trial . | Total out of 7279 participants (%) . | Atherosclerotic CVD incidence rate (95% CI) . | CVD mortality rate (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . |
| Positive trials | |||||
| WOSCOPS | 659 (9.1) | 15.7 (12.0–20.0) | 1.7 (0.8–3.4) | 86.3 (83.5–88.9) | 35.4 (31.7–39.1) |
| AFCAPS/TexCAPS | 635 (8.7) | 13.5 (10.3–17.4) | 3.8 (2.3–6.0) | 77.6 (74.2–80.8) | 38.1 (34.3–42.0) |
| PROSPER | 448 (6.2) | 23.6 (18.8–29.2) | 8.8 (6.1–12.3) | 99.6 (98.4–99.9) | 89.5 (86.3–92.2) |
| ASCOT-LLA | 358 (4.9) | 22.1 (16.6–29.0) | 4.8 (2.5–8.1) | 95.3 (92.5–97.2) | 72.3 (67.4–76.9) |
| MRC/BHF HPS | 145 (2.0) | 17.6 (10.4–27.8) | 7.2 (3.1–14.2) | 96.6 (92.1–98.9) | 87.6 (81.1–92.5) |
| CARDS | 407 (5.6) | 21.4 (16.2–27.7) | 7.3 (4.6–11.1) | 92.4 (89.4–94.8) | 76.2 (71.7–80.2) |
| MEGA | 2240 (30.8) | 10.3 (8.8–12.0) | 2.3 (1.6–3.1) | 55.5 (53.4–57.6) | 26.3 (24.5–28.2) |
| JUPITER | 332 (4.6) | 10.6 (6.8–15.8) | 2.8 (1.1–5.8) | 68.1 (62.8–73.1) | 26.8 (22.1–31.9) |
| HOPE-3a | 1215 (16.7) | 7.8 (6.1–9.9) | 1.9 (1.2–3.0) | 66.0 (63.3–68.7)b | 10.7 (9.0–12.6)c |
| Neutral trialsc | |||||
| ALLHAT-LLT | 31 (0.4) | 17.7 (4.8–45.4) | 0.0d | 100 (88.8–100) | 67.7 (48.6–83.3) |
| ASPEN | 185 (2.5) | 15.1 (9.2–23.3) | 4.1 (1.5–8.9) | 95.1 (91.0–97.8) | 82.2 (75.9–87.4) |
| . | . | Event rates per 1000 person-years . | ACC/AHA 2013 . | ESC 2016 . | |
|---|---|---|---|---|---|
| Randomised clinical trial . | Total out of 7279 participants (%) . | Atherosclerotic CVD incidence rate (95% CI) . | CVD mortality rate (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . |
| Positive trials | |||||
| WOSCOPS | 659 (9.1) | 15.7 (12.0–20.0) | 1.7 (0.8–3.4) | 86.3 (83.5–88.9) | 35.4 (31.7–39.1) |
| AFCAPS/TexCAPS | 635 (8.7) | 13.5 (10.3–17.4) | 3.8 (2.3–6.0) | 77.6 (74.2–80.8) | 38.1 (34.3–42.0) |
| PROSPER | 448 (6.2) | 23.6 (18.8–29.2) | 8.8 (6.1–12.3) | 99.6 (98.4–99.9) | 89.5 (86.3–92.2) |
| ASCOT-LLA | 358 (4.9) | 22.1 (16.6–29.0) | 4.8 (2.5–8.1) | 95.3 (92.5–97.2) | 72.3 (67.4–76.9) |
| MRC/BHF HPS | 145 (2.0) | 17.6 (10.4–27.8) | 7.2 (3.1–14.2) | 96.6 (92.1–98.9) | 87.6 (81.1–92.5) |
| CARDS | 407 (5.6) | 21.4 (16.2–27.7) | 7.3 (4.6–11.1) | 92.4 (89.4–94.8) | 76.2 (71.7–80.2) |
| MEGA | 2240 (30.8) | 10.3 (8.8–12.0) | 2.3 (1.6–3.1) | 55.5 (53.4–57.6) | 26.3 (24.5–28.2) |
| JUPITER | 332 (4.6) | 10.6 (6.8–15.8) | 2.8 (1.1–5.8) | 68.1 (62.8–73.1) | 26.8 (22.1–31.9) |
| HOPE-3a | 1215 (16.7) | 7.8 (6.1–9.9) | 1.9 (1.2–3.0) | 66.0 (63.3–68.7)b | 10.7 (9.0–12.6)c |
| Neutral trialsc | |||||
| ALLHAT-LLT | 31 (0.4) | 17.7 (4.8–45.4) | 0.0d | 100 (88.8–100) | 67.7 (48.6–83.3) |
| ASPEN | 185 (2.5) | 15.1 (9.2–23.3) | 4.1 (1.5–8.9) | 95.1 (91.0–97.8) | 82.2 (75.9–87.4) |
AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT: Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT-LLA: Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial: The Lipid Lowering Arm; ASPEN: Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS: Collaborative Atorvastatin Diabetes Study; CI: confidence interval; CVD: cardiovascular disease; HOPE-3: Heart Outcomes Prevention Evaluation 3; JUPITER: Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; MRC/BHF HPS: Heart Protection Study of Cholesterol Lowering with Simvastatin; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; WOSCOPS: West of Scotland Coronary Prevention Study.
HOPE-3 published their results in April 2016, therefore results of this randomised clinical trial were not taken into account in ACC/AHA 2013 and ESC 2016 guidelines.
Due to major exclusion criteria for HOPE-3 (indication for statin therapy), all individuals qualifying for statin therapy by guidelines valid at the time of recruitment (ESC 2007 and Adult Treatment Panel III guidelines) are considered ineligible for HOPE-3.
Neutral trials: 14 out of 31 persons eligible for ALLHAT-LLT, and 161 out of 185 persons eligible for ASPEN would qualify for at the least one out of nine randomised clinical trials reporting positive findings on clinical cardiovascular end points.
Among ALLHAT-LLT eligible individuals, no cardiovascular death cases were observed in our study population.
Persons eligible for each randomised clinical trial with their corresponding event rates, and American College of Cardiology/American Heart Association (ACC/AHA) 2013 and European Society of Cardiology (ESC) 2016 definite treatment recommendations.
| . | . | Event rates per 1000 person-years . | ACC/AHA 2013 . | ESC 2016 . | |
|---|---|---|---|---|---|
| Randomised clinical trial . | Total out of 7279 participants (%) . | Atherosclerotic CVD incidence rate (95% CI) . | CVD mortality rate (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . |
| Positive trials | |||||
| WOSCOPS | 659 (9.1) | 15.7 (12.0–20.0) | 1.7 (0.8–3.4) | 86.3 (83.5–88.9) | 35.4 (31.7–39.1) |
| AFCAPS/TexCAPS | 635 (8.7) | 13.5 (10.3–17.4) | 3.8 (2.3–6.0) | 77.6 (74.2–80.8) | 38.1 (34.3–42.0) |
| PROSPER | 448 (6.2) | 23.6 (18.8–29.2) | 8.8 (6.1–12.3) | 99.6 (98.4–99.9) | 89.5 (86.3–92.2) |
| ASCOT-LLA | 358 (4.9) | 22.1 (16.6–29.0) | 4.8 (2.5–8.1) | 95.3 (92.5–97.2) | 72.3 (67.4–76.9) |
| MRC/BHF HPS | 145 (2.0) | 17.6 (10.4–27.8) | 7.2 (3.1–14.2) | 96.6 (92.1–98.9) | 87.6 (81.1–92.5) |
| CARDS | 407 (5.6) | 21.4 (16.2–27.7) | 7.3 (4.6–11.1) | 92.4 (89.4–94.8) | 76.2 (71.7–80.2) |
| MEGA | 2240 (30.8) | 10.3 (8.8–12.0) | 2.3 (1.6–3.1) | 55.5 (53.4–57.6) | 26.3 (24.5–28.2) |
| JUPITER | 332 (4.6) | 10.6 (6.8–15.8) | 2.8 (1.1–5.8) | 68.1 (62.8–73.1) | 26.8 (22.1–31.9) |
| HOPE-3a | 1215 (16.7) | 7.8 (6.1–9.9) | 1.9 (1.2–3.0) | 66.0 (63.3–68.7)b | 10.7 (9.0–12.6)c |
| Neutral trialsc | |||||
| ALLHAT-LLT | 31 (0.4) | 17.7 (4.8–45.4) | 0.0d | 100 (88.8–100) | 67.7 (48.6–83.3) |
| ASPEN | 185 (2.5) | 15.1 (9.2–23.3) | 4.1 (1.5–8.9) | 95.1 (91.0–97.8) | 82.2 (75.9–87.4) |
| . | . | Event rates per 1000 person-years . | ACC/AHA 2013 . | ESC 2016 . | |
|---|---|---|---|---|---|
