-
PDF
- Split View
-
Views
-
Cite
Cite
Hans-Christoph Diener, James Aisenberg, Jack Ansell, Dan Atar, Günter Breithardt, John Eikelboom, Michael D. Ezekowitz, Christopher B. Granger, Jonathan L. Halperin, Stefan H. Hohnloser, Elaine M. Hylek, Paulus Kirchhof, Deirdre A. Lane, Freek W.A. Verheugt, Roland Veltkamp, Gregory Y.H. Lip, Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2, European Heart Journal, Volume 38, Issue 12, 21 March 2017, Pages 860–868, https://doi.org/10.1093/eurheartj/ehw069
Close - Share Icon Share
The choice of oral anticoagulant (OAC) for patients with atrial fibrillation (AF) may be influenced by individual clinical features or by patterns of risk factors and comorbidities. We reviewed analyses of subgroups of patients from trials of vitamin K antagonists vs. non-vitamin K oral anticoagulants (NOACs) for stroke prevention in AF with the aim to identify patient groups who might benefit from a particular OAC more than from another. In addition, we discuss the timing of initiation of anticoagulation. In the second of a two-part review, we discuss the use of NOAC for stroke prevention in the following subgroups of patients with AF: (vii) secondary stroke prevention in patients after stroke or transient ischaemic attack (TIA), (viii) patients with acute stroke requiring thrombolysis or thrombectomy, (ix) those initiating or restarting OAC treatment after stroke or TIA, (x) those with renal impairment on dialysis, (xi) the elderly, (xii) those at high risk of gastrointestinal bleeding, and (xiii) those with hypertension. In addition, we discuss adherence and compliance. Finally, we present a summary of treatment suggestions. In specific subgroups of patients with AF, evidence supports the use of particular NOACs and/or particular doses of anticoagulant. The appropriate choice of treatment for these subgroups will help to promote optimal clinical outcomes.
Introduction
This review, like part 1, is based on sub-analyses of the major trials of non-vitamin K oral anticoagulants (NOACs).1–4 In the absence of data from the main trials, our suggestions are based on expert opinion only. On the basis of our review of the data, we offer suggestions—reflecting a consensus of the authors—for choice of NOAC and/or dose in subgroups of patients with atrial fibrillation (AF) and the timing of initiation of anticoagulation after stroke or intracranial bleeding. There is a clear need to evaluate some of the proposed management strategies in prospective, randomized trials. At the time of writing of this report, there is insufficient evidence to support firm recommendations. This report is thus meant to support clinical decision making when used in conjunction with treatment guidelines5 and the European Heart Rhythm Association guide on practical aspects of NOAC therapy.6 We added a section on adherence and reversal agents. In the future, when more data are available these issues might have an impact on the choice of OAC and treatment decisions may change.
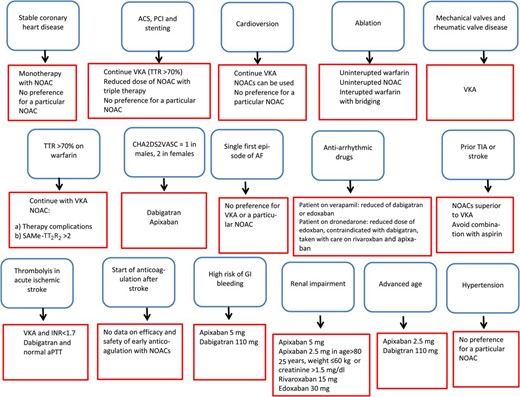
Secondary stroke prevention
Warfarin is superior to aspirin and placebo in prevention of recurrent stroke after transient ischaemic attack (TIA) or stroke in patients with AF.7 All randomized trials comparing NOACs with warfarin had subgroups of patients with prior stroke or TIA.8–10 Detailed data for edoxaban have not been published.4 The AVERROES trial, which compared apixaban with aspirin in patients with AF, also had a secondary stroke prevention subgroup.11 The stroke–TIA subgroups were too small to allow statistical comparisons of the NOACs with warfarin, but tests of heterogeneity found no differences in safety or efficacy among patients with and without prior stroke or TIA. In a meta-analysis of 14 527 patients with prior stroke or TIA from RE-LY, ARISTOTLE, and ROCKET AF, NOACs were associated with a significant reduction in the incidence of stroke and systemic embolism compared with warfarin [odds ratio (OR) 0.85; 95% confidence interval (CI) 0.74–0.99].12 The NOACs were also associated with less major bleeding than warfarin (OR 0.86; 95% CI 0.75–0.99), mainly due to a reduction in the incidence of haemorrhagic stroke (OR 0.44; 95% CI 0.32–0.62).12 It should be noted, however, that the time in therapeutic range for the warfarin-treated patients in these trials was on average <70%. For secondary stroke prevention, apixaban superior efficacy compared with aspirin [hazard ratio (HR) 0.29; 95% CI 0.15–0.60], with a comparable risk of bleeding.11
After TIA or stroke, combination therapy with an OAC and antiplatelet agent is not advisable. Compared with an OAC alone, combination therapy did not prevent ischaemic endpoints, but increased the risk of major bleeding.13 For patients suffering ischaemic stroke or TIA during well-titrated warfarin therapy, substitution with an NOAC is reasonable.
Based on our interpretation of available data we suggest:
| First choice | NOACs as a group are superior to warfarin for secondary stroke prevention in patients with AF |
| Comment | Aspirin should not be used for secondary stroke prevention in patients with AF. The combination of antiplatelet therapy plus OAC in patients with AF does not prevent major ischaemic events better than does OAC monotherapy and should be restricted to specific high-risk periods |
| First choice | NOACs as a group are superior to warfarin for secondary stroke prevention in patients with AF |
| Comment | Aspirin should not be used for secondary stroke prevention in patients with AF. The combination of antiplatelet therapy plus OAC in patients with AF does not prevent major ischaemic events better than does OAC monotherapy and should be restricted to specific high-risk periods |
| First choice | NOACs as a group are superior to warfarin for secondary stroke prevention in patients with AF |
| Comment | Aspirin should not be used for secondary stroke prevention in patients with AF. The combination of antiplatelet therapy plus OAC in patients with AF does not prevent major ischaemic events better than does OAC monotherapy and should be restricted to specific high-risk periods |
| First choice | NOACs as a group are superior to warfarin for secondary stroke prevention in patients with AF |
| Comment | Aspirin should not be used for secondary stroke prevention in patients with AF. The combination of antiplatelet therapy plus OAC in patients with AF does not prevent major ischaemic events better than does OAC monotherapy and should be restricted to specific high-risk periods |
Patients with acute stroke requiring thrombolysis or thrombectomy
Anticoagulants, including NOACs, present special challenges for the emergency management of ischaemic stroke. Intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) within 4.5 h after symptom onset is currently the only licensed medical therapy for stroke. As per licence, anticoagulation is a contraindication to thrombolysis because it can increase the risk of intracerebral haemorrhage.
In a recent series, almost 10% of acute ischaemic stroke patients were taking vitamin K antagonists (VKAs) at the time of the event.14 However, up to 20% of patients with acute stroke are unable to convey information about anticoagulation status when presenting in the emergency room. Rapid assessment of coagulation status at presentation is necessary to guide a decision for or against thrombolysis. For those taking a VKA, this can be done quickly by using a point-of-care device to measure the international normalized ratio (INR).15 Beyond the qualitative determination of whether a patient is anticoagulated, the threshold intensity at which thrombolysis can safely be used is uncertain.16 Data from two large observational registries in the USA and Europe suggest that thrombolysis does not increase the risk of intracerebral haemorrhagic complications in patients on VKA when the INR is ≤1.7.17,18
In randomized trials of anticoagulation, the annual risk of ischaemic stroke among patients with AF ranged from 1–2% for primary to 2–3% for secondary stroke prevention.19 Experience with patients taking VKA suggests that low levels or an absence of anticoagulation with NOACs might allow thrombolysis with rtPA. Extrapolation of intracerebral haemorrhagic risk may not be appropriate, and safety thresholds for the NOACs have not been established. An observational study in 78 patients on NOACs undergoing systemic thrombolysis and or thrombectomy showed no increased bleeding risk.20 During long-term therapy, the risk of spontaneous intracerebral haemorrhage in patients treated with NOACs was consistently about half that during VKA therapy, and pharmacodynamic differences may contribute to this difference in rates of intracranial haemorrhage (ICH).19 In preclinical experiments, haemorrhagic transformation of brain infarcts after thrombolysis is elevated in rodents exposed to VKA but not in those given NOACs when compared with animals that were not anticoagulated.21,22
Management of ischaemic stroke in patients treated with NOACs must balance efficacy against safety concerns.23–25 Currently, no emergency point-of-care test is available to test quantitatively for the anticoagulant effect of any of the NOACs. For dabigatran, the activated partial thromboplastin time can be used as a qualitative screening test. Diluted thrombin time or the ecarin clotting-time assays allow quantitative assessment of anticoagulation intensity corresponding to dabigatran plasma levels. For rivaroxaban, apixaban, and edoxaban, substance-specific Factor Xa assays are needed. The EHRA recommendations have defined levels of anticoagulant effect that are deemed to be safe for intravenous thrombolysis, but confirmation of safety is needed.25 In view of the relatively short half-life of NOACs in patients with normal renal clearance, another approach is to consider thrombolysis only when more than 2–4 half-lives have elapsed since NOAC dosing. Interventional mechanical thrombectomy is strongly recommended in anticoagulated patients with proximal intracranial vessel occlusion.25 Finally, the advent of specific reversal agents for NOACs, without prothrombotic side effects, may in the future allow rapid termination of the anticoagulant effect before starting thrombolysis. Whether this approach is safe and feasible needs to be determined. Evidence from large prospective registries is needed to evaluate this important management issue.
Based on our interpretation of available data we suggest:
| Choice of treatment | After careful assessment of potential risks and benefits of intravenous thrombolysis, rtPA may be given if coagulation tests specific for the individual NOAC reveal low or absent anticoagulant intensity (off label) |
| Interventional mechanical thrombectomy is an alternative to pharmacological thrombolysis for patients with acute ischaemic stroke with proximal intracranial arterial occlusions who are effectively anticoagulated with an NOAC |
| Choice of treatment | After careful assessment of potential risks and benefits of intravenous thrombolysis, rtPA may be given if coagulation tests specific for the individual NOAC reveal low or absent anticoagulant intensity (off label) |
| Interventional mechanical thrombectomy is an alternative to pharmacological thrombolysis for patients with acute ischaemic stroke with proximal intracranial arterial occlusions who are effectively anticoagulated with an NOAC |
| Choice of treatment | After careful assessment of potential risks and benefits of intravenous thrombolysis, rtPA may be given if coagulation tests specific for the individual NOAC reveal low or absent anticoagulant intensity (off label) |
| Interventional mechanical thrombectomy is an alternative to pharmacological thrombolysis for patients with acute ischaemic stroke with proximal intracranial arterial occlusions who are effectively anticoagulated with an NOAC |
| Choice of treatment | After careful assessment of potential risks and benefits of intravenous thrombolysis, rtPA may be given if coagulation tests specific for the individual NOAC reveal low or absent anticoagulant intensity (off label) |
| Interventional mechanical thrombectomy is an alternative to pharmacological thrombolysis for patients with acute ischaemic stroke with proximal intracranial arterial occlusions who are effectively anticoagulated with an NOAC |
Patients initiating or restarting anticoagulant treatment after transient ischaemic attack or ischaemic stroke
No prospective studies have investigated the risk or benefit of initiation or resumption of OAC treatment, including NOAC therapy, early after TIA or ischaemic stroke in patients with AF. Patients with a TIA or stroke within the past 7–30 days were excluded from the randomized NOAC trials.1–4 Therefore, recommendations on the initiation of anticoagulation, based on the EHRA consensus opinion, accord with the 1–3–6–12 day rule.6 In patients with TIA, OAC can begin on Day 1 after exclusion of intracerebral haemorrhage by brain imaging (CT or MRI). In patients with mild stroke and small ischaemic defect initiation of OAC can start on Day 3. The beginning of OAC should be delayed by 6 days in patients with moderate strokes and by 12–14 days in patients with severe strokes. Additional factors to consider are the size of the infarct on brain imaging and risk factors for bleeding such as advanced age, uncontrolled hypertension, severe small vessel disease, and need for triple antithrombotic therapy in patients with a recent acute coronary syndrome or coronary stent. Whether this concept is valid at present under investigation in large prospective registries.
Based on our interpretation of available data we suggest:
| Timing of treatment according to the 1–3–6–12 day rule | In patients with AF and TIA, OAC including NOACAs treatment may be initiated on the first day after neuroimaging has excluded ICH. The 1–3–6–12 day rule is not based on evidence and has not been derived from controlled trials |
| In patients with mild ischaemic stroke, OAC treatment may be initiated after 3 days. | |
| In patients with strokes of moderate severity, anticoagulation may be started after 5–7 days. | |
| In patients with severe strokes, anticoagulation may be initiated after 12–14 days. | |
| Comment | Brain imaging should be repeated before anticoagulation in patients with moderate or severe stroke to exclude haemorrhagic transformation |
| Timing of treatment according to the 1–3–6–12 day rule | In patients with AF and TIA, OAC including NOACAs treatment may be initiated on the first day after neuroimaging has excluded ICH. The 1–3–6–12 day rule is not based on evidence and has not been derived from controlled trials |
| In patients with mild ischaemic stroke, OAC treatment may be initiated after 3 days. | |
| In patients with strokes of moderate severity, anticoagulation may be started after 5–7 days. | |
| In patients with severe strokes, anticoagulation may be initiated after 12–14 days. | |
| Comment | Brain imaging should be repeated before anticoagulation in patients with moderate or severe stroke to exclude haemorrhagic transformation |
| Timing of treatment according to the 1–3–6–12 day rule | In patients with AF and TIA, OAC including NOACAs treatment may be initiated on the first day after neuroimaging has excluded ICH. The 1–3–6–12 day rule is not based on evidence and has not been derived from controlled trials |
| In patients with mild ischaemic stroke, OAC treatment may be initiated after 3 days. | |
| In patients with strokes of moderate severity, anticoagulation may be started after 5–7 days. | |
| In patients with severe strokes, anticoagulation may be initiated after 12–14 days. | |
| Comment | Brain imaging should be repeated before anticoagulation in patients with moderate or severe stroke to exclude haemorrhagic transformation |
| Timing of treatment according to the 1–3–6–12 day rule | In patients with AF and TIA, OAC including NOACAs treatment may be initiated on the first day after neuroimaging has excluded ICH. The 1–3–6–12 day rule is not based on evidence and has not been derived from controlled trials |
| In patients with mild ischaemic stroke, OAC treatment may be initiated after 3 days. | |
| In patients with strokes of moderate severity, anticoagulation may be started after 5–7 days. | |
| In patients with severe strokes, anticoagulation may be initiated after 12–14 days. | |
| Comment | Brain imaging should be repeated before anticoagulation in patients with moderate or severe stroke to exclude haemorrhagic transformation |
Patients with a high risk of gastrointestinal bleeding
Several of the NOACs increase the risk of major gastrointestinal bleeding (MGIB) relative to adjusted-dose warfarin in patients with AF. In RE-LY, dabigatran 150 mg twice daily was associated with a higher rate of MGIB compared with warfarin [relative risk (RR) 1.50], but the MGIB risk with dabigatran 110 mg twice daily was comparable with that of warfarin (RR 1.10).1 An increased RR of MGIB with dabigatran was seen only in patients aged ≥75 years26 and with respect to lower but not upper gastrointestinal bleeding.26
Most post-market studies confirm the RRs of MGIB seen in RE-LY. A propensity-matched analysis from the US Center for Medicare and Medicaid Services (CMS) database showed an increased risk of MGIB in patients receiving dabigatran (pooled data from 150 to 75 mg twice daily doses) compared with warfarin (HR 1.28).27 The increased risk in dabigatran users involved women aged ≥75 years and men aged ≥85 years. The MGIB rate in patients taking dabigatran 75 mg twice daily was comparable with that of warfarin (HR 1.01). A US Veteran's Affairs database study (pooled dabigatran doses) showed an increased rate of MGIB among warfarin users who switched to dabigatran compared with those who remained on warfarin.28 A smaller non-FDA CMS database study confirmed an increased rate of MGIB with dabigatran (pooled doses) compared with warfarin.29 Two population-based cohort studies in US subjects suggest that the increased MGIB risk for dabigatran vs. warfarin involves mainly patients aged >75 years.30,31 However, two observational studies from Denmark failed to confirm excess MGIB with dabigatran compared with warfarin.32,33 A community-based study suggested that dabigatran-related gastrointestinal bleeding was associated with clinical outcomes comparable with those of warfarin-related bleeding.34 A study from Hong Kong in 5041 patients newly prescribed dabigatran showed a reduced risk of gastrointestinal bleeding in patients taking gastroprotective agents.35
In ROCKET AF, patients receiving rivaroxaban 20 mg once daily had a significantly higher risk of MGIB than did those on warfarin (3.2 vs. 2.2%; P < 0.001),3 but the incidence of both life-threatening and fatal gastrointestinal bleeds was similar in the two arms.36 In ROCKET AF, a greater MGIB risk was noted with rivaroxaban compared with warfarin in patients aged ≥75 years.37 This interaction between age and MGIB risk was confirmed in a population-based cohort study.30 Rivaroxaban has been associated with upper gastrointestinal bleeding more frequently than lower gastrointestinal bleeding.38 However, two studies failed to confirm a significant difference in RR of MGIB between rivaroxaban and warfarin.39,40
The ARISTOTLE trial showed a comparable rate of MGIB in the apixaban 5 mg twice daily and the warfarin arms (HR 0.89).2 The ENGAGE AF study showed an increased risk with high-dose edoxaban (60 mg daily) vs. warfarin (HR 1.23), with comparable HRs for upper and lower gastrointestinal bleeding. On the other hand, low-dose edoxaban (30 mg daily) was associated with a decreased risk of MGIB (HR 0.67).4 Sub-analyses and post-market data regarding MGIB with apixaban or edoxaban are not yet available.
Based on our interpretation of available data we suggest:
| First choice | For patients with a high risk of gastrointestinal bleeding, apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used |
| Second choice | Dabigatran 150 mg twice daily, edoxaban 60 mg once daily, or rivaroxaban 20 mg once daily |
| Comments | Gastrointestinal bleeding, even in the setting of anticoagulation, does usually not cause death or permanent major disability. Thus, the choice of OAC should be driven mainly by stroke prevention considerations. The label ‘high risk of gastrointestinal bleeding’ is imprecise. For example, patients with H. pylori-related ulcer haemorrhage may no longer be at high risk of bleeding once the infection has been eradicated. The gastrointestinal bleeding risk associated with any anticoagulant is increased by concurrent use of antiplatelet agents, including aspirin.41 As with warfarin, NOAC agents should be restarted as soon as deemed safe to do so once gastrointestinal bleeding has been controlled. The gastrointestinal bleeding risk of dabigatran and edoxaban are dose-dependent. The increased gastrointestinal bleeding risk of dabigatran and rivaroxaban are most evident in patients ≥75 years old. Gastrointestinal tract cancer screening and surveillance strategies (e.g. colonoscopy) increase early detection of occult tumours and may thereby reduce the incidence of neoplasm-associated gastrointestinal bleeding in patients receiving OACs.42 Age-appropriate colorectal cancer screening should be undertaken prior to initiation of OAC43 |
| First choice | For patients with a high risk of gastrointestinal bleeding, apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used |
| Second choice | Dabigatran 150 mg twice daily, edoxaban 60 mg once daily, or rivaroxaban 20 mg once daily |
| Comments | Gastrointestinal bleeding, even in the setting of anticoagulation, does usually not cause death or permanent major disability. Thus, the choice of OAC should be driven mainly by stroke prevention considerations. The label ‘high risk of gastrointestinal bleeding’ is imprecise. For example, patients with H. pylori-related ulcer haemorrhage may no longer be at high risk of bleeding once the infection has been eradicated. The gastrointestinal bleeding risk associated with any anticoagulant is increased by concurrent use of antiplatelet agents, including aspirin.41 As with warfarin, NOAC agents should be restarted as soon as deemed safe to do so once gastrointestinal bleeding has been controlled. The gastrointestinal bleeding risk of dabigatran and edoxaban are dose-dependent. The increased gastrointestinal bleeding risk of dabigatran and rivaroxaban are most evident in patients ≥75 years old. Gastrointestinal tract cancer screening and surveillance strategies (e.g. colonoscopy) increase early detection of occult tumours and may thereby reduce the incidence of neoplasm-associated gastrointestinal bleeding in patients receiving OACs.42 Age-appropriate colorectal cancer screening should be undertaken prior to initiation of OAC43 |
| First choice | For patients with a high risk of gastrointestinal bleeding, apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used |
| Second choice | Dabigatran 150 mg twice daily, edoxaban 60 mg once daily, or rivaroxaban 20 mg once daily |
| Comments | Gastrointestinal bleeding, even in the setting of anticoagulation, does usually not cause death or permanent major disability. Thus, the choice of OAC should be driven mainly by stroke prevention considerations. The label ‘high risk of gastrointestinal bleeding’ is imprecise. For example, patients with H. pylori-related ulcer haemorrhage may no longer be at high risk of bleeding once the infection has been eradicated. The gastrointestinal bleeding risk associated with any anticoagulant is increased by concurrent use of antiplatelet agents, including aspirin.41 As with warfarin, NOAC agents should be restarted as soon as deemed safe to do so once gastrointestinal bleeding has been controlled. The gastrointestinal bleeding risk of dabigatran and edoxaban are dose-dependent. The increased gastrointestinal bleeding risk of dabigatran and rivaroxaban are most evident in patients ≥75 years old. Gastrointestinal tract cancer screening and surveillance strategies (e.g. colonoscopy) increase early detection of occult tumours and may thereby reduce the incidence of neoplasm-associated gastrointestinal bleeding in patients receiving OACs.42 Age-appropriate colorectal cancer screening should be undertaken prior to initiation of OAC43 |
| First choice | For patients with a high risk of gastrointestinal bleeding, apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used |
| Second choice | Dabigatran 150 mg twice daily, edoxaban 60 mg once daily, or rivaroxaban 20 mg once daily |
| Comments | Gastrointestinal bleeding, even in the setting of anticoagulation, does usually not cause death or permanent major disability. Thus, the choice of OAC should be driven mainly by stroke prevention considerations. The label ‘high risk of gastrointestinal bleeding’ is imprecise. For example, patients with H. pylori-related ulcer haemorrhage may no longer be at high risk of bleeding once the infection has been eradicated. The gastrointestinal bleeding risk associated with any anticoagulant is increased by concurrent use of antiplatelet agents, including aspirin.41 As with warfarin, NOAC agents should be restarted as soon as deemed safe to do so once gastrointestinal bleeding has been controlled. The gastrointestinal bleeding risk of dabigatran and edoxaban are dose-dependent. The increased gastrointestinal bleeding risk of dabigatran and rivaroxaban are most evident in patients ≥75 years old. Gastrointestinal tract cancer screening and surveillance strategies (e.g. colonoscopy) increase early detection of occult tumours and may thereby reduce the incidence of neoplasm-associated gastrointestinal bleeding in patients receiving OACs.42 Age-appropriate colorectal cancer screening should be undertaken prior to initiation of OAC43 |
Patients with renal impairment and on dialysis
Chronic kidney disease (CKD) is an important risk factor for both stroke and bleeding in anticoagulated patients with AF.4,44–48 Each of the NOACs is eliminated via the kidneys to some degree: 80% for dabigatran, 50% for edoxaban, 33% for rivaroxaban, and 27% for apixaban. This results in substantially different plasma concentrations across the spectrum of creatinine clearance. For example, the area under the plasma concentration curve for dabigatran is 3.2 times greater in a patient with a creatinine clearance of 30 mL/min than in a patient with a clearance of 80 mL/min (US Dabigatran FDA Package Insert: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022512s000lbl.pdf). This relationship between NOAC plasma concentration and kidney function underlies the advice to reduce the doses of each of the NOACs in patients with CKD, as shown in Table1 (see also the EHRA practical guide).6
Dose reduction of non-vitamin K oral anticoagulants for reduced creatinine clearance
| Drug . | Dose reduction criteria . | Reduced dose . |
|---|---|---|
| Dabigatran | Creatinine clearance <50 mL/min | 110 mg twice a day is recommended in ESC guidelines |
| Rivaroxaban | Creatinine clearance <50 mL/min | Use 15 mg once a day |
| Apixaban | 2 of three criteria: age ≥80 years, weight ≤60 kg, creatinine ≥1.5 mg/dL | Use 2.5 mg twice a day |
| Edoxaban | Creatinine clearance ≤50 mL/min | Use 30 mg once a day |
| Drug . | Dose reduction criteria . | Reduced dose . |
|---|---|---|
| Dabigatran | Creatinine clearance <50 mL/min | 110 mg twice a day is recommended in ESC guidelines |
| Rivaroxaban | Creatinine clearance <50 mL/min | Use 15 mg once a day |
| Apixaban | 2 of three criteria: age ≥80 years, weight ≤60 kg, creatinine ≥1.5 mg/dL | Use 2.5 mg twice a day |
| Edoxaban | Creatinine clearance ≤50 mL/min | Use 30 mg once a day |
ESC, European Society of Cardiology.
Dose reduction of non-vitamin K oral anticoagulants for reduced creatinine clearance
| Drug . | Dose reduction criteria . | Reduced dose . |
|---|---|---|
| Dabigatran | Creatinine clearance <50 mL/min | 110 mg twice a day is recommended in ESC guidelines |
| Rivaroxaban | Creatinine clearance <50 mL/min | Use 15 mg once a day |
| Apixaban | 2 of three criteria: age ≥80 years, weight ≤60 kg, creatinine ≥1.5 mg/dL | Use 2.5 mg twice a day |
| Edoxaban | Creatinine clearance ≤50 mL/min | Use 30 mg once a day |
| Drug . | Dose reduction criteria . | Reduced dose . |
|---|---|---|
| Dabigatran | Creatinine clearance <50 mL/min | 110 mg twice a day is recommended in ESC guidelines |
| Rivaroxaban | Creatinine clearance <50 mL/min | Use 15 mg once a day |
| Apixaban | 2 of three criteria: age ≥80 years, weight ≤60 kg, creatinine ≥1.5 mg/dL | Use 2.5 mg twice a day |
| Edoxaban | Creatinine clearance ≤50 mL/min | Use 30 mg once a day |
ESC, European Society of Cardiology.
With the dose reductions (based at least in part on renal function) that were part of the protocols in three of the four warfarin-comparator trials, the results were consistent for patients with creatinine clearance of 30–49 mL/min.46–48 These findings provide confidence that NOACs can be safe and effective, compared with warfarin, for patients with moderate renal impairment. The AVERROES trial found that the benefit of apixaban compared with aspirin was similar in patients with and without Stage III CKD.45 In the ARISTOTLE trial, the major bleeding rate in patients with moderate renal impairment was lower with apixaban than with warfarin.48 In contrast, major bleeding was similar with dabigatran (both doses) and warfarin in the RE-LY trial47 and with rivaroxaban 20 mg daily and warfarin.46
There are no clinical outcome data regarding the use of NOACs for patients with creatinine clearance (calculated by the Cockroft–Gault equation) of <30 mL/min. This includes patients on haemodialysis,49 for whom warfarin provides uncertain benefit.50 Until trial outcome data are available, warfarin is the preferred anticoagulant for these patient subgroups.49 The FDA has approved apixaban for patients on haemodialysis without safety data from this population.
The FDA review of the ENGAGE AF trial raised a question of efficacy among patients with high normal creatinine clearance (>95 mL/min), and resulting lower plasma concentration of drug: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM421613.pdf). There was a statistically lower treatment effect (interaction P = 0.002) for prevention of ischaemic stroke with edoxaban compared with warfarin for patients with creatinine clearance >95 mL/min, and a higher stroke rate with edoxaban in this subgroup. Whether this was due to under-dosing of edoxaban, particular effectiveness of warfarin in this subgroup, or a combination of factors is not known.
Based on our interpretation of available data we suggest:
| First choice | Patients with AF and stage III CKD (creatinine clearance 30–49 mL/min) may be treated with apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥ 1.5 mg/dL (133 µmol/L are present), rivaroxaban 15 mg daily, or edoxaban 30 mg once daily |
| Second choice | Dabigatran 110 mg twice daily |
| Not recommended | Dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | Patients with AF and stage III CKD (creatinine clearance 30–49 mL/min) may be treated with apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥ 1.5 mg/dL (133 µmol/L are present), rivaroxaban 15 mg daily, or edoxaban 30 mg once daily |
| Second choice | Dabigatran 110 mg twice daily |
| Not recommended | Dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | Patients with AF and stage III CKD (creatinine clearance 30–49 mL/min) may be treated with apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥ 1.5 mg/dL (133 µmol/L are present), rivaroxaban 15 mg daily, or edoxaban 30 mg once daily |
| Second choice | Dabigatran 110 mg twice daily |
| Not recommended | Dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | Patients with AF and stage III CKD (creatinine clearance 30–49 mL/min) may be treated with apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥ 1.5 mg/dL (133 µmol/L are present), rivaroxaban 15 mg daily, or edoxaban 30 mg once daily |
| Second choice | Dabigatran 110 mg twice daily |
| Not recommended | Dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | For patients with AF on haemodialysis, no anticoagulation or VKA therapy is appropriate |
| Not recommended | Dabigatran, rivaroxaban, apixaban*, or edoxaban |
| First choice | For patients with AF on haemodialysis, no anticoagulation or VKA therapy is appropriate |
| Not recommended | Dabigatran, rivaroxaban, apixaban*, or edoxaban |
| First choice | For patients with AF on haemodialysis, no anticoagulation or VKA therapy is appropriate |
| Not recommended | Dabigatran, rivaroxaban, apixaban*, or edoxaban |
| First choice | For patients with AF on haemodialysis, no anticoagulation or VKA therapy is appropriate |
| Not recommended | Dabigatran, rivaroxaban, apixaban*, or edoxaban |
| First choice | Patients with AF and creatinine clearance of >95 mL/min may be treated with dabigatran 150 twice daily, rivaroxaban 20 mg once daily or apixaban 5 mg twice daily. No preference for NOACS over VKAs |
| Second choice | Edoxaban 60 mg once daily (not recommended in USA based on FDA indication approval) |
| First choice | Patients with AF and creatinine clearance of >95 mL/min may be treated with dabigatran 150 twice daily, rivaroxaban 20 mg once daily or apixaban 5 mg twice daily. No preference for NOACS over VKAs |
| Second choice | Edoxaban 60 mg once daily (not recommended in USA based on FDA indication approval) |
| First choice | Patients with AF and creatinine clearance of >95 mL/min may be treated with dabigatran 150 twice daily, rivaroxaban 20 mg once daily or apixaban 5 mg twice daily. No preference for NOACS over VKAs |
| Second choice | Edoxaban 60 mg once daily (not recommended in USA based on FDA indication approval) |
| First choice | Patients with AF and creatinine clearance of >95 mL/min may be treated with dabigatran 150 twice daily, rivaroxaban 20 mg once daily or apixaban 5 mg twice daily. No preference for NOACS over VKAs |
| Second choice | Edoxaban 60 mg once daily (not recommended in USA based on FDA indication approval) |
Non-vitamin K oral anticoagulants and age
The risks of both bleeding and stroke increase with age. Older age is the reason often given for not prescribing anticoagulants for individuals aged over 80 years.51,52 The Birmingham Atrial Fibrillation Treatment of the Aged Study conclusively showed that individuals aged ≥75 years (mean 81.5 years) benefit from anticoagulation compared with aspirin.53 There were 24 primary events (21 strokes, 2 ICH events, and 1 systemic embolic event) in the warfarin arm and 48 primary events (44 strokes, 1 ICH, and 3 systemic embolic events) in the aspirin arm (annual risk 1.8 vs. 3.8%; RR 0.48; 95% CI 0.28–0.80). The annual risk of extracranial bleeding was 1.4% with warfarin vs. 1.6% with aspirin (RR 0.87; 95% CI 0.43–1.73).
Given the high risk for ischaemic stroke, anticoagulant therapy offers net clinical benefit for older adults, including those at risk of falls.54 Compared with VKAs, all of the NOACs reduced the incidence of ICH. All of the AF trials confirmed the increased risk of major bleeding among older adults compared with younger individuals (Table2). In the RE-LY trial, there was a significant treatment-by-age interaction for major bleeding.26 Compared with warfarin, the 110 mg twice daily dabigatran dose was associated with a lower risk of major bleeding among patients <75 years old and similar risk among those ≥75 years. The higher dose of dabigatran, 150 mg twice daily, was associated with a lower risk of bleeding in the younger group, but trended to higher risk among those patients ≥75 years. Both doses reduced ICH compared with warfarin, regardless of patient age.
| . | No. of events (%/year) . | No. of events (%/year) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| ARISTOTLE | Apixaban 5 mg twice daily | Warfarin | ||
| <65 | 56 (1.2) | 72 (1.5) | 0.78 (0.55–1.11) | 0.63 |
| 65 to <75 | 120 (2.0) | 166 (2.8) | 0.71 (0.56–0.89) | |
| ≥75 | 151 (3.3) | 224 (5.2) | 0.64 (0.52–0.79) | |
| RE-LY | Dabigatran 110 mg twice daily | Warfarin | ||
| <75 | 138 (1.89) | 215 (3.04) | 0.62 (0.50–0.77) | 0.0003 |
| ≥75 | 204 (4.43) | 206 (4.37) | 1.01 (0.83–1.23) | |
| Dabigatran 150 mg | Warfarin | |||
| <75 | 153 (2.12) | 215 (3.04) | 0.70 (0.57–0.86) | 0.0001 |
| ≥75 | 246 (5.10) | 206 (4.37) | 1.18 (0.98–1.42) | |
| ROCKET AF | Rivaroxaban 20 mg once daily | Warfarin | ||
| <65 | 59 (2.21) | 59 (2.16) | 1.02 (0.71–1.46) | 0.59 |
| 65 to <75 | 113 (3.03) | 123 (3.24) | 0.94 (0.73–1.21) | |
| ≥75 | 223 (4.86) | 204 (4.40) | 1.11 (0.92–1.34) | |
| ENGAGE AF-TIMI | Edoxaban 60 mg once daily | Warfarin | 0.57 | |
| <75 | (2.02) | (2.62) | ||
| ≥75 | (4.01) | (4.83) |
| . | No. of events (%/year) . | No. of events (%/year) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| ARISTOTLE | Apixaban 5 mg twice daily | Warfarin | ||
| <65 | 56 (1.2) | 72 (1.5) | 0.78 (0.55–1.11) | 0.63 |
| 65 to <75 | 120 (2.0) | 166 (2.8) | 0.71 (0.56–0.89) | |
| ≥75 | 151 (3.3) | 224 (5.2) | 0.64 (0.52–0.79) | |
| RE-LY | Dabigatran 110 mg twice daily | Warfarin | ||
| <75 | 138 (1.89) | 215 (3.04) | 0.62 (0.50–0.77) | 0.0003 |
| ≥75 | 204 (4.43) | 206 (4.37) | 1.01 (0.83–1.23) | |
| Dabigatran 150 mg | Warfarin | |||
| <75 | 153 (2.12) | 215 (3.04) | 0.70 (0.57–0.86) | 0.0001 |
| ≥75 | 246 (5.10) | 206 (4.37) | 1.18 (0.98–1.42) | |
| ROCKET AF | Rivaroxaban 20 mg once daily | Warfarin | ||
| <65 | 59 (2.21) | 59 (2.16) | 1.02 (0.71–1.46) | 0.59 |
| 65 to <75 | 113 (3.03) | 123 (3.24) | 0.94 (0.73–1.21) | |
| ≥75 | 223 (4.86) | 204 (4.40) | 1.11 (0.92–1.34) | |
| ENGAGE AF-TIMI | Edoxaban 60 mg once daily | Warfarin | 0.57 | |
| <75 | (2.02) | (2.62) | ||
| ≥75 | (4.01) | (4.83) |
The trials were different in the baseline risk for bleeding complications.
| . | No. of events (%/year) . | No. of events (%/year) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| ARISTOTLE | Apixaban 5 mg twice daily | Warfarin | ||
| <65 | 56 (1.2) | 72 (1.5) | 0.78 (0.55–1.11) | 0.63 |
| 65 to <75 | 120 (2.0) | 166 (2.8) | 0.71 (0.56–0.89) | |
| ≥75 | 151 (3.3) | 224 (5.2) | 0.64 (0.52–0.79) | |
| RE-LY | Dabigatran 110 mg twice daily | Warfarin | ||
| <75 | 138 (1.89) | 215 (3.04) | 0.62 (0.50–0.77) | 0.0003 |
| ≥75 | 204 (4.43) | 206 (4.37) | 1.01 (0.83–1.23) | |
| Dabigatran 150 mg | Warfarin | |||
| <75 | 153 (2.12) | 215 (3.04) | 0.70 (0.57–0.86) | 0.0001 |
| ≥75 | 246 (5.10) | 206 (4.37) | 1.18 (0.98–1.42) | |
| ROCKET AF | Rivaroxaban 20 mg once daily | Warfarin | ||
| <65 | 59 (2.21) | 59 (2.16) | 1.02 (0.71–1.46) | 0.59 |
| 65 to <75 | 113 (3.03) | 123 (3.24) | 0.94 (0.73–1.21) | |
| ≥75 | 223 (4.86) | 204 (4.40) | 1.11 (0.92–1.34) | |
| ENGAGE AF-TIMI | Edoxaban 60 mg once daily | Warfarin | 0.57 | |
| <75 | (2.02) | (2.62) | ||
| ≥75 | (4.01) | (4.83) |
| . | No. of events (%/year) . | No. of events (%/year) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| ARISTOTLE | Apixaban 5 mg twice daily | Warfarin | ||
| <65 | 56 (1.2) | 72 (1.5) | 0.78 (0.55–1.11) | 0.63 |
| 65 to <75 | 120 (2.0) | 166 (2.8) | 0.71 (0.56–0.89) | |
| ≥75 | 151 (3.3) | 224 (5.2) | 0.64 (0.52–0.79) | |
| RE-LY | Dabigatran 110 mg twice daily | Warfarin | ||
| <75 | 138 (1.89) | 215 (3.04) | 0.62 (0.50–0.77) | 0.0003 |
| ≥75 | 204 (4.43) | 206 (4.37) | 1.01 (0.83–1.23) | |
| Dabigatran 150 mg | Warfarin | |||
| <75 | 153 (2.12) | 215 (3.04) | 0.70 (0.57–0.86) | 0.0001 |
| ≥75 | 246 (5.10) | 206 (4.37) | 1.18 (0.98–1.42) | |
| ROCKET AF | Rivaroxaban 20 mg once daily | Warfarin | ||
| <65 | 59 (2.21) | 59 (2.16) | 1.02 (0.71–1.46) | 0.59 |
| 65 to <75 | 113 (3.03) | 123 (3.24) | 0.94 (0.73–1.21) | |
| ≥75 | 223 (4.86) | 204 (4.40) | 1.11 (0.92–1.34) | |
| ENGAGE AF-TIMI | Edoxaban 60 mg once daily | Warfarin | 0.57 | |
| <75 | (2.02) | (2.62) | ||
| ≥75 | (4.01) | (4.83) |
The trials were different in the baseline risk for bleeding complications.
In the ARISTOTLE trial, the rate of major bleeding with apixaban 5 mg twice daily compared with warfarin was lower for the older age groups (65–74, ≥75 years; Table2). The dose of apixaban, 5 mg twice daily, was reduced to 2.5 mg twice daily in patients with two of the following characteristics: age ≥80 years, weight ≤60 kg, and creatinine ≥1.5 mg/dL (133 μmol).55 There was no treatment-by-age interaction for major bleeding among participants enrolled in the ROCKET AF trial, which found similar rates of bleeding with rivaroxaban and warfarin in each age stratum. A reduced dose of rivaroxaban, 15 mg per day, was used in those with reduced renal function (30–49 mL/min). In the ENGAGE AF trial, edoxaban 60 mg daily was associated with a lower risk of major bleeding among patients aged <75 years compared with warfarin, and similar rates among those ≥75 years of age. The edoxaban dose was reduced by half in patients with reduced renal function (30–50 mL/min), with weight ≤60 kg, or with concomitant use of verapamil, quinidine, or dronedarone.
Based on our interpretation of available data we suggest:
| First choice | In patients older than 75 years, we suggest apixaban 5 mg twice daily [2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL (133 µmol/L)] |
| Second choice | Dabigatran 110 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | In patients older than 75 years, we suggest apixaban 5 mg twice daily [2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL (133 µmol/L)] |
| Second choice | Dabigatran 110 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | In patients older than 75 years, we suggest apixaban 5 mg twice daily [2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL (133 µmol/L)] |
| Second choice | Dabigatran 110 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
| First choice | In patients older than 75 years, we suggest apixaban 5 mg twice daily [2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL (133 µmol/L)] |
| Second choice | Dabigatran 110 mg twice daily, rivaroxaban 20 mg once daily, or edoxaban 60 mg once daily |
Patients with hypertension
Hypertension is a powerful risk factor for stroke in patients with and without AF, and a risk factor for bleeding in anticoagulated patients. The NOACs have been extensively evaluated for stroke prevention in patients with AF who are eligible for OAC treatment with VKAs in the presence or absence of hypertension (Tables3 and 4). There are no specific data on the risk of bleeding in patients with or without hypertension during therapy with dabigatran or rivaroxaban. Results for apixaban and edoxaban are shown in Table4. In patients with hypertension, HRs vary from 0.69 to 0.80 for safety and 0.64 to 0.84 for efficacy compared with warfarin, but confidence intervals are wide and overlapping, and the inherent limitations of cross-trial comparisons preclude preferential recommendations for one anticoagulant agent over another.
Stroke or systemic embolism (%/year) in relation to the presence or absence of hypertension in the four trials comparing non-vitamin K oral anticoagulants with warfarin in patients with atrial fibrillation
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| RE-LY1 | Dabigatran 110 mg twice daily | Yes | 9488 | 1.46 | 1.78 | 0.82a | |
| No | 2549 | 1.79 | 1.36 | 1.31a | 0.06 | ||
| Dabigatran 150 mg twice daily | Yes | 9545 | 1.20 | 1.78 | 0.64a | ||
| No | 2453 | 0.78 | 1.36 | 0.57a | 0.58 | ||
| ROCKET AF3 | Rivaroxaban 20 mg once daily | Yes | 12 801 | 2.73 | 3.47 | 0.79 (0.65–0.97) | |
| No | 1342 | 2.18 | 3.06 | 0.71 (0.74–1.45) | 0.85 | ||
| ARISTOTLE2 | Apixaban 5 mg twice daily | Yes | 15 916 | 1.31 | 1.59 | 0.82 (0.68–1.00) | |
| No | 2285 | 0.99 | 1.67 | 0.60 (0.35–1.02) | 0.27 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 1.51 | 1.80 | 0.84* | |
| No | 1351 | 2.49 | 1.79 | 1.38* | 0.09 |
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| RE-LY1 | Dabigatran 110 mg twice daily | Yes | 9488 | 1.46 | 1.78 | 0.82a | |
| No | 2549 | 1.79 | 1.36 | 1.31a | 0.06 | ||
| Dabigatran 150 mg twice daily | Yes | 9545 | 1.20 | 1.78 | 0.64a | ||
| No | 2453 | 0.78 | 1.36 | 0.57a | 0.58 | ||
| ROCKET AF3 | Rivaroxaban 20 mg once daily | Yes | 12 801 | 2.73 | 3.47 | 0.79 (0.65–0.97) | |
| No | 1342 | 2.18 | 3.06 | 0.71 (0.74–1.45) | 0.85 | ||
| ARISTOTLE2 | Apixaban 5 mg twice daily | Yes | 15 916 | 1.31 | 1.59 | 0.82 (0.68–1.00) | |
| No | 2285 | 0.99 | 1.67 | 0.60 (0.35–1.02) | 0.27 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 1.51 | 1.80 | 0.84* | |
| No | 1351 | 2.49 | 1.79 | 1.38* | 0.09 |
aEstimated.
bIncluding protocol-mandated dose reduction.
Stroke or systemic embolism (%/year) in relation to the presence or absence of hypertension in the four trials comparing non-vitamin K oral anticoagulants with warfarin in patients with atrial fibrillation
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| RE-LY1 | Dabigatran 110 mg twice daily | Yes | 9488 | 1.46 | 1.78 | 0.82a | |
| No | 2549 | 1.79 | 1.36 | 1.31a | 0.06 | ||
| Dabigatran 150 mg twice daily | Yes | 9545 | 1.20 | 1.78 | 0.64a | ||
| No | 2453 | 0.78 | 1.36 | 0.57a | 0.58 | ||
| ROCKET AF3 | Rivaroxaban 20 mg once daily | Yes | 12 801 | 2.73 | 3.47 | 0.79 (0.65–0.97) | |
| No | 1342 | 2.18 | 3.06 | 0.71 (0.74–1.45) | 0.85 | ||
| ARISTOTLE2 | Apixaban 5 mg twice daily | Yes | 15 916 | 1.31 | 1.59 | 0.82 (0.68–1.00) | |
| No | 2285 | 0.99 | 1.67 | 0.60 (0.35–1.02) | 0.27 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 1.51 | 1.80 | 0.84* | |
| No | 1351 | 2.49 | 1.79 | 1.38* | 0.09 |
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| RE-LY1 | Dabigatran 110 mg twice daily | Yes | 9488 | 1.46 | 1.78 | 0.82a | |
| No | 2549 | 1.79 | 1.36 | 1.31a | 0.06 | ||
| Dabigatran 150 mg twice daily | Yes | 9545 | 1.20 | 1.78 | 0.64a | ||
| No | 2453 | 0.78 | 1.36 | 0.57a | 0.58 | ||
| ROCKET AF3 | Rivaroxaban 20 mg once daily | Yes | 12 801 | 2.73 | 3.47 | 0.79 (0.65–0.97) | |
| No | 1342 | 2.18 | 3.06 | 0.71 (0.74–1.45) | 0.85 | ||
| ARISTOTLE2 | Apixaban 5 mg twice daily | Yes | 15 916 | 1.31 | 1.59 | 0.82 (0.68–1.00) | |
| No | 2285 | 0.99 | 1.67 | 0.60 (0.35–1.02) | 0.27 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 1.51 | 1.80 | 0.84* | |
| No | 1351 | 2.49 | 1.79 | 1.38* | 0.09 |
aEstimated.
bIncluding protocol-mandated dose reduction.
International Society of Thrombosis and Hemostasis56 major bleeding (%/year) in relation to the presence or absence of hypertension in the four trials comparing non-vitamin K oral anticoagulants with warfarin in patients with atrial fibrillation
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| ARISTOTLE2 | Apixaban 5 mg twice dailyb | Yes | 15 916 | 2.07 | 3.00 | 0.69 (0.59–0.80) | |
| No | 2285 | 2.60 | 3.73 | 0.70 (0.48–1.00) | 0.96 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 2.72 | 3.42 | 0.80a | |
| No | 1351 | 3.17 | 3.42 | 0.93a | 0.68 |
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| ARISTOTLE2 | Apixaban 5 mg twice dailyb | Yes | 15 916 | 2.07 | 3.00 | 0.69 (0.59–0.80) | |
| No | 2285 | 2.60 | 3.73 | 0.70 (0.48–1.00) | 0.96 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 2.72 | 3.42 | 0.80a | |
| No | 1351 | 3.17 | 3.42 | 0.93a | 0.68 |
aEstimated.
bIncluding protocol-mandated dose reduction.
International Society of Thrombosis and Hemostasis56 major bleeding (%/year) in relation to the presence or absence of hypertension in the four trials comparing non-vitamin K oral anticoagulants with warfarin in patients with atrial fibrillation
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| ARISTOTLE2 | Apixaban 5 mg twice dailyb | Yes | 15 916 | 2.07 | 3.00 | 0.69 (0.59–0.80) | |
| No | 2285 | 2.60 | 3.73 | 0.70 (0.48–1.00) | 0.96 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 2.72 | 3.42 | 0.80a | |
| No | 1351 | 3.17 | 3.42 | 0.93a | 0.68 |
| Trial . | Drug and dose . | Hypertension . | No. of patients . | NOAC . | Warfarin . | HR (95% CI) . | P-interaction . |
|---|---|---|---|---|---|---|---|
| ARISTOTLE2 | Apixaban 5 mg twice dailyb | Yes | 15 916 | 2.07 | 3.00 | 0.69 (0.59–0.80) | |
| No | 2285 | 2.60 | 3.73 | 0.70 (0.48–1.00) | 0.96 | ||
| ENGAGE AF4 | Edoxaban 60 mg once dailyb | Yes | 19 754 | 2.72 | 3.42 | 0.80a | |
| No | 1351 | 3.17 | 3.42 | 0.93a | 0.68 |
aEstimated.
bIncluding protocol-mandated dose reduction.
Based on our interpretation of available data we suggest:
| Choice of NOAC | No particular NOAC is superior to another NOAC in terms of safety or efficacy in patients with AF and hypertension |
| Choice of NOAC | No particular NOAC is superior to another NOAC in terms of safety or efficacy in patients with AF and hypertension |
| Choice of NOAC | No particular NOAC is superior to another NOAC in terms of safety or efficacy in patients with AF and hypertension |
| Choice of NOAC | No particular NOAC is superior to another NOAC in terms of safety or efficacy in patients with AF and hypertension |
Adherence
Non-adherence to chronic OAC treatment increases the risks of both ischaemic and haemorrhagic complications.57,58 Enthusiasm for the convenience of fixed-dose NOACs has been paralleled by concerns about patient adherence given the shorter half-lives of these agents compared with VKAs, and inability to reliably and readily measure the anticoagulant effect of NOACs.59
No published data are available from the phase III trials regarding adherence to NOACs, other than overall discontinuation rates. There are limited data from experience in clinical practice, with five studies reporting adherence and/or persistence rates for dabigatran,60–64 and two reporting persistence data for rivaroxaban.40,65 Those reporting dabigatran adherence data used 80% or more as the threshold for good adherence, determined by the proportion of days covered (number of days in which the medication was taken as prescribed).60–62 One small study (n = 99) reported 88% adherence to dabigatran over a variable follow-up period,64 while larger studies report median adherence rates of 67–77%.60–62 A prospective registry (n = 1204) reported an overall persistence rate of 81.5% on rivaroxaban.65 Compared with warfarin, persistence was better with dabigatran (63 vs. 39%) at 1 year63 and with rivaroxaban (81.5 vs. 68.3%) at 6 months,40 but methodological, demographic, and clinical differences between these studies including length of follow-up may account for the differences in reported rates of adherence and persistence with therapy.
Reducing the complexity of a medication regimen or frequency of dosing does not necessarily improve adherence,66 although the proportion of doses taken is generally greater with once-daily vs. twice-daily dosing.67–69 There are no significant differences in persistence rates between dosing regimens.68 Drug-action depends on both the frequency and timing of dosing, and there is insufficient evidence to advocate once or twice daily dosing to improve adherence to NOAC therapy.
To date, no interventions have been shown to improve adherence to NOAC therapy. The impact of adherence to apixaban is under investigation in the Assessment of an Education and Guidance program for Eliquis Adherence in Non-valvular atrial fibrillation study (NCT0188435), in which ‘usual care’ is compared with ‘usual care plus education supported by a virtual clinic’, with adherence recorded using an electronic device that gathers data based on the timing of removal of medication from the device.
Patient engagement in treatment decisions, and education about AF, stroke, and drug-specific information (Table5) are essential to improve adherence. The mode of delivery and complexity of information should be adapted to the individual patient.70,71 The importance of sustained adherence must be communicated so patients are aware of the potential consequences of non-adherence. Adherence should be measured. Identifying the patterns of and reasons for non-adherence are valuable in developing individualized strategies to improve adherence and outcomes.69
Key points in counselling patients taking an oral anticoagulant to improve adherence
|
|
Key points in counselling patients taking an oral anticoagulant to improve adherence
|
|
Based on our interpretation of available data we suggest:
| Choice of OAC | OACs should not be used in patients where intentional non-adherence is known (i.e. choosing not to take medication) |
| When medication, non-adherence is unintentional (due to cognitive impairment or other impediments), strategies such as pill-boxes or engagement of a family member or caregiver to oversee administration of OAC medication should be used. NOACs may be more appropriate than VKA agents in this situation, given their fixed dose and simpler regimen | |
| The decision of which NOAC to prescribe should not be based primarily on once vs. twice daily dosing, but this may be a factor in the decision-making process for some patients (i.e. polypharmacy, patient preference) | |
| There is no evidence to support the use of a particular NOAC |
| Choice of OAC | OACs should not be used in patients where intentional non-adherence is known (i.e. choosing not to take medication) |
| When medication, non-adherence is unintentional (due to cognitive impairment or other impediments), strategies such as pill-boxes or engagement of a family member or caregiver to oversee administration of OAC medication should be used. NOACs may be more appropriate than VKA agents in this situation, given their fixed dose and simpler regimen | |
| The decision of which NOAC to prescribe should not be based primarily on once vs. twice daily dosing, but this may be a factor in the decision-making process for some patients (i.e. polypharmacy, patient preference) | |
| There is no evidence to support the use of a particular NOAC |
| Choice of OAC | OACs should not be used in patients where intentional non-adherence is known (i.e. choosing not to take medication) |
| When medication, non-adherence is unintentional (due to cognitive impairment or other impediments), strategies such as pill-boxes or engagement of a family member or caregiver to oversee administration of OAC medication should be used. NOACs may be more appropriate than VKA agents in this situation, given their fixed dose and simpler regimen | |
| The decision of which NOAC to prescribe should not be based primarily on once vs. twice daily dosing, but this may be a factor in the decision-making process for some patients (i.e. polypharmacy, patient preference) | |
| There is no evidence to support the use of a particular NOAC |
| Choice of OAC | OACs should not be used in patients where intentional non-adherence is known (i.e. choosing not to take medication) |
| When medication, non-adherence is unintentional (due to cognitive impairment or other impediments), strategies such as pill-boxes or engagement of a family member or caregiver to oversee administration of OAC medication should be used. NOACs may be more appropriate than VKA agents in this situation, given their fixed dose and simpler regimen | |
| The decision of which NOAC to prescribe should not be based primarily on once vs. twice daily dosing, but this may be a factor in the decision-making process for some patients (i.e. polypharmacy, patient preference) | |
| There is no evidence to support the use of a particular NOAC |
Limitations and caveats
Authors' contributions
Conceived and designed the research: all authors contributed to the review. Drafted the manuscript: each author drafted one section of the manuscript. G.B. and J.L.H, performed the final editing. Made critical revision of the manuscript for key intellectual content: all authors.
Acknowledgements
We acknowledge the editorial support of Rebecca Craven.
Conflict of interest: Dr Aisenberg has received honoraria for speaking or participating on advisory boards for Boehringer Ingelheim, Pfizer, and Portola. Dr Ansell has received honoraria or consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Daiichi-Sankyo, Janssen, Instrumentation Laboratories, Roche Diagnostics, Alere Home Monitoring, and Perosphere. Dr Ansell has equity interest in Perosphere. Dr Atar received fees, honoraria from Boehringer Ingelheim, Bayer, BMS/Pfizer, Daiichi-Sankyo, Medtronic, Nycomed-Takeda, Cardiome, and AstraZeneca. Dr Breithardt reports honoraria from Bayer HealthCare and Bristol-Myers Squibb and Pfizer, and consulting and advisory board fees from Bayer HealthCare, Bristol-Myers Squibb and Pfizer, and Sanofi-Aventis. Dr Diener received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, MSD, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth, and Yamanouchi. Financial support for research projects was provided by AstraZeneca, GSK, Boehringer Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi-Aventis, Syngis, and Talecris. The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. HCD has no ownership interest and does not own stocks of any pharmaceutical company. Dr Eikelboom has received honoraria and/or research grants from AstraZeneca, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Janssen, and Sanofi-Aventis. Dr Ezekowitz has served as a consultant for Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Daiichi-Sankyo, Merck, J & J, Bayer, and Medtronic. Dr Granger received grant funding and reports consulting from BMS, Pfizer, Daiichi-Sankyo, BI, Bayer, and Janssen. Dr Halperin reports consulting fees from Bayer HealthCare AG, Boehringer Ingelheim, Daiichi-Sankyo, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals, Pfizer, Sanofi-Aventis, AstraZeneca, Biotronik, Boston Scientific, Janssen, and Medtronic. Dr Hohnloser has received consulting fees from Bayer, BMS, Sanofi-Aventis, St Jude Medical, Boehringer Ingelheim, Cardiome, and Medtronic Vascular; and lecture fees from Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and St Jude Medical. Dr Hylek served on advisory boards for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, Medtronic, Pfizer, received honoraria for conference lecture from Bayer, Boehringer Ingelheim, and Pfizer. Dr Kirchhof reports consulting fees and honoraria from 3M Medica, MEDA Pharma, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer/BMS, Sanofi, Servier, Siemens, and TAKEDA; research grants from 3M Medica/MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, Sanofi, St. Jude Medical, German Federal Ministry for Education and Research, Fondation Leducq, German Research Foundation, and the European Union; travel support received from the European Society of Cardiology, the European Heart Rhythm Association, and from the German Atrial Fibrillation Competence NETwork. Dr Lane has received investigator-initiated educational grants from Bayer Healthcare, Boehringer Ingelheim and Bristol-Myers Squibb. She has also been on the speaker bureau for Boehringer Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer and is a Steering Committee member for a Phase IV trial sponsored by Bristol-Myers Squibb. Dr Lip (i) Guideline membership/reviewing: ESC Guidelines on Atrial Fibrillation, 2010 and Focused Update, 2012; ESC Guidelines on Heart Failure, 2012; American College of Chest Physicians Antithrombotic Therapy Guidelines for Atrial Fibrillation, 2012; NICE Guidelines on Atrial Fibrillation, 2006 and 2014; NICE Quality Standards on Atrial Fibrillation 2015; ESC Cardio-oncology Task Force, 2015; ESC Working Group on Thrombosis position documents (2011–). Chairman, Scientific Documents Committee, European Heart Rhythm Association (EHRA). Reviewer for various guidelines and position statements from ESC, EHRA, NICE, etc. (ii) Steering Committees/trials: Includes steering committees for various Phase II and III studies, Health Economics & Outcomes Research, etc. Investigator in various clinical trials in cardiovascular disease, including those on antithrombotic therapies in AF, acute coronary syndrome, lipids, etc. (iii) Consultant/Advisor/Speaker: Consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Biotronik, Medtronic, Portola, Boehringer Ingelheim, Microlife, and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi-Sankyo Dr Veltkamp has received consulting honoraria, research support, travel grants, and speakers' honoraria from Bayer HealthCare, Boehringer Ingelheim, BMS Pfizer, Daiichi-Sankyo, Roche Diagnostics, CSL Behring, St. Jude Medical, Medtronic, and Sanofi-Aventis. Dr Verheugt received honoraria for speaker fees and consultancy honoraria from Bayer Healthcare, Boehringer Ingelheim, BMS/Pfizer, and Daiichi-Sankyo.
References
- acute cerebrovascular accidents
- anticoagulants
- anticoagulation
- atrial fibrillation
- anticoagulants, oral
- hypertension
- transient ischemic attack
- hemodialysis
- thrombolytic therapy
- cerebrovascular accident
- nonvalvular atrial fibrillation
- ischemic stroke
- gastrointestinal bleeding
- thrombectomy
- vitamins
- renal impairment
- vitamin k antagonists
- stroke prevention
- direct oral anticoagulants



