-
PDF
- Split View
-
Views
-
Cite
Cite
FD Richard Hobbs, Clare J Taylor, Geert Jan Geersing, Frans H Rutten, Judith R Brouwer, the European Primary Care Cardiovascular Society (EPCCS) SPAF working group, European Primary Care Cardiovascular Society (EPCCS) consensus guidance on stroke prevention in atrial fibrillation (SPAF) in primary care, European Journal of Preventive Cardiology, Volume 23, Issue 5, 1 March 2016, Pages 460–473, https://doi.org/10.1177/2047487315571890
Close - Share Icon Share
Abstract
Atrial fibrillation affects 1–2% of the general population and 10% of those over 75, and is responsible for around a quarter of all strokes. These strokes are largely preventable by the use of anticoagulation therapy, although many eligible patients are not treated. Recent large clinical trials have added to the evidence base on stroke prevention and international clinical guidelines have been updated.
Consensus practical recommendations from primary care physicians with an interest in vascular disease and vascular specialists.
A focussed all-day meeting, with presentation of summary evidence under each section of this guidance and review of European guidelines on stroke prevention in atrial fibrillation, was used to generate a draft document, which then underwent three cycles of revision and debate before all panel members agreed with the consensus statements.
Six areas were identified that included how to identify patients with atrial fibrillation, how to determine their stroke risk and whether to recommend modification of this risk, and what management options are available, with practical recommendations on maximising benefit and minimising risk if anticoagulation is recommended and the reasons why antiplatelet therapy is no longer recommended. The summary evidence is presented for each area and simple summary recommendations are highlighted, with areas of remaining uncertainty listed.
Atrial fibrillation-related stroke is a major public health priority for most health systems. This practical guidance can assist generalist community physicians to translate the large evidence base for this cause of preventable stroke and implement this at a local level.
Background
Despite the large evidence base for preventing stroke in atrial fibrillation (AF), and the recent guideline updates with recent clinical outcome data and clinical experience (see web version for full details and references), the European Primary Cardiovascular Care Society (EPCCS) felt that wider implementation of the available guidelines (European Society of Cardiology (ESC) and others) in primary care settings would benefit from adding contextual changes or clarifications of the evidence to aid the uptake of guidance in primary care. The EPCCS therefore established a Stroke Prevention in Atrial Fibrillation (SPAF) working group to develop an evidence-guided pragmatic guide on SPAF in primary care.
The EPCCS Consensus Group made its recommendations based on ‘the trade-off between the benefits and harms of any intervention, taking into account the quality of the underpinning evidence’. The wording used in our recommendations (see Box 1) denotes the certainty with which the recommendation is made (the strength of the recommendation). There should be discussion with the patient about the risks and benefits of the interventions, and their values and preferences. This discussion aims to help clinician and patient to reach a fully informed decision.
Box 1. Strength of recommendations
The following colour coding will be used throughout the document, to indicate the strength of the individual recommendations.
Interventions that should (or should not) be used: a ‘strong’ recommendation
‘Offer’ (and similar words such as ‘refer’ or ‘advise’) indicates confidence that, for the vast majority of patients, an intervention will do more good than harm, and be cost-effective. Similar forms of words (for example, ‘Do not offer…’) are used when we are confident that an intervention will not be of benefit for most patients.
Interventions that could be used
‘Consider’ indicates confidence that an intervention will do more good than harm for most patients, and be cost-effective, but other options may be similarly cost-effective. The choice of intervention, and whether or not to have the intervention at all, is more likely to depend on the patient’s values and preferences than for a strong recommendation, and so the healthcare professional should spend more time considering and discussing the options with the patient.
Terminology used with permission from NICE.
To highlight the summary recommendations, the writing committee chose to indicate where the position taken is clearly evidence-based (green), and where it is more inferred and consensus-based (blue). It is also specified when studies were carried out in primary care settings, and therefore the evidence is most relevant.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with about 1–2% of the general population estimated to be affected.1,2 It is a particularly common disorder in the elderly, with over 5% of people over the age of 65 suffering from AF, and around 10% of people over the age of 75.3–5 As a consequence of the ageing population, the prevalence of AF is predicted to rise.6 Rising prevalence has also been attributed to better survival of patients following acute coronary events, and a greater awareness among healthcare professionals of the importance of diagnosing AF.
Clinical picture of patients with atrial fibrillation
Patients with AF may have symptoms such as palpitations, lack of energy, dizziness, chest discomfort and shortness of breath, which may impair quality of life.7 The degree of these symptoms varies considerably, from patients who are completely asymptomatic to those who are quite disabled by the arrhythmia. The use of rate and rhythm control to improve symptoms is beyond the scope of this guideline, which will focus exclusively on stroke prevention.
It is important to note that, for many patients with AF, the condition is often asymptomatic - or associated with minor symptoms that are ignored or unrecognised by patients -- therefore, if the arrhythmia is to be identified in all, some type of AF screening is needed.
Perhaps the most important consequence of AF is the risk of embolic stroke. Patients with AF are at an almost fivefold higher risk of stroke compared with age-matched individuals with normal sinus rhythm, as shown in the Framingham study,8 as well as at a twice as high risk of all-cause mortality and heart failure. About 20--25% of all ischaemic strokes are attributable to embolism as a result of AF.9 Not only do patients with AF have more strokes, they also develop more recurrent strokes, both fatal and nonfatal.8 In addition, strokes are likely to be more severe in patients with AF, than in patients who have a stroke not associated with AF, regardless of age.10 Following a stroke, patients with AF are more likely to be left with long-term disability and may require long-term care.11,12 This disability is a major source of concern for patients and is associated with high costs for healthcare systems. Moreover, AF is often associated with other underlying pathology, such as cardiovascular comorbidity (coronary artery disease, heart failure, hypertension) and/or non-cardiovascular comorbidity (chronic obstructive pulmonary disease (COPD), renal failure, etc.). This is especially prevalent in the elderly, and highlights the role general practice should play in managing this multi-morbid disorder. AF has considerable impact on individuals and health systems. It is also associated with increased mortality, heart failure and high rates of hospitalisation because of stroke. Admission and readmission rates are the most important factors driving healthcare expenditure.18–20
Types of atrial fibrillation
ESC guidelines distinguish various types of AF, mainly based on duration, for example paroxysmal (usually ≤48 hours), persistent (≥7 days) and long-standing or permanent (>1 year).1,2 These classifications are somewhat arbitrary and their use in clinical practice might be limited. They may, however, be relevant to determine how to treat the arrhythmia itself, rather than the stroke risk associated with AF. The risk of stroke is considered similar for all types of AF.13
It is also important to consider the distinction between valvular and non-valvular types of AF, as this affects management. In the ESC guidelines,1,2 the term valvular AF is used to indicate that AF is related to rheumatic valvular disease (predominantly mitral stenosis) or prosthetic heart valves.
Atrial flutter, a different type of atrial arrhythmia, is sometimes considered along with AF. Patients with atrial flutter are often referred for consideration of curative management, but in the meantime stroke risk should be considered and managed in the same way as AF.
In summary, all types of AF, with the exception of clinically significant valvular AF as defined above, should be regarded as the same in terms of stroke risk. Defining the type of AF can provide a diagnostic label, which can be useful when considering rate or rhythm management, but stroke risk is similar. Importantly, re-establishing sinus rhythm will not remove the stroke risk.14–17
Role of the general practitioner in stroke prevention in atrial fibrillation
AF often co-exists with other chronic diseases, and these comorbidities may have caused or exacerbated each other. Over extended periods of time, AF may cause substantial cardiac remodelling that can impact on the management of both conditions. A recent epidemiological study showed that 5% of over 65-year-olds had AF, and at least three other chronic conditions.21 This implies that the general practitioner can play a crucial, central role, as he or she is aware of and can manage different conditions.
Practical recommendations
Based on current evidence and experience, we recommend the following in a primary care setting:
All patients with AF, regardless of AF type, are at increased risk of stroke as they age or develop certain comorbid conditions and therefore all AF patients should be offered assessment of their stroke risk (see How to decide whether to treat stroke risk in atrial fibrillation?).
Patients who are treated for AF and returned to sinus rhythm should be risk assessed as if they were still in AF and should remain on their stroke prevention therapy if it was indicated prior to rhythm control. If a decision is taken to stop anticoagulation in these patients, it should be a specialist decision.
How is atrial fibrillation detected? Should we screen for atrial fibrillation?
Rationale for opportunistic case-finding
AF is one of the most important causes of preventable stroke, is associated with more severe strokes and is therefore a major health risk to modify in patients and an important disease target for health systems. AF meets all the Wilson-Jungner criteria to be a condition worth screening for. Patients with AF may present with symptoms, and AF should be considered in anyone complaining of palpitations (fluttering or irregular heart beat), dizziness or fainting spells, chest discomfort, shortness of breath and/or reduced exercise tolerance. However, not all patients with AF will have symptoms and may, therefore, be unaware they have an arrhythmia. The observation that even short episodes of silent AF (as measured with implanted devices and by Holter electrocardiograms (ECGs)) convey an increased risk of stroke22,23 offers the rationale for opportunistic screening.
Opportunistic screening by pulse palpation in general practice detected a large number of patients with previously undiagnosed AF compared with usual care (1.64% per year by screening vs. 1.04% per year with care as usual), yielding a feasible number of 169 needed to screen.24 These data suggest that active screening for AF in patients of 65 years and older can identify patients eligible for anticoagulation treatment according to CHADS2 criteria.25 New technologies, such as modified sphygmomanometers capable of detecting an irregular pulse, may also improve pick-up rates.
Confirming the diagnosis in suspected atrial fibrillation
In patients with suspected AF, an ECG, preferably 12-lead ECG, can confirm the diagnosis. Loss of P-waves and completely irregular R-R distances are characteristic features of AF on ECG; however, ECG changes may be subtle, so judgement by a competent ECG-reader is required to confidently diagnose AF.5 Adequate interpretation of a single-lead ECG may be considered as convincing as a 12-lead ECG for detection or exclusion of AF.26
Conventionally, an ECG should contain a total of 30 seconds of AF to confirm the diagnosis, but this criterion is consensus not evidence-based, and was developed for considering which patients to offer cardioversion or pacing. Therefore, a standard 12-lead ECG of 10 seconds is perhaps sufficient in practice settings.
Practical recommendations
Based on current evidence and experiences, we recommend the following case finding efforts in a primary care setting:
Interventions that should be used
Opportunistic case finding should be carried out for timely detection of AF in all patients over 65 years of age, and in anyone who receives routine cardiovascular follow-up:
○ Pulse palpation, at least once a year could be incorporated into already existing medical visits, for instance during an annual cardiac disease review, and/or at flu vaccinations or pharmacy visits.
In the case of a positive pulse palpation:
○ 12-lead ECG follow-up should be performed shortly after pulse assessment. 12-lead ECG follow-up should be done by a practitioner who is competent in ECG interpretation.
Alternative approach
Modified sphygmomanometers or other devices using single-lead ECG registrations to detect an irregular pulse may be used instead of pulse palpation, but only where they have been subject to independent validation with a 12-lead ECG.
If not enough expertise is available in the primary care setting to confidently read a 12-lead ECG, it should be reviewed by a specialist. 12-lead ECG may also provide other useful information on cardiac functioning.
Additional work recommended in this area/gaps in the evidence:
See web version
How to decide whether to treat stroke risk in atrial fibrillation?
Risk assessment for stroke prevention in atrial fibrillation
When giving antithrombotic agents to reduce stroke risk in AF, both thromboembolic risk and bleeding risk need to be considered. ‘Whom not to treat?’ is a question at least as important to ask as ‘whom to treat?’, as each form of antithrombotic therapy has an inherent, and possibly severe, bleeding risk.
The CHADS2 score is a valuable tool that has been used for some time to assess stroke risk in patients with AF,27,28 but observational data highlighted the need for a more robust stroke risk score. The CHA2DS2-VASc score has since been developed, which includes additional risk factors and gives a maximum score of 9 compared with a maximum of 6 in the CHADS2 score (see Table 1 for components of the CHA2DS2-VASc score). Age can contribute 2 points, rather than 1, if the patient is ≥75 years old. Vascular disease and female sex also add an extra point,1,2 the latter only contributing a score, however, if other stroke risk factors are present (i.e. if the only ‘risk’ factor is being female, the CHA2DS2-VASc score is 0). The improved risk stratification with CHA2DS2-VASc as opposed to CHADS2 score has been validated in several studies,29–33 and CHA2DS2-VASc is the recommended score for assessment of stroke risk in the ESC guidelines.2 Event rates of hospital admission and death caused by thromboembolism (including peripheral artery embolism, ischaemic stroke, and pulmonary embolism) for each CHADS2 and CHA2DS2-VASc category are shown in Table 2.
| . | CHADS2 score . | CHA2DS2-VASc score . | ||
|---|---|---|---|---|
| Condition . | . | Points . | . | Points . |
| Congestive heart failure (or left ventricular systolic dysfunction) | C | 1 | C | 1 |
| Hypertension: blood pressure consistently above 140/90 mmHg (or treated hypertension on medication) | H | 1 | H | 1 |
| Age ≥ 75 years | A | 1 | A2 | 2 |
| Diabetes mellitus | D | 1 | D | 1 |
| Stroke or TIA or thromboembolism in history | S2 | 2 | S2 | 2 |
| Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque) | V | 1 | ||
| Age 65–74 years | A | 1 | ||
| Sex category (i.e. female gender) | Sc | 1 | ||
| . | CHADS2 score . | CHA2DS2-VASc score . | ||
|---|---|---|---|---|
| Condition . | . | Points . | . | Points . |
| Congestive heart failure (or left ventricular systolic dysfunction) | C | 1 | C | 1 |
| Hypertension: blood pressure consistently above 140/90 mmHg (or treated hypertension on medication) | H | 1 | H | 1 |
| Age ≥ 75 years | A | 1 | A2 | 2 |
| Diabetes mellitus | D | 1 | D | 1 |
| Stroke or TIA or thromboembolism in history | S2 | 2 | S2 | 2 |
| Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque) | V | 1 | ||
| Age 65–74 years | A | 1 | ||
| Sex category (i.e. female gender) | Sc | 1 | ||
This table shows the components of the CHADS2 (Gage et al., JAMA 2001)28 and CHA2DS2-VASc scores (Lip et al., Chest 2010)30 tools to assess stroke risk in patients with AF. These risk assessment tools help to determine who should and who should not receive anticoagulation. CHA2DS2-VASc improves risk stratification in patients with CHADS2=0 or 1, and allows for identification of patients at truly low risk.
| . | CHADS2 score . | CHA2DS2-VASc score . | ||
|---|---|---|---|---|
| Condition . | . | Points . | . | Points . |
| Congestive heart failure (or left ventricular systolic dysfunction) | C | 1 | C | 1 |
| Hypertension: blood pressure consistently above 140/90 mmHg (or treated hypertension on medication) | H | 1 | H | 1 |
| Age ≥ 75 years | A | 1 | A2 | 2 |
| Diabetes mellitus | D | 1 | D | 1 |
| Stroke or TIA or thromboembolism in history | S2 | 2 | S2 | 2 |
| Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque) | V | 1 | ||
| Age 65–74 years | A | 1 | ||
| Sex category (i.e. female gender) | Sc | 1 | ||
| . | CHADS2 score . | CHA2DS2-VASc score . | ||
|---|---|---|---|---|
| Condition . | . | Points . | . | Points . |
| Congestive heart failure (or left ventricular systolic dysfunction) | C | 1 | C | 1 |
| Hypertension: blood pressure consistently above 140/90 mmHg (or treated hypertension on medication) | H | 1 | H | 1 |
| Age ≥ 75 years | A | 1 | A2 | 2 |
| Diabetes mellitus | D | 1 | D | 1 |
| Stroke or TIA or thromboembolism in history | S2 | 2 | S2 | 2 |
| Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque) | V | 1 | ||
| Age 65–74 years | A | 1 | ||
| Sex category (i.e. female gender) | Sc | 1 | ||
This table shows the components of the CHADS2 (Gage et al., JAMA 2001)28 and CHA2DS2-VASc scores (Lip et al., Chest 2010)30 tools to assess stroke risk in patients with AF. These risk assessment tools help to determine who should and who should not receive anticoagulation. CHA2DS2-VASc improves risk stratification in patients with CHADS2=0 or 1, and allows for identification of patients at truly low risk.
| Score/risk category . | 1-year follow-up . | 5-years follow-up . | 10-year follow-up . |
|---|---|---|---|
| . | Annual event rate . | Annual event rate . | Annual event rate . |
| CHADS2 score: | |||
| 0 | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| 1 | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| 2 | 7.34 (6.88–7.82) | 5.58 (5.35–5.83) | 5.40 (5.18–5.63) |
| 3 | 15.47 (14.62–16.36) | 10.29 (9.87–10.73) | 9.89 (9.50–10.31) |
| 4 | 21.55 (20.03–23.18) | 14.00 (13.22–14.82) | 13.70 (12.95–14.48) |
| 5 | 19.71 (16.93–22.93) | 12.98 (11.52–14.63) | 12.57 (11.18–14.14) |
| 6 | 22.36 (14.58–34.30) | 16.75 (11.91–23.56) | 17.17 (12.33–23.92) |
| Low risk (0) | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| Intermediate risk (1) | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| High risk (2–6) | 12.27 (11.84–12.71) | 8.30 (8.08–8.51) | 7.97 (7.77–8.17) |
| CHA2DS2-VASc risk score: | |||
| 0 | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| 1 | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| 2 | 3.71 (3.36–4.09) | 3.01 (2.83–3.20) | 2.92 (2.76–3.09) |
| 3 | 5.92 (5.53–6.34) | 4.41 (4.21–4.61) | 4.28 (4.10–4.47) |
| 4 | 9.27 (8.71–9.86) | 6.69 (6.41–6.99) | 6.46 (6.20–6.74) |
| 5 | 15.26 (14.35–16.24) | 10.42 (9.95–10.91) | 9.97 (9.53–10.43) |
| 6 | 19.74 (18.21–21.41) | 12.85 (12.07–13.69) | 12.52 (11.78–13.31) |
| 7 | 21.50 (18.75–24.64) | 13.92 (12.49–15.51) | 13.96 (12.57–15.51) |
| 8 | 22.38 (16.29–30.76) | 14.07 (10.80–18.33) | 14.10 (10.90–18.23) |
| 9 | 23.64 (10.62–52.61) | 16.08 (8.04–32.15) | 15.89 (7.95–31.78) |
| Low risk (0) | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| Intermediate risk (1) | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| High risk (2–9) | 8.82 (8.55–9.09) | 6.01 (5.88–6.14) | 5.72 (5.60–5.84) |
| Score/risk category . | 1-year follow-up . | 5-years follow-up . | 10-year follow-up . |
|---|---|---|---|
| . | Annual event rate . | Annual event rate . | Annual event rate . |
| CHADS2 score: | |||
| 0 | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| 1 | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| 2 | 7.34 (6.88–7.82) | 5.58 (5.35–5.83) | 5.40 (5.18–5.63) |
| 3 | 15.47 (14.62–16.36) | 10.29 (9.87–10.73) | 9.89 (9.50–10.31) |
| 4 | 21.55 (20.03–23.18) | 14.00 (13.22–14.82) | 13.70 (12.95–14.48) |
| 5 | 19.71 (16.93–22.93) | 12.98 (11.52–14.63) | 12.57 (11.18–14.14) |
| 6 | 22.36 (14.58–34.30) | 16.75 (11.91–23.56) | 17.17 (12.33–23.92) |
| Low risk (0) | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| Intermediate risk (1) | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| High risk (2–6) | 12.27 (11.84–12.71) | 8.30 (8.08–8.51) | 7.97 (7.77–8.17) |
| CHA2DS2-VASc risk score: | |||
| 0 | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| 1 | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| 2 | 3.71 (3.36–4.09) | 3.01 (2.83–3.20) | 2.92 (2.76–3.09) |
| 3 | 5.92 (5.53–6.34) | 4.41 (4.21–4.61) | 4.28 (4.10–4.47) |
| 4 | 9.27 (8.71–9.86) | 6.69 (6.41–6.99) | 6.46 (6.20–6.74) |
| 5 | 15.26 (14.35–16.24) | 10.42 (9.95–10.91) | 9.97 (9.53–10.43) |
| 6 | 19.74 (18.21–21.41) | 12.85 (12.07–13.69) | 12.52 (11.78–13.31) |
| 7 | 21.50 (18.75–24.64) | 13.92 (12.49–15.51) | 13.96 (12.57–15.51) |
| 8 | 22.38 (16.29–30.76) | 14.07 (10.80–18.33) | 14.10 (10.90–18.23) |
| 9 | 23.64 (10.62–52.61) | 16.08 (8.04–32.15) | 15.89 (7.95–31.78) |
| Low risk (0) | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| Intermediate risk (1) | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| High risk (2–9) | 8.82 (8.55–9.09) | 6.01 (5.88–6.14) | 5.72 (5.60–5.84) |
Event rates (95% CI) of hospital admission and death caused by thromboembolism (including peripheral artery embolism, ischaemic stroke and pulmonary embolism) per 100 person years, for each CHADS2 and CHA2DS2-VASc category. Risk profiles are largely similar with different lengths of follow-up. Adapted from Olesen et al., BMJ 201129.
| Score/risk category . | 1-year follow-up . | 5-years follow-up . | 10-year follow-up . |
|---|---|---|---|
| . | Annual event rate . | Annual event rate . | Annual event rate . |
| CHADS2 score: | |||
| 0 | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| 1 | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| 2 | 7.34 (6.88–7.82) | 5.58 (5.35–5.83) | 5.40 (5.18–5.63) |
| 3 | 15.47 (14.62–16.36) | 10.29 (9.87–10.73) | 9.89 (9.50–10.31) |
| 4 | 21.55 (20.03–23.18) | 14.00 (13.22–14.82) | 13.70 (12.95–14.48) |
| 5 | 19.71 (16.93–22.93) | 12.98 (11.52–14.63) | 12.57 (11.18–14.14) |
| 6 | 22.36 (14.58–34.30) | 16.75 (11.91–23.56) | 17.17 (12.33–23.92) |
| Low risk (0) | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| Intermediate risk (1) | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| High risk (2–6) | 12.27 (11.84–12.71) | 8.30 (8.08–8.51) | 7.97 (7.77–8.17) |
| CHA2DS2-VASc risk score: | |||
| 0 | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| 1 | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| 2 | 3.71 (3.36–4.09) | 3.01 (2.83–3.20) | 2.92 (2.76–3.09) |
| 3 | 5.92 (5.53–6.34) | 4.41 (4.21–4.61) | 4.28 (4.10–4.47) |
| 4 | 9.27 (8.71–9.86) | 6.69 (6.41–6.99) | 6.46 (6.20–6.74) |
| 5 | 15.26 (14.35–16.24) | 10.42 (9.95–10.91) | 9.97 (9.53–10.43) |
| 6 | 19.74 (18.21–21.41) | 12.85 (12.07–13.69) | 12.52 (11.78–13.31) |
| 7 | 21.50 (18.75–24.64) | 13.92 (12.49–15.51) | 13.96 (12.57–15.51) |
| 8 | 22.38 (16.29–30.76) | 14.07 (10.80–18.33) | 14.10 (10.90–18.23) |
| 9 | 23.64 (10.62–52.61) | 16.08 (8.04–32.15) | 15.89 (7.95–31.78) |
| Low risk (0) | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| Intermediate risk (1) | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| High risk (2–9) | 8.82 (8.55–9.09) | 6.01 (5.88–6.14) | 5.72 (5.60–5.84) |
| Score/risk category . | 1-year follow-up . | 5-years follow-up . | 10-year follow-up . |
|---|---|---|---|
| . | Annual event rate . | Annual event rate . | Annual event rate . |
| CHADS2 score: | |||
| 0 | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| 1 | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| 2 | 7.34 (6.88–7.82) | 5.58 (5.35–5.83) | 5.40 (5.18–5.63) |
| 3 | 15.47 (14.62–16.36) | 10.29 (9.87–10.73) | 9.89 (9.50–10.31) |
| 4 | 21.55 (20.03–23.18) | 14.00 (13.22–14.82) | 13.70 (12.95–14.48) |
| 5 | 19.71 (16.93–22.93) | 12.98 (11.52–14.63) | 12.57 (11.18–14.14) |
| 6 | 22.36 (14.58–34.30) | 16.75 (11.91–23.56) | 17.17 (12.33–23.92) |
| Low risk (0) | 1.67 (1.47–1.89) | 1.28 (1.19–1.38) | 1.24 (1.16–1.33) |
| Intermediate risk (1) | 4.75 (4.45–5.07) | 3.70 (3.55–3.86) | 3.56 (3.42–3.70) |
| High risk (2–6) | 12.27 (11.84–12.71) | 8.30 (8.08–8.51) | 7.97 (7.77–8.17) |
| CHA2DS2-VASc risk score: | |||
| 0 | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| 1 | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| 2 | 3.71 (3.36–4.09) | 3.01 (2.83–3.20) | 2.92 (2.76–3.09) |
| 3 | 5.92 (5.53–6.34) | 4.41 (4.21–4.61) | 4.28 (4.10–4.47) |
| 4 | 9.27 (8.71–9.86) | 6.69 (6.41–6.99) | 6.46 (6.20–6.74) |
| 5 | 15.26 (14.35–16.24) | 10.42 (9.95–10.91) | 9.97 (9.53–10.43) |
| 6 | 19.74 (18.21–21.41) | 12.85 (12.07–13.69) | 12.52 (11.78–13.31) |
| 7 | 21.50 (18.75–24.64) | 13.92 (12.49–15.51) | 13.96 (12.57–15.51) |
| 8 | 22.38 (16.29–30.76) | 14.07 (10.80–18.33) | 14.10 (10.90–18.23) |
| 9 | 23.64 (10.62–52.61) | 16.08 (8.04–32.15) | 15.89 (7.95–31.78) |
| Low risk (0) | 0.78 (0.58–1.04) | 0.69 (0.59–0.81) | 0.66 (0.57–0.76) |
| Intermediate risk (1) | 2.01 (1.70–2.36) | 1.51 (1.37–1.67) | 1.45 (1.32–1.58) |
| High risk (2–9) | 8.82 (8.55–9.09) | 6.01 (5.88–6.14) | 5.72 (5.60–5.84) |
Event rates (95% CI) of hospital admission and death caused by thromboembolism (including peripheral artery embolism, ischaemic stroke and pulmonary embolism) per 100 person years, for each CHADS2 and CHA2DS2-VASc category. Risk profiles are largely similar with different lengths of follow-up. Adapted from Olesen et al., BMJ 201129.
Risk assessment for bleeding risks from anticoagulation
Bleeding is an important, potentially serious, side effect of anticoagulation and should be considered for all patients prior to treatment initiation.
Bleeding risk can be assessed with the HAS-BLED score, as introduced in the 2010 ESC Guidelines (see Table 3 for components of the HAS-BLED score).1,34 The score takes into account nine risk factors for bleeding that should be considered before starting antithrombotic treatment. The most important factor determining both stroke and bleeding risk appears to be age,2,35–38 which justifies its double weight in the CHA2DS2-VASc score. An observational retrospective study has confirmed increased bleeding rates in incremental HAS-BLED scores.35 Table 4 shows the incidence of major bleeding per HAS-BLED category as seen in non-selected patients receiving anticoagulation.
| Clinical characteristic . | . | . | Points . |
|---|---|---|---|
| Hypertension | Uncontrolled, >160 mmHg systolic | H | 1 |
| Abnormal renal and liver function | Dialysis, transplant, Cr ≥200 µmol/L, | A | 1 or 2 |
| (1 point each) | Cirrhosis, bilirubin >2× normal, AST/ALT/AP >3× normal | ||
| Stroke history | S | 1 | |
| Bleeding or predisposition to bleeding | B | 1 | |
| Labile INR | Unstable/high INRs, time in therapeutic range < 60% | L | 1 |
| Elderly | Age > 65 | E | 1 |
| Drugs or alcohol | Antiplatelet agents, NSAIDs | D | 1 or 2 |
| (1 point each) | ≥ 8 drinks/week |
| Clinical characteristic . | . | . | Points . |
|---|---|---|---|
| Hypertension | Uncontrolled, >160 mmHg systolic | H | 1 |
| Abnormal renal and liver function | Dialysis, transplant, Cr ≥200 µmol/L, | A | 1 or 2 |
| (1 point each) | Cirrhosis, bilirubin >2× normal, AST/ALT/AP >3× normal | ||
| Stroke history | S | 1 | |
| Bleeding or predisposition to bleeding | B | 1 | |
| Labile INR | Unstable/high INRs, time in therapeutic range < 60% | L | 1 |
| Elderly | Age > 65 | E | 1 |
| Drugs or alcohol | Antiplatelet agents, NSAIDs | D | 1 or 2 |
| (1 point each) | ≥ 8 drinks/week |
| Clinical characteristic . | . | . | Points . |
|---|---|---|---|
| Hypertension | Uncontrolled, >160 mmHg systolic | H | 1 |
| Abnormal renal and liver function | Dialysis, transplant, Cr ≥200 µmol/L, | A | 1 or 2 |
| (1 point each) | Cirrhosis, bilirubin >2× normal, AST/ALT/AP >3× normal | ||
| Stroke history | S | 1 | |
| Bleeding or predisposition to bleeding | B | 1 | |
| Labile INR | Unstable/high INRs, time in therapeutic range < 60% | L | 1 |
| Elderly | Age > 65 | E | 1 |
| Drugs or alcohol | Antiplatelet agents, NSAIDs | D | 1 or 2 |
| (1 point each) | ≥ 8 drinks/week |
| Clinical characteristic . | . | . | Points . |
|---|---|---|---|
| Hypertension | Uncontrolled, >160 mmHg systolic | H | 1 |
| Abnormal renal and liver function | Dialysis, transplant, Cr ≥200 µmol/L, | A | 1 or 2 |
| (1 point each) | Cirrhosis, bilirubin >2× normal, AST/ALT/AP >3× normal | ||
| Stroke history | S | 1 | |
| Bleeding or predisposition to bleeding | B | 1 | |
| Labile INR | Unstable/high INRs, time in therapeutic range < 60% | L | 1 |
| Elderly | Age > 65 | E | 1 |
| Drugs or alcohol | Antiplatelet agents, NSAIDs | D | 1 or 2 |
| (1 point each) | ≥ 8 drinks/week |
Incidence of major bleeds per HAS-BLED category as seen in non-selected AF patients receiving anticoagulation.
| HAS-BLED score . | Incidence (%/year) of major bleeding events . |
|---|---|
| 0 | 0 |
| 1 | 0.83 |
| 2 | 1.88 |
| 3 | 5.72 |
| 4 | 5.61 |
| ≥5 | 16.48 |
| HAS-BLED score . | Incidence (%/year) of major bleeding events . |
|---|---|
| 0 | 0 |
| 1 | 0.83 |
| 2 | 1.88 |
| 3 | 5.72 |
| 4 | 5.61 |
| ≥5 | 16.48 |
N=937 patients. Median follow-up was 952 (IQR 785–1074) days. C-statistic as a quantitative variable: 0.71 and 0.68 as a dichotomised variable. Adapted from Roldan et al., Chest 2013.41
Incidence of major bleeds per HAS-BLED category as seen in non-selected AF patients receiving anticoagulation.
| HAS-BLED score . | Incidence (%/year) of major bleeding events . |
|---|---|
| 0 | 0 |
| 1 | 0.83 |
| 2 | 1.88 |
| 3 | 5.72 |
| 4 | 5.61 |
| ≥5 | 16.48 |
| HAS-BLED score . | Incidence (%/year) of major bleeding events . |
|---|---|
| 0 | 0 |
| 1 | 0.83 |
| 2 | 1.88 |
| 3 | 5.72 |
| 4 | 5.61 |
| ≥5 | 16.48 |
N=937 patients. Median follow-up was 952 (IQR 785–1074) days. C-statistic as a quantitative variable: 0.71 and 0.68 as a dichotomised variable. Adapted from Roldan et al., Chest 2013.41
HAS-BLED should guide the patient and clinician to reduce modifiable bleeding risks (namely high blood pressure, liver and kidney function, INR control and use of interacting medications or alcohol), but not determine whether to offer anticoagulation or not – that decision is based on stroke risk estimation.
Falls are sometimes given as an argument not to anticoagulate a frail patient, yet evidence suggests that patients with low impact falls need to fall a great number of times before it actually increases the bleeding risk. Patient preferences should determine treatment; some patients are willing to endure many bleeds to avoid one stroke and its consequences, whereas others may accept stroke risk rather than the inconvenience of taking anticoagulants.39
In general, it should be noted that with an area under the curve below 70,38,40,41 these risk scores remain less than perfect predictors of individual risk.
Practical recommendations
Based on current evidence and experiences, we recommend the following stroke and bleeding risk assessment strategy in primary care in patients who have been diagnosed with AF:
Stroke and bleeding risk interventions that should be used
CHA2DS2-VASc score is superior to CHADS2 score for assessing stroke risk in AF, notably in identifying those that should not receive anticoagulation.
Alternatively, as CHADS2 is simpler to use, patients’ risk of stroke can be initially assessed using CHADS2 but if their score is 1 or less, then a CHA2DS2-VASC score should be performed to identify those patients who do not require anticoagulation.
Patients with a CHA2DS2-VASc score of 0 should not be offered antiplatelet or anticoagulation therapy.
Patients with a CHA2DS2-VASc score of 2 or above should be offered anticoagulation. In patients with a CHA2DS2-VASc score of 1, consider anticoagulation and base any decision to treat or not treat on patient preference after balancing the benefits with risks of treatment.
As a second step, HAS-BLED should be used to assess bleeding risk, with the aim of modifying this risk through addressing individual risk factors that can be altered.
HAS-BLED should not be used to decide whether to offer anticoagulation in someone with a CHA2DS2-VASc score of 2 or above, but consider its use to balance the benefits of anticoagulation in patients with a CHA2DS2-VASc score of 1.
On a regular basis, and at least once a year, the risk status of patients with AF should be re-evaluated depending on change in risk factors (change of age category, new hypertension, etc).
Alternative risk assessment approaches that may be used:
A more pragmatic strong risk application might simply be to consider age, as women over 65 with AF and an additional risk factor qualify for anticoagulation according to CHA2DS2-VASc stroke risk stratification, and so do men over 75.
People under 65 with no additional risk factor to their AF, in contrast, do not need anticoagulation.
Risk assessment should also be done in patients younger than 65 years of age who have multiple risk factors.
Additional work recommended in this area/gaps in the evidence:
See web version.
What are the management options to treat stroke risk in atrial fibrillation?
What is the evidence for anticoagulation in patients with atrial fibrillation in primary care?
Different antithrombotic strategies to prevent stroke in AF have been investigated over time. Early studies suggested that antiplatelet agents were effective in reducing stroke risk with one meta-analysis showing that acetyl salicylic acid (ASA) reduced the stroke rate by 22%,42 and addition of clopidogrel led to a risk reduction of 28%.43 Vitamin K antagonists show risk reductions of 66%.44,45
The BAFTA study specifically studied an elderly (≥75 years old) primary care population, and compared warfarin with ASA in a randomised controlled trial, showing that warfarin was much more effective than ASA at reducing stroke (see Table 5). Strokes occurring in the elderly are more likely to be embolic strokes.46 The BAFTA data are supported by the individual patient data (IPD) meta-analysis of the totality of available warfarin and aspirin data in preventing AF stroke by age, which showed that aspirin became less effective and more likely to cause bleeding with increasing age, with no benefits observed beyond age 75.47
Nature of primary events with warfarin or aspirin in an elderly community population with atrial fibrillation.
| . | Warfarin . | Aspirin . | Warfarin vs. aspirin . | |||
|---|---|---|---|---|---|---|
| . | n=488 . | n=485 . | . | |||
| . | n . | risk per year . | n . | risk per year . | RR (95% CI) . | p . |
| Stroke | 21 | 1.6% | 44 | 3.4% | 0.46 (0.26–0.79) | 0.003 |
| By severity | ||||||
| Fatal | 13 | 1.0% | 21 | 1.6% | 0.59 (0.27–1.24) | 0.14 |
| Disabling-non fatal | 8 | 0.6% | 23 | 1.8% | 0.33 (0.13–0.77) | 0.005 |
| Type of stroke | ||||||
| Ischaemic | 10 | 0.8% | 32 | 2.5% | 0.30 (0.13–0.63) | 0.0004 |
| Haemorrhagic | 6 | 0.5% | 5 | 0.4% | 1.15 (0.29–4.77) | 0.83 |
| Unknown | 5 | 0.4% | 7 | 0.5% | 0.69 (0.17–2.51) | 0.53 |
| Other intracranial haemorrhage | 2 | 0.2% | 1 | 0.1% | 1.92 (0.10–113.3) | 0.65 |
| Systemic embolism | 1 | 0.1% | 3 | 0.2% | 0.32 (0.01–3.99) | 0.36 |
| Total number of events | 24 | 1.8% | 48 | 3.8% | 0.48 (0.28–0.80) | 0.0027 |
| . | Warfarin . | Aspirin . | Warfarin vs. aspirin . | |||
|---|---|---|---|---|---|---|
| . | n=488 . | n=485 . | . | |||
| . | n . | risk per year . | n . | risk per year . | RR (95% CI) . | p . |
| Stroke | 21 | 1.6% | 44 | 3.4% | 0.46 (0.26–0.79) | 0.003 |
| By severity | ||||||
| Fatal | 13 | 1.0% | 21 | 1.6% | 0.59 (0.27–1.24) | 0.14 |
| Disabling-non fatal | 8 | 0.6% | 23 | 1.8% | 0.33 (0.13–0.77) | 0.005 |
| Type of stroke | ||||||
| Ischaemic | 10 | 0.8% | 32 | 2.5% | 0.30 (0.13–0.63) | 0.0004 |
| Haemorrhagic | 6 | 0.5% | 5 | 0.4% | 1.15 (0.29–4.77) | 0.83 |
| Unknown | 5 | 0.4% | 7 | 0.5% | 0.69 (0.17–2.51) | 0.53 |
| Other intracranial haemorrhage | 2 | 0.2% | 1 | 0.1% | 1.92 (0.10–113.3) | 0.65 |
| Systemic embolism | 1 | 0.1% | 3 | 0.2% | 0.32 (0.01–3.99) | 0.36 |
| Total number of events | 24 | 1.8% | 48 | 3.8% | 0.48 (0.28–0.80) | 0.0027 |
The BAFTA (Birmingham Atrial Fibrillation Treatment of the Aged) Study was a randomised controlled trial comparing warfarin (target INR 2.0–3.0) with aspirin (75 mg/day) for stroke prevention in atrial fibrillation in a community population over 75 years of age. Primary events are shown for both treatments, along with the relative risk (RR) for warfarin versus aspirin. Adapted from: Mant et al., Lancet 200746.
Nature of primary events with warfarin or aspirin in an elderly community population with atrial fibrillation.
| . | Warfarin . | Aspirin . | Warfarin vs. aspirin . | |||
|---|---|---|---|---|---|---|
| . | n=488 . | n=485 . | . | |||
| . | n . | risk per year . | n . | risk per year . | RR (95% CI) . | p . |
| Stroke | 21 | 1.6% | 44 | 3.4% | 0.46 (0.26–0.79) | 0.003 |
| By severity | ||||||
| Fatal | 13 | 1.0% | 21 | 1.6% | 0.59 (0.27–1.24) | 0.14 |
| Disabling-non fatal | 8 | 0.6% | 23 | 1.8% | 0.33 (0.13–0.77) | 0.005 |
| Type of stroke | ||||||
| Ischaemic | 10 | 0.8% | 32 | 2.5% | 0.30 (0.13–0.63) | 0.0004 |
| Haemorrhagic | 6 | 0.5% | 5 | 0.4% | 1.15 (0.29–4.77) | 0.83 |
| Unknown | 5 | 0.4% | 7 | 0.5% | 0.69 (0.17–2.51) | 0.53 |
| Other intracranial haemorrhage | 2 | 0.2% | 1 | 0.1% | 1.92 (0.10–113.3) | 0.65 |
| Systemic embolism | 1 | 0.1% | 3 | 0.2% | 0.32 (0.01–3.99) | 0.36 |
| Total number of events | 24 | 1.8% | 48 | 3.8% | 0.48 (0.28–0.80) | 0.0027 |
| . | Warfarin . | Aspirin . | Warfarin vs. aspirin . | |||
|---|---|---|---|---|---|---|
| . | n=488 . | n=485 . | . | |||
| . | n . | risk per year . | n . | risk per year . | RR (95% CI) . | p . |
| Stroke | 21 | 1.6% | 44 | 3.4% | 0.46 (0.26–0.79) | 0.003 |
| By severity | ||||||
| Fatal | 13 | 1.0% | 21 | 1.6% | 0.59 (0.27–1.24) | 0.14 |
| Disabling-non fatal | 8 | 0.6% | 23 | 1.8% | 0.33 (0.13–0.77) | 0.005 |
| Type of stroke | ||||||
| Ischaemic | 10 | 0.8% | 32 | 2.5% | 0.30 (0.13–0.63) | 0.0004 |
| Haemorrhagic | 6 | 0.5% | 5 | 0.4% | 1.15 (0.29–4.77) | 0.83 |
| Unknown | 5 | 0.4% | 7 | 0.5% | 0.69 (0.17–2.51) | 0.53 |
| Other intracranial haemorrhage | 2 | 0.2% | 1 | 0.1% | 1.92 (0.10–113.3) | 0.65 |
| Systemic embolism | 1 | 0.1% | 3 | 0.2% | 0.32 (0.01–3.99) | 0.36 |
| Total number of events | 24 | 1.8% | 48 | 3.8% | 0.48 (0.28–0.80) | 0.0027 |
The BAFTA (Birmingham Atrial Fibrillation Treatment of the Aged) Study was a randomised controlled trial comparing warfarin (target INR 2.0–3.0) with aspirin (75 mg/day) for stroke prevention in atrial fibrillation in a community population over 75 years of age. Primary events are shown for both treatments, along with the relative risk (RR) for warfarin versus aspirin. Adapted from: Mant et al., Lancet 200746.
Comorbidity in atrial fibrillation
Patients with AF often have other chronic conditions, which may be a cause or consequence of the arrhythmia or simply co-exist. Multi-morbidity increases with age, and is common in people over 65 years old. Patients between 65 and 84 were found to have on average 2.6 (SD 2.09) morbidities, and 64.9% (95% CI 64.7–65.1) of people in this age group have multi-morbidity. In patients of 85 years and older the mean number of morbidities is 3.62 (SD 2.30), and 81.5% (95% CI 81.1–81.9) of individuals have multi-morbidity. Socioeconomic deprivation is associated with earlier onset of multi-morbidity.21
Stroke risk management in atrial fibrillation patients with rate or rhythm control strategies
In symptomatic AF patients, it is important to optimise and control heart rate and it may also be appropriate to try to re-establish and maintain sinus-rhythm. Now that all guidelines advocate consideration of the use of antithrombotic therapy in all patients based on risk calculation, it is important to consider that control of rate and rhythm should only be attempted alongside controlling for the stroke risk. Importantly, antithrombotic therapy should be continued, even if rhythm control is obtained.14–17
Practical recommendations
Based on current evidence and experiences, we recommend the following stroke risk reduction management in primary care patients who have been diagnosed with AF:
Management strategies that should be used
Patients with a CHA2DS2-VASc score of 2 or above should be offered anticoagulation.
Management strategies that should not be used
Patients assessed as low risk on the CHA2DS2-VASc score (0) should not be offered antiplatelet or anticoagulation therapy, but may be offered standard advice regarding improving vascular risk factors (smoking cessation, BP and cholesterol control).
Patients with a CHA2DS2-VASc score of 1, should not be offered single antiplatelet therapy.
Patients at high stroke risk, with a CHA2DS2-VASc score of 2 or more, should not be offered single or dual antiplatelet therapy.
Management strategies that may be used
In patients with a CHA2DS2-VASc score of 1, consider anticoagulation and base any decision to treat or not to treat on patient preference after balancing the benefits with risks of treatment. In this situation, the decision to treat with anticoagulation or not should be based on patient preferences of their primary desire to reduce their stroke risk or to avoid bleeding risk. As their stroke risk is moderate rather than high, it is also very important to attempt to modify any bleeding risk factors.
Only in those intolerant of, or declining, anticoagulation may a combination of antiplatelets be considered (although the bleeding risk of this strategy will approach that of anticoagulation).
What are the therapeutic options to treat stroke risk in atrial fibrillation?
Although vitamin K antagonists (VKAs), and to a lesser extent other antithrombotic agents, importantly reduce the risk of stroke in patients with AF, they have several drawbacks, and a lot of effort has been dedicated to developing new anticoagulant agents. VKAs require intensive monitoring of international normalised ratio (INR) to ensure the drug is effective yet safe by maintaining figures within a therapeutic range (INR target 2.5, range 2–3 for NVAF). Moreover, it is important to achieve INR control above 65% time in therapeutic range (TTR), as shown in Figure 1. Four new oral anticoagulants (NOACs) are now available, namely dabigatran etexilate, a direct thrombin inhibitor,48,49 and the direct factor Xa inhibitors apixaban,50 rivaroxaban,51 and edoxaban.51,52 These newer agents offer additional options to current treatments for SPAF, as outlined below.
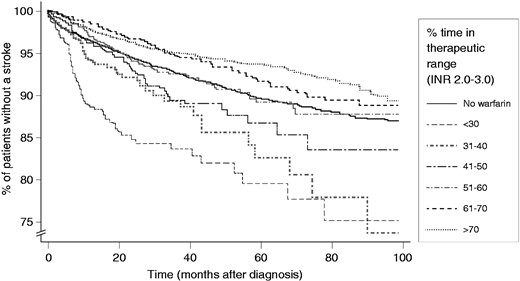
Percent of patients free from stroke over time, stratified by time spent in therapeutic range (INR 2.0–3.0). Adapted from Gallagher et al., Thromb and Haem 2011.60
Efficacy of NOACs versus warfarin in non-valvular AF
In recent years, new classes of anticoagulants have been developed and tested. Data of large trials on their efficacy and safety have been evaluated extensively, including ‘real life’ follow-up data. In several, large randomised controlled trials (RCTs) of AF patients without severe valve disease or mechanical valves, NOACs have been shown to be effective at reducing stroke risk.48–52 These RCTs were designed as non-inferiority studies; thus powered to show that NOACs are at least as good as warfarin in the prevention of stroke in AF. Fuller study details are available in the web version of this paper.
A systematic review53 that evaluated the results of the NOAC versus warfarin trials (RE-LY, ROCKET-AF and ARISTOTLE) concluded that overall mortality was decreased in patients with AF taking NOACs (risk difference estimated to be 8 [95% CI 3–11] fewer deaths per 1000 patients, RR 0.88, 95% CI 0.82–0.96). In the meta-analysis that also included ENGAGE AF-TIMI, all-cause mortality was also significantly reduced with NOACs (2022 events in 29292 patients [6.9%]) versus warfarin (2245 events in 29221 patients [7.7%], RR 0.90, 95% CI 0.85–0.95, p=0.0003).54
Safety of NOACs versus warfarin
Anticoagulation comes with an inherent bleeding risk, thus bleeding events were closely monitored and documented in the NOAC trials. When combining all data of the first three large trials, namely RELY, ROCKET-AF and ARISTOTLE, on direct thrombin inhibitors and Factor Xa inhibitors, fatal bleeds were found to be significantly reduced in comparison with anticoagulation with warfarin (RR 0.60, 95% CI 0.46–0.77, estimated risk difference is 1 fewer death per 1000 patients). Reduction in major bleeds did not reach statistical significance (RR 0.80, 95% CI 0.63–1.01),45,53 but a substantial reduction in intracranial haemorrhage was observed (204 events in 29287 [0.70%] patients on NOAC versus 425 events in 29211 [1.45%] patients on warfarin, RR 0.48, 95% CI 0.39–0.59, p<0.0001).52,54 The data of individual trials are summarised in Table 9 in the web version of this paper.
The meta-analysis of all NOAC RCTs also showed that NOACs were associated with a higher rate of GI bleeds (751 events in 29287 [2.6%] patients on NOACs vs. 591 events in 29211 [2.0%] warfarin-treated patients, RR 1.25, 95% CI 1.01–1.55, p=0.0430).52
Meta-analyses of the NOAC trials did not find a difference in the rate of MI with NOACs versus warfarin on combined data of all trials evaluated (RR 0.95, 95% CI 0.81–1.11,53 and RR 0.97, 95% CI 0.78–1.20 52).
NOACs and mechanical valves (without AF)
The recent RE-ALIGN study reported that dabigatran should not be used in patients with mechanical heart valves, as more thromboembolic and bleeding events were observed in patients on dabigatran than in the warfarin-treated patients (to a higher target INR than in NVAF). The same contraindication is currently extended to all NOACs in this ‘high flow’ setting.
Potential barriers to NOAC use
Reversibility of the anticoagulant effect
Although there are no specific antidotes yet for NOACs, nonspecific reversal strategies, such as administration of prothrombin complex or recombinant factor VII, may be applied.
A large advantage of NOACs in comparison with VKAs is that NOACs have a much shorter half-life. Thus, the duration of the effect is much shorter, thereby decreasing the need for an antidote. Clinical impact of major bleeds, as seen by, for instance, mortality, or the need for hospitalisation or intensive care unit stay, indeed appears to be less with the new oral anticoagulants than with VKAs, despite the fact that an antidote exists for the latter.55
Specific antidotes are currently under development, such as an antibody against dabigatran 56 and recombinant factor Xa.57
Renal impairment
Severe chronic kidney disease, defined as an eGFR<30 ml/min or <15 ml/min depending on type of NOAC, is a contraindication to NOAC use. There are limited trial data for VKA and NOACs in frail elderly patients.
Costs
Although NOACs may still be more expensive than VKA, INR monitoring is also expensive. Logistics and available resources may vary between countries or by region and will determine specific cost-effect balances. Cost-effectiveness of AF-related stroke prevention strategies is being explored, and efforts are dedicated to determine the impact of NOACs on health economics. Available evidence suggests that NOACs are cost-effective alternatives to warfarin with regard to efficacy and safety, although the final balance will depend on individual healthcare settings.58 Comparisons between NOACs are emerging, but are based on indirect comparisons.
Treatment adherence
Because of the shorter half-life of NOACs compared with VKAs, compliance is very important. It is therefore vital for physicians to emphasise the need for daily treatment adherence.
An advantage of NOACs may lie in their fixed dose, compared with VKA doses that need to be adjusted based on INR measurements. However, this lack of need for monitoring drug levels reinforces the importance of stressing drug adherence with patients. It should not be underestimated that although the new agents are more convenient, they are still powerful anticoagulants. A dosage box with all medications to dispense may facilitate compliance (although not for dabigatran which requires original packaging). Rivaroxaban needs to be taken with food as taking it on an empty stomach may reduce therapeutic drug levels by up to 40%.
Real-life data
Observational studies comparing the actual impact of NOAC use in daily clinical care are now emerging; some show similar and comparable results on the efficacy and safety of NOAC use. From a primary care perspective, such observational studies will help show efficacy and safety of NOAC use in frail elderly patients not included in the large trials.
In summary, NOACs are at least as safe as VKA. Barriers for implementation still exist but these may improve with the growing body of trial data on safety and subgroup analyses. Moreover, generalisability may expand as a result of having more registries. In addition, growing confidence in reversal strategies should facilitate implementation.
Practical recommendations
Based on current evidence and experiences, we recommend the following anticoagulation treatment strategy in a primary care setting:
Management strategies that should be used
All AF patients at high risk of stroke should be offered anticoagulation.
In patients with mechanical valves or severe valve disease (defined by a specialist), this anticoagulation should be high-intensity VKA.
For patients with AF but without mechanical valves or clinically significant valve disease, both warfarin, to adjusted INR target of 2.5, and NOACs are anticoagulant options.
NOACs may represent a more convenient, at least as safe, and at least equally as effective option in the prevention of stroke in AF compared with VKAs. Treatment adherence is an important issue with NOAC use. Thus, on the basis of these issues, as well as cost and access, NOACs and VKAs both represent good treatment options for SPAF.
Patients should be fully counselled, including written information, on the risks and benefits of anticoagulation or on changing to or initiating a NOAC.
Patient preferences should guide decision-making over whether to initiate anticoagulation, and on what to prescribe, including estimation of a patient’s compliance.
The patient groups in whom use of a NOAC is preferable to warfarin are patients who are unable or unwilling to take warfarin, and patients who are difficult to maintain at a stable INR (less than 65% time in therapeutic range).
If prescribing NOACs, the importance of treatment adherence must be emphasised. Compliance may be facilitated with the use of a dosage box, except for dabigatran which should only be dispensed in its original packaging (although dabigatran is available in blister packages so, if desired, individual doses can be cut out with their original blister preserved and put in dosage box).
Management strategies that may be used
ASA alone has no role in SPAF.
In patients at all ages who are unable or unwilling to take VKAs or NOACs, ASA may be used in combination with clopidogrel as antiplatelet therapy to prevent stroke.
Additional work recommended in this area/gaps in the evidence
See web version.
Practical considerations for atrial fibrillation stroke prevention in primary care
A large evidence base on the effectiveness of warfarin regimes, the limitations of antiplatelets, and the licensing of several new anticoagulants, has meant that traditional management strategies of SPAF have been challenged in recent years. The practicalities of screening, diagnosis, stroke risk assessment, treatment initiation, monitoring and, where appropriate, cessation of therapy will need to be determined at a local level according to the structure of the healthcare system and expertise of the team. The full web version of this paper provides a list of practical guidance on: Finding the patient with atrial fibrillation; Making the diagnosis of atrial fibrillation; Risk assessment; Preferable anticoagulant; NOAC use with impaired renal function; and initiating and monitoring treatment.
Practical guidance on the use of NOACs
The European Heart Rhythm Association (EHRA) has assembled an excellent practical guide on the use of NOACs, to help physicians use NOACs in specific clinical situations.59 The different clinical scenarios as outlined in the EHRA practical guide, which are useful for both physician and patients to learn how to use these new agents safely and effectively, are summarised in the web version.
All recommendations given in this consensus document are summarised in the flow chart in Figure 2 for easy reference.
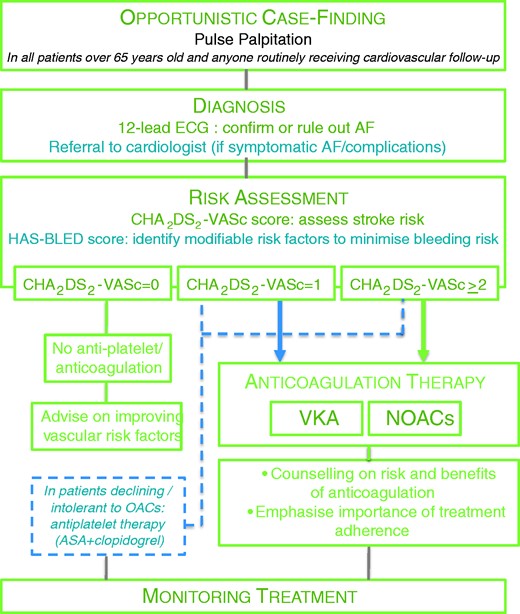
Flow chart of recommendations. Management of stroke prevention in atrial fibrillation as recommended in this document. Strength of recommendations is indicated by colour, with recommendations that should be used in green, and interventions that may be considered in blue. See text for explanation of HAS-BLED and CHA2DS2-VASc scores. AF: atrial fibrillation; OACs: oral anticoagulants; NOACs: novel oral anticoagulants; ASA: acetyl salicylic acid; VKA: vitamin K antagonists.
Conclusions
Atrial fibrillation is a common disorder, especially in those aged over 75, and is a major cause of preventable embolic stroke. Anticoagulation therapy, available for 50 years as vitamin K antagonist derivatives like warfarin, can reduce this risk by up to two-thirds, but its use has been complicated by major food and drug interactions, significant bleeding risks, a narrow therapeutic range and the need to monitor. As a consequence, in most countries, only around half of those eligible for anticoagulation are on treatment and many of those treated are poorly controlled. The evidence base for atrial fibrillation stroke risk is considerable and growing, resulting in recent major changes to clinical guidance internationally. This guidance has particular implications for primary care with many new recommendations on better AF diagnosis, more reliable methods of determining stroke risk to guide treatment choice for patients and bleeding risks to manage the risk factors better, and revised treatment options, with the exclusion of low-dose aspirin as an option for most patients, the importance of good therapeutic control if warfarin is used, and the availability of a range of new rapid-onset, short-acting, anticoagulants with few interactions and no monitoring requirements.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The idea, rationale, and methods in generating the guidance originated with the EPCCS. All preparation, presentations, document drafting and printing was done by the EPCCS and its Secretariat. The EPCCS approached the sponsors. The sponsors had no input or role in producing or commenting on the guidance. A full list of competing interests is available in the web version.
Funding
The costs of producing this document were met by the EPCCS which itself received an unrestricted educational grant from Bayer, Boehringer Ingelheim and Pfizer/BMS to support these costs. FDR Hobbs is supported by NIHR BRC and CLAHRC.




Comments