| Randomised clinical trial . | Total out of 7279 participants (%) . | Atherosclerotic CVD incidence rate (95% CI) . | CVD mortality rate (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . | Proportion of adults with definite treatment recommendations (95% CI) . |
| Positive trials | |||||
| WOSCOPS | 659 (9.1) | 15.7 (12.0–20.0) | 1.7 (0.8–3.4) | 86.3 (83.5–88.9) | 35.4 (31.7–39.1) |
| AFCAPS/TexCAPS | 635 (8.7) | 13.5 (10.3–17.4) | 3.8 (2.3–6.0) | 77.6 (74.2–80.8) | 38.1 (34.3–42.0) |
| PROSPER | 448 (6.2) | 23.6 (18.8–29.2) | 8.8 (6.1–12.3) | 99.6 (98.4–99.9) | 89.5 (86.3–92.2) |
| ASCOT-LLA | 358 (4.9) | 22.1 (16.6–29.0) | 4.8 (2.5–8.1) | 95.3 (92.5–97.2) | 72.3 (67.4–76.9) |
| MRC/BHF HPS | 145 (2.0) | 17.6 (10.4–27.8) | 7.2 (3.1–14.2) | 96.6 (92.1–98.9) | 87.6 (81.1–92.5) |
| CARDS | 407 (5.6) | 21.4 (16.2–27.7) | 7.3 (4.6–11.1) | 92.4 (89.4–94.8) | 76.2 (71.7–80.2) |
| MEGA | 2240 (30.8) | 10.3 (8.8–12.0) | 2.3 (1.6–3.1) | 55.5 (53.4–57.6) | 26.3 (24.5–28.2) |
| JUPITER | 332 (4.6) | 10.6 (6.8–15.8) | 2.8 (1.1–5.8) | 68.1 (62.8–73.1) | 26.8 (22.1–31.9) |
| HOPE-3a | 1215 (16.7) | 7.8 (6.1–9.9) | 1.9 (1.2–3.0) | 66.0 (63.3–68.7)b | 10.7 (9.0–12.6)c |
| Neutral trialsc | |||||
| ALLHAT-LLT | 31 (0.4) | 17.7 (4.8–45.4) | 0.0d | 100 (88.8–100) | 67.7 (48.6–83.3) |
| ASPEN | 185 (2.5) | 15.1 (9.2–23.3) | 4.1 (1.5–8.9) | 95.1 (91.0–97.8) | 82.2 (75.9–87.4) |
AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT: Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT-LLA: Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial: The Lipid Lowering Arm; ASPEN: Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS: Collaborative Atorvastatin Diabetes Study; CI: confidence interval; CVD: cardiovascular disease; HOPE-3: Heart Outcomes Prevention Evaluation 3; JUPITER: Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; MRC/BHF HPS: Heart Protection Study of Cholesterol Lowering with Simvastatin; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; WOSCOPS: West of Scotland Coronary Prevention Study.
HOPE-3 published their results in April 2016, therefore results of this randomised clinical trial were not taken into account in ACC/AHA 2013 and ESC 2016 guidelines.
Due to major exclusion criteria for HOPE-3 (indication for statin therapy), all individuals qualifying for statin therapy by guidelines valid at the time of recruitment (ESC 2007 and Adult Treatment Panel III guidelines) are considered ineligible for HOPE-3.
Neutral trials: 14 out of 31 persons eligible for ALLHAT-LLT, and 161 out of 185 persons eligible for ASPEN would qualify for at the least one out of nine randomised clinical trials reporting positive findings on clinical cardiovascular end points.
Among ALLHAT-LLT eligible individuals, no cardiovascular death cases were observed in our study population.
Atherosclerotic CVD incidence rates were lowest in individuals eligible for HOPE-3 (7.8 per 1000 person-years), MEGA (10.3 per 1000 person-years), and Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) (10.6 per 1000 person-years), while the highest atherosclerotic CVD incidence rate was noted in individuals eligible for Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) (23.6 per 1000 person-years) (Table 3). Similarly, CVD mortality rates were lowest in individuals eligible for HOPE-3 (1.9 per 1000 person-years), WOSCOPS (1.7 per 1000 person-years), MEGA (2.3 per 1000 person-years) and JUPITER (2.8 per 1000 person-years), while the highest CVD mortality rate was noted in individuals eligible for PROSPER (8.8 per 1000 person-years) (Table 3).
Overall 2415 men (79.4%) and 1869 women (44.1%) would receive a definite positive recommendation for lipid-lowering therapy based on the ACC/AHA guidelines. Corresponding numbers for the ESC guidelines were 1276 for men (42.0%) and 1044 for women (26.4%). Proportions of trial-eligible individuals recommended for definite lipid-lowering treatment by the ACC/AHA 2013 and ESC 2016 guidelines varied not only among RCTs, but per guideline as well. The lowest proportions were observed in individuals eligible for MEGA (ACC/AHA 55.5%, ESC 26.3%), HOPE-3 (ACC/AHA 66.0%, ESC 10.7%) and JUPITER (ACC/AHA 68.1%, ESC 26.8%). The highest proportions (>75% for both ACC/AHA and ESC guidelines) were observed in RCTs enrolling individuals with diabetes (MRC/BHF HPS, CARDS and ASPEN) and older individuals (PROSPER) (Table 3, Figure 1). When definite and consider treatment recommendations were combined, we noticed that the vast majority of trial eligible adults qualify for statin treatment under both guidelines (Table 4 in Supplementary Material). However, individuals eligible for MEGA, JUPITER and HOPE-3 still had the lowest probability of being recommended for statin therapy by either guideline.
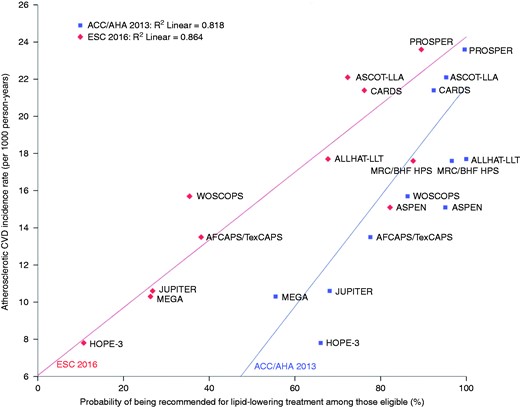
Probability of being recommended for lipid-lowering treatment by American College of Cardiology/American Heart Association (ACC/AHA) 2013 and European Society of Cardiology (ESC) 2016 guidelines and corresponding atherosclerotic cardiovascular disease (CVD) incidence rates, by trial.
A clear gradient can be seen with the likelihood of guidelines recommending lipid-lowering treatment related to the atherosclerotic CVD risk in the trial population. See Table 3 for corresponding data. Example: among all Rotterdam Study participants eligible for Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), the atherosclerotic CVD incidence rate was 10.6 per 1000 person-years of follow-up. A total of 68.1% and 26.8% would be recommended lipid-lowering treatment following the ACC/AHA 2013 and ESC 2016 guidelines, respectively.
AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT: Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT-LLA: Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial, The Lipid Lowering Arm; ASPEN: Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS: Collaborative Atorvastatin Diabetes Study; HOPE-3: Heart Outcomes Prevention Evaluation 3; MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; MRC/BHF HPS: Heart Protection Study of Cholesterol Lowering with Simvastatin; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; WOSCOPS: West of Scotland Coronary Prevention Study.
In Figure 2, proportions of cardiovascular deaths during follow-up in middle-aged participants (45–65 years) are presented by levels of estimated 10-year risk based on the advocated PCE and SCORE equations for the ACC/AHA (Figure 2(a) and (b)) and ESC guidelines (Figure 2(c) and (d)), respectively. Most individuals who died from CVD were deemed at high risk according to the PCE (i.e. ≥7.5% of 10-year risk of atherosclerotic CVD) and correspondingly got a positive treatment recommendation following the ACC/AHA guidelines (Figure 2(a)). On the other hand, for the ESC guidelines, it is notable that most individuals who died from CVD were deemed to be at low risk according to SCORE (i.e. <5% of 10-year risk of CVD mortality). Out of 26 participants under 65 years of age at low SCORE risk who died from CVD, 88.5% were not recommended lipid-lowering therapy by the ESC guidelines (Figure 2(c)), while 61.5% of these individuals would have qualified for one or more RCTs (Figure 2(d)).
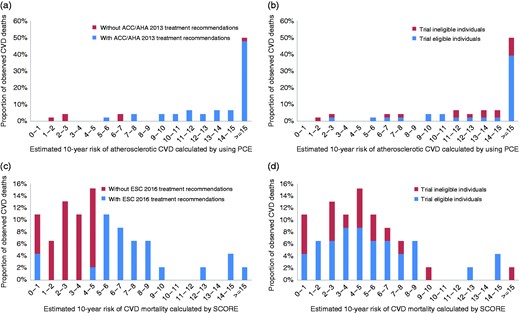
Proportion of cardiovascular deaths by estimated 10-year risk, observed in individuals aged 45–65 years during the first 10 years of follow-up. (a) Proportion of cardiovascular deaths by estimated 10-year risk of atherosclerotic cardiovascular disease (CVD) using the Pooled Cohort equations (PCEs), stratified by definite American College of Cardiology/American Heart Association (ACC/AHA) 2013 guideline treatment recommendations. (b) Proportion of cardiovascular deaths by 10-year risk of atherosclerotic CVD using the PCEs, stratified by eligibility for at least one out 11 randomised clinical trials (RCTs). (c) Proportion of cardiovascular deaths by estimated 10-year risk of CVD mortality using the Systematic Coronary Risk Evaluation (SCORE) equations, stratified by definite European Society of Cardiology (ESC) 2016 guideline recommendations. (d) Proportion of cardiovascular deaths by estimated 10-year risk of CVD mortality using the SCORE equations, stratified by eligibility for at least one out 11 RCTs.
In Figure 3, the number of RCTs that a single individual would be eligible for is plotted against the probability of a positive recommendation for lipid-lowering therapy by the ACC/AHA and ESC guidelines. In the overall study population, there was a clear gradient between the increase in the number of RCTs that one is eligible for and the likelihood of being recommended for lipid-lowering treatment (p-for-linear-trend <0.001 for both ACC/AHA and ESC). None of the non-diabetic individuals were eligible for more than three trials, but results in the overall population were not driven by individuals with diabetes (p-for-linear-trend in individuals without diabetes <0.001 for both ACC/AHA and ESC). In middle-aged individuals (≤65 years), both guidelines were substantially less likely to recommend lipid-lowering treatment, especially in those with a limited amount of evidence available (i.e. one or two RCTs). A stronger graded association between the amount of evidence and likelihood of a positive treatment recommendation was observed in women as compared to men for both guidelines. Overall, when compared to the ACC/AHA guidelines, the ESC guidelines appeared more conservative in recommending lipid-lowering treatment in the entire population and in all subgroups, especially in younger individuals.
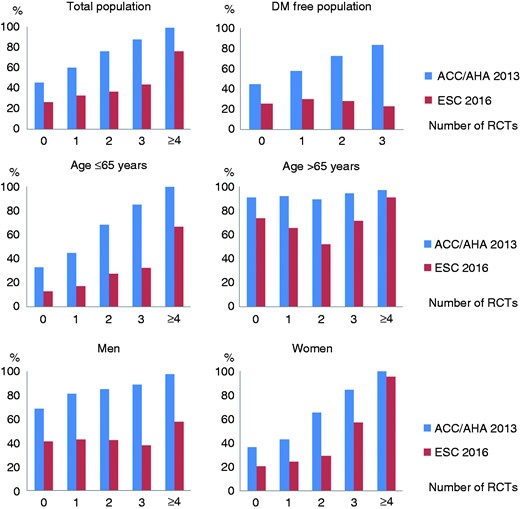
Probability of being recommended for lipid-lowering treatment by American College of Cardiology/American Heart Association (ACC/AHA) 2013 and European Society of Cardiology (ESC) 2016 guidelines, by number of trials an individual would be eligible for. Likelihood of being recommended for lipid-lowering treatment according to American and European guidelines, by total number of randomised clinical trials (RCTs) an individual would be eligible for. Results are depicted for the entire Rotterdam Study population and within clinically relevant subgroups.
DM: diabetes mellitus.
Discussion
Our study is the first to investigate to what extent statin RCTs for primary prevention are reflected in clinical practice guidelines at population level. Previous studies evaluating the applicability of selected RCTs applied only the main entry criteria17 and were limited to a handful of RCTs at most.18 We, however, applied an extensive list of inclusion and exclusion criteria to emulate trial eligibility and evaluated all RCTs published to date.
Based on prospective population-based data from middle-aged and older individuals free of atherosclerotic CVD, our results demonstrate that the proportions of the population eligible for 11 RCTs on statin use for primary prevention of atherosclerotic CVD varied considerably by trial, ranging from 0.4–30.8% of the overall population. Trials done in individuals with diabetes were well represented in both ACC/AHA 2013 and ESC 2016 guidelines. For other trials, the probability of being recommended for lipid-lowering therapy was positively related to the observed atherosclerotic CVD risk, with higher probabilities for ACC/AHA as compared to ESC guidelines, reflecting the low overall cardiovascular risk treatment threshold in the current ACC/AHA guidelines. Finally, we noted a positive relation between the number of RCTs that an individual would have been eligible for and the likelihood of being recommended lipid-lowering treatment by either guideline.
Clinical implications
The initial RCTs on statins only enrolled individuals at high risk of developing atherosclerotic CVD, either with dyslipidaemia (WOSCOPS and AFCAPS/TexCAPS), elderly (PROSPER), hypertensives (Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients in the Anglo-Scandinavian Cardiac Outcomes Trial: The Lipid Lowering Arm (ASCOT-LLA) and ALLHAT-LLT), or individuals with diabetes (MRC/BHF HPS, CARDS and ASPEN), whilst more recent RCTs enrolled individuals at low-to-intermediate risk of atherosclerotic CVD (JUPITER, MEGA and HOPE-3). The evidence coming from older RCTs recruiting individuals at higher risk for atherosclerotic CVD is better reflected in both guidelines (Figure 1). In particular RCTs targeting individuals with diabetes have a very high penetrance in contemporary guidelines since we observed the closest alignment between the ACC/AHA and ESC guidelines in this group of patients. The most recent RCTs targeting populations at low-to-intermediate risk are less well-represented in the current guidelines, and also have more discrepant recommendations between the guidelines (Figure 1).
Currently, a trial targeting an even lower risk population for primary prevention of atherosclerotic CVD with atorvastatin Eliminate Coronary Artery Disease trial (ECAD) is ongoing.19 However, the majority of the middle-aged and older population is already recommended for lipid-lowering treatment under the regime of the current guidelines.11,18,20,21 Therefore, extending guideline recommendations on lipid-lowering treatment to even lower risk individuals – despite statins having been proven efficacious in JUPITER, MEGA and HOPE-3 – is considered unwanted by many.22–25 Since statins have been proven to be both cost-effective and safe,26 a potential solution to this issue could be through identification of individuals at increased risk of atherosclerotic CVD among those deemed at predicted lower risk. For instance, by using additional tests with strong predictive properties, such as the quantification of coronary calcium.27 In any case, particularly in individuals at lower risk, the balance between the benefits of safe low-cost generic statins and the burden of taking medication demands joint decision-making among patients and practitioners.
Cooney and colleagues demonstrated that a substantial number of cardiovascular deaths occur in individuals considered to be at low 10-year SCORE risk, simply because they constitute the majority of the general population.28 Our findings are in line with these observations, since we confirmed that the majority of middle-aged individuals who died from a CVD during follow-up had an estimated 10-year risk of CVD mortality <5% and thereby would not have qualified for lipid-lowering treatment under the ESC guidelines. Many of them would, however, have met the entry criteria of one or more RCTs (Figure 2(d)). This would support the argument for reconsidering the ESC treatment recommendations in younger individuals, for instance by lowering the treatment threshold similar to the latest ACC/AHA guidelines,4,12 extending the SCORE risk equations to also predict non-fatal outcomes,29 making a transition from 10-year risk to lifetime risk assessment,30 reconsidering the dominant role of age in the current 10-year CVD risk calculators,29 or using hybrid algorithms based on both cardiovascular risk estimation and statin trial eligibility.18
Those eligible for trials but not recommended for lipid-lowering treatment apparently have a low 10-year risk of CVD based on the calculators. However, considering that these adults have risk factors that made them eligible for these trials and given the nature of the cumulative risk factor exposure, they will likely have an increased lifetime risk of CVD.30–32 Therefore, statin therapy for primary prevention of atherosclerotic CVD could be considered in these individuals, since early treatment with statins may have a legacy effect in later life even after only several years of use.29,33 This does imply that the numbers needed to treat will likely be relatively high when only considering the period shortly after statin initiation, but this could drop substantially when considering a longer-term perspective in these individuals.
Limitations
A number of limitations of our study need to be addressed. First, the Rotterdam Study population is almost entirely white, thus extrapolation of our findings to other ethnicities should be done with caution and validation of our findings in diverse populations seems warranted. Second, a small proportion of the studied population (9.3%) was using statins at baseline, which might have led to an underestimation of event rates in these individuals. Yet, exclusion of statin users at baseline did not materially change the results (Figure 1 in Supplementary Material). Third, we emphasise that HOPE-3 did not contribute to the current recommendations in the prevailing ACC/AHA and the ESC guidelines. Our results for this trial, therefore, should be interpreted as providing the potential evidence for expanding recommendations on lipid-lowering therapy in future revisions of the current guidelines, rather than a representation of evidence in the current guidelines. Fourth, the exact details for all minor inclusion and exclusion criteria for 11 RCTs under study, such as rare conditions and allergies, were not available (Table 1 in Supplementary Material).11 Due to the rarity of these entry criteria, this is unlikely to have affected our findings. Finally, similar to RCTs, population-based cohort studies require active participation and are therefore subject to the healthy volunteer effect.34 This leads to underestimation of the prevalence of cardiovascular risk factors and CVD event rates in the source population.
Conclusions
As compared to RCTs targeted at high-risk populations, RCTs targeted at low-to-intermediate risk populations are less likely to be reflected in the ACC/AHA 2013 and ESC 2016 guideline recommendations for lipid-lowering treatment in primary prevention of atherosclerotic CVD. At population level, the ESC guidelines were far more conservative in recommending lipid-lowering treatment in low-to-intermediate risk individuals as compared to the ACC/AHA guidelines, especially in middle-aged individuals. HOPE-3, the most recent addition to the evidence base for statin use in primary prevention of atherosclerotic CVD, targeted individuals at low-to-intermediate risk that could benefit from statin treatment, but who are currently not well-captured by prevailing European guidelines. These novel insights should foster future updates to clinical practice guidelines for lipid-lowering in primary prevention of atherosclerotic CVD.
Acknowledgements
The dedication, commitment and contribution of inhabitants, general practitioners and pharmacists of the Ommoord district to the Rotterdam Study are gratefully acknowledged by the authors.
Author contribution
JP, PG, OHF and MJGL contributed to the study concept and design. All authors contributed to the acquisition, analysis, or interpretation of data. JP and MJGL drafted the manuscript and all other authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J Pavlović is supported by Erasmus Mundus Western Balkans (ERAWEB), a project funded by the European Commission. M Kavousi is supported by the Netherlands Organisation for Health Research and Development (ZonMw) (VENI 91616079). MJG Leening is supported by the Prins Bernhard Cultuurfonds Fellowship (30140588), and De Drie Lichten Foundation (04/14). OH Franco works in ErasmusAGE, a centre for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.); Metagenics Inc.; and AXA. The remaining authors report no potential conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organisation for Scientific Research (NWO); the Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. None of the funders had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- atherosclerosis
- primary prevention
- statins
- cardiovascular diseases
- diabetes mellitus
- diabetes mellitus, type 2
- cholesterol
- american heart association
- randomization
- guidelines
- lipids
- mortality
- lipid-lowering therapy
- clinical practice guideline
- older adult
- treatment guidelines
- allhat trial
- primary prevention of cardiovascular disease
- american college of cardiology
- prevention
- european society of cardiology




Comments