-
PDF
- Split View
-
Views
-
Cite
Cite
Hein Heidbuchel, Peter Verhamme, Marco Alings, Matthias Antz, Hans-Christoph Diener, Werner Hacke, Jonas Oldgren, Peter Sinnaeve, A. John Camm, Paulus Kirchhof, ESC Scientific Document Group , Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary, European Heart Journal, Volume 38, Issue 27, 14 July 2017, Pages 2137–2149, https://doi.org/10.1093/eurheartj/ehw058
Close - Share Icon Share
Abstract
In 2013, the European Heart Rhythm Association (EHRA) published a Practical Guide on the use of non-VKA oral anticoagulants (NOACs) in patients with atrial fibrillation (AF) (Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, European Heart Rhythm A. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–651; Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013;34:2094–2106). The document received widespread interest, not only from cardiologists but also from neurologists, geriatricians, and general practitioners, as became evident from the distribution of >350 000 copies of its pocket version (the EHRA Key Message Booklet) world-wide. Since 2013, numerous new studies have appeared on different aspects of NOAC therapy in AF patients. Therefore, EHRA updated the Practical Guide, including new information but also providing balanced guiding in the many areas where prospective data are still lacking. The outline of the original guide that addressed 15 clinical scenarios has been preserved, but all chapters have been rewritten. Main changes in the Update comprise a discussion on the definition of ‘non-valvular AF’ and eligibility for NOAC therapy, inclusion of finalized information on the recently approved edoxaban, tailored dosing information dependent on concomitant drugs, and/or clinical characteristics, an expanded chapter on neurologic scenarios (ischaemic stroke or intracranial haemorrhage under NOAC), an updated anticoagulation card and more specifics on start-up and follow-up issues. There are also many new flow charts, like on appropriate switching between anticoagulants (VKA to NOAC or vice versa), default scenarios for acute management of coronary interventions, step-down schemes for long-term combined antiplatelet-anticoagulant management in coronary heart disease, management of bleeding, and cardioversion under NOAC therapy. The Updated Guide is available in full in EP Europace (Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, Advisors. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–1507), while additional resources can be found at the related ESC/EHRA website (www.NOACforAF.eu).
Introduction
The proper use of non-vitamin K antagonist (VKA) oral anticoagulants (NOACs) for thromboembolic prevention in patients with non-valvular atrial fibrillation (AF) requires different approaches in many daily care settings compared with VKAs. Guidelines4–6 mainly discuss the indications for anticoagulation in general (e.g. based on the CHA2DS2-VASc score; NOAC vs. VKA). This Practical Guide supplements the Guidelines, providing guidance on how to use NOACs in specific clinical situations. The main changes from the original European Heart Rhythm Association (EHRA) Practical Guide that was published in 20131,2 are summarized in this Executive Summary. The full text of the Update is published in EP Europace.3 The Update will also be presented in an new version of the slide kit (downloadable for free by EHRA members) and a Key Message booklet, which can be obtained through EHRA and ESC. Stay tuned to the www.NOACforAF.eu Web site for up-to-date information. You can also provide your feedback via that Web site.
Definition of ‘non-valvular atrial fibrillation’ and eligibility for NOACs
Valvular AF refers to AF that occurs in the presence of mechanical prosthetic heart valves or of moderate-to-severe mitral stenosis (usually of rheumatic origin). Both types of patients were excluded from all NOAC trials and are not eligible for NOAC therapy. Atrial fibrillation patients often have other valvular abnormalities many of which were included in the NOAC trials. They were shown to be suitable NOAC candidates based on the consistent beneficial findings in these subgroups (with the exception of higher bleeding rates with rivaroxaban compared with VKA in patients with valvular disease).7–9Table 1 summarizes the eligibility recommendations for NOAC therapy for other patient subgroups, acknowledging that limited data are available for some groups.10 The full Guide describes the rationale for this eligibility guidance.
Valvular indications and contra-indications for NOAC therapy in atrial fibrillation patients
| . | Eligible . | Contra-indicated . |
|---|---|---|
| Mechanical prosthetic valve | ✓ | |
| Moderate-to-severe mitral stenosis (usually of rheumatic origin) | ✓ | |
| Mild-to-moderate other native valvular disease | ✓ | |
| Severe aortic stenosis | ✓ Limited data Most will undergo intervention | |
| Bioprosthetic valvea | ✓ (except for the first 3 months post-operatively) | |
| Mitral valve repaira | ✓ (except for the first 3–6 months post-operatively) | |
| PTAV and TAVI | ✓ (but no prospective data; may require combination with single or double antiplatelets: consider bleeding risk)10 | |
| Hypertrophic cardiomyopathy | ✓ (but no prospective data) |
| . | Eligible . | Contra-indicated . |
|---|---|---|
| Mechanical prosthetic valve | ✓ | |
| Moderate-to-severe mitral stenosis (usually of rheumatic origin) | ✓ | |
| Mild-to-moderate other native valvular disease | ✓ | |
| Severe aortic stenosis | ✓ Limited data Most will undergo intervention | |
| Bioprosthetic valvea | ✓ (except for the first 3 months post-operatively) | |
| Mitral valve repaira | ✓ (except for the first 3–6 months post-operatively) | |
| PTAV and TAVI | ✓ (but no prospective data; may require combination with single or double antiplatelets: consider bleeding risk)10 | |
| Hypertrophic cardiomyopathy | ✓ (but no prospective data) |
PTAV, percutaneous transluminal aortic valvuloplasty; TAVI, transcatheter aortic valve implantation.
aAmerican guidelines do not recommend NOAC in patients with biological heart valves or after valve repair.12
Valvular indications and contra-indications for NOAC therapy in atrial fibrillation patients
| . | Eligible . | Contra-indicated . |
|---|---|---|
| Mechanical prosthetic valve | ✓ | |
| Moderate-to-severe mitral stenosis (usually of rheumatic origin) | ✓ | |
| Mild-to-moderate other native valvular disease | ✓ | |
| Severe aortic stenosis | ✓ Limited data Most will undergo intervention | |
| Bioprosthetic valvea | ✓ (except for the first 3 months post-operatively) | |
| Mitral valve repaira | ✓ (except for the first 3–6 months post-operatively) | |
| PTAV and TAVI | ✓ (but no prospective data; may require combination with single or double antiplatelets: consider bleeding risk)10 | |
| Hypertrophic cardiomyopathy | ✓ (but no prospective data) |
| . | Eligible . | Contra-indicated . |
|---|---|---|
| Mechanical prosthetic valve | ✓ | |
| Moderate-to-severe mitral stenosis (usually of rheumatic origin) | ✓ | |
| Mild-to-moderate other native valvular disease | ✓ | |
| Severe aortic stenosis | ✓ Limited data Most will undergo intervention | |
| Bioprosthetic valvea | ✓ (except for the first 3 months post-operatively) | |
| Mitral valve repaira | ✓ (except for the first 3–6 months post-operatively) | |
| PTAV and TAVI | ✓ (but no prospective data; may require combination with single or double antiplatelets: consider bleeding risk)10 | |
| Hypertrophic cardiomyopathy | ✓ (but no prospective data) |
PTAV, percutaneous transluminal aortic valvuloplasty; TAVI, transcatheter aortic valve implantation.
aAmerican guidelines do not recommend NOAC in patients with biological heart valves or after valve repair.12
Expanded data on all four NOAC drugs
Although already provisionally present in the original Practical Guide, all the latest information on edoxaban, the most recently approved NOAC, has been included in the Update. The standard dose of edoxaban is 60 mg once daily (OD), with prespecified dose reductions in patients with a reduced kidney function (CrCl estimated by the Cockcroft-Gault formula of ≤49 mL/min), the concomitant use of certain drugs (e.g. dronedarone), and in patients weighing ≤60 kg. The table from the original Guide that highlights all known drug–drug interactions and clinical factors that impact NOAC plasma levels has been updated and re-organized (Table 2). The table aims to provide physicians with a clear rationale to optimize the NOAC dose for particular patients, preventing both under- and overtreatment. The table uses a color-coded scheme to indicate situations with a contraindication for concomitant NOAC use (‘red’), necessity to reduce its dose (‘orange’), or consideration of dose reduction in the presence of other ‘yellow’ factors. Some cells with missing pharmacokinetic interaction data have now been filled in (although some retain the ‘no data yet’ label …), drugs are classified according to therapeutic area for easier reference, and there has been a separate color coding for interactions that lead to reduced NOAC plasma levels (vs. the more usual scenario of increased plasma levels).
Effect on NOAC plasma levels (‘area under the curve, AUC’) from drug–drug interactions and clinical factors, and recommendations towards NOAC dose adaptation
 | |||||
 |
 | |||||
 |
Red: contra-indicated/not recommended. Orange: reduce dose (from 150 mg BID to 110 mg BID for dabigatran; from 20 to 15 mg OD for rivaroxaban; from 5 mg BID to 2.5 mg BID for apixaban). Yellow: consider dose reduction if two or more ‘yellow’ factors are present.
Hatching: no clinical or PK data available. BCRP, breast cancer resistance protein; NSAID, non-steroidal anti-inflammatory drugs; H2B, H2-blockers; PPI, proton pump inhibitors; P-gp, P-glycoprotein; GI, gastro-intestinal.
aBased on in vitro investigations, comparing the IC50 for P-gp inhibition to maximal plasma levels at therapeutic dose, and/or on interaction analysis of efficacy and safety endpoints in the phase-3 clinical trials. No direct PK interaction data available.
bSome interactions lead to reduced NOAC plasma levels in contrast to most interactions that lead to increased NOAC plasma levels. This may also constitute a contraindication for simultaneous use, and such cases are colored brown. The label for edoxaban mentions that co-administration is possible in these cases, despite a decreased plasma level, which are deemed not clinically relevant (blue). Since not tested prospectively, however, such concomitant use should be used with caution, and avoided when possible.
cThe SmPC specifies dose reduction from 5 mg BID to 2.5 mg BID if two of three criteria are fulfilled: age ≥80 years, weight ≤60 kg, and serum creatinine ≥1.5 mg/dL.
dAge had no significant effect after adjusting for weight and renal function.
Effect on NOAC plasma levels (‘area under the curve, AUC’) from drug–drug interactions and clinical factors, and recommendations towards NOAC dose adaptation
 | |||||
 |
 | |||||
 |
Red: contra-indicated/not recommended. Orange: reduce dose (from 150 mg BID to 110 mg BID for dabigatran; from 20 to 15 mg OD for rivaroxaban; from 5 mg BID to 2.5 mg BID for apixaban). Yellow: consider dose reduction if two or more ‘yellow’ factors are present.
Hatching: no clinical or PK data available. BCRP, breast cancer resistance protein; NSAID, non-steroidal anti-inflammatory drugs; H2B, H2-blockers; PPI, proton pump inhibitors; P-gp, P-glycoprotein; GI, gastro-intestinal.
aBased on in vitro investigations, comparing the IC50 for P-gp inhibition to maximal plasma levels at therapeutic dose, and/or on interaction analysis of efficacy and safety endpoints in the phase-3 clinical trials. No direct PK interaction data available.
bSome interactions lead to reduced NOAC plasma levels in contrast to most interactions that lead to increased NOAC plasma levels. This may also constitute a contraindication for simultaneous use, and such cases are colored brown. The label for edoxaban mentions that co-administration is possible in these cases, despite a decreased plasma level, which are deemed not clinically relevant (blue). Since not tested prospectively, however, such concomitant use should be used with caution, and avoided when possible.
cThe SmPC specifies dose reduction from 5 mg BID to 2.5 mg BID if two of three criteria are fulfilled: age ≥80 years, weight ≤60 kg, and serum creatinine ≥1.5 mg/dL.
dAge had no significant effect after adjusting for weight and renal function.
Also the impact of the different NOACs on standard and specific coagulation assays has been revised and made more specific where possible. Information on the activated clotting time and quantitative trough plasma levels for all drugs have been added.
The addition of edoxaban also called for updates of the recommendations concerning switching anticoagulants (Figure 1). When switching from VKA therapy to NOAC (upper panel), the proposed scheme in the Practical Guide unifies instructions from Summary of Product Characteristics (SmPC) for the different NOACs, that state that NOAC can be started when international normalized ratio (INR) is ≤3 for rivaroxaban, ≤2.5 for edoxaban, and ≤2 for apixaban and dabigatran. The Guide advises uniformly that if the INR is 2.0–2.5, the NOAC can be started immediately or the next day. For INR >2.5, the actual INR value and the half-life of the VKA need to be taken into account to estimate the time when the INR value will likely drop to within this threshold range. At that time, a new INR measurement can be scheduled. Inadequate transitioning from NOAC to VKA has been shown to be associated with increased stroke rates.11–13 Therefore, a more rigorous switching scheme has been proposed (Figure 1, lower panel), taking into account that NOACs (especially the FXa inhibitors) may have an effect on the INR, influencing the measurement while on combined treatment during the overlap phase. INR should be measured just before the next intake of the NOAC during concomitant administration, and be retested 24 h after the last dose of the NOAC (i.e. sole VKA therapy) to assure adequate anticoagulation. It is also recommended to closely monitor INR within the first month until stable therapeutic values have been attained. At the end of the ENGAGE-AF trial, patients on edoxaban transitioning to VKA received up to 14 days of a half dose of the NOAC until INR was within range, in combination with the above intensive INR testing strategy.14 Whether the half-dose bridging regimen also applies to other NOACs is unknown.
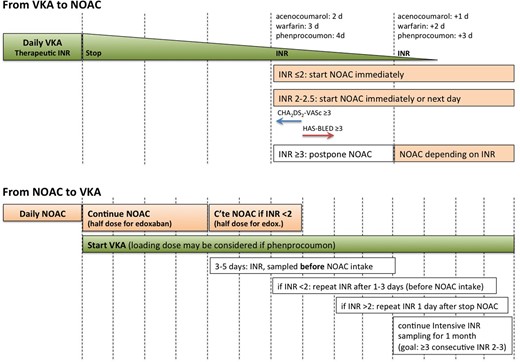
Switching between vitamin-K antagonists (VKA) and non-VKA oral anticoagulants (NOACs) and vice versa.
Peri-procedural management of NOAC-treated patients
The table on the timing of intake of last NOAC before elective surgery (Table 3) has been simplified since edoxaban follows the same regime as the other FXa inhibitors. The table stresses that pre-operative bridging with low-molecular-weight heparins (LMWH) is inappropriate in the context of NOAC therapy, since it will only increase perioperative bleeding risk.15 The time of last intake depends on the type of intervention, distinguishing interventions that do not necessarily require discontinuation of anticoagulation and can be done at trough level, those with minor bleeding risk (i.e. infrequent or with low clinical impact) usually requiring last intake ≥24 h before (if normal renal function; longer if reduced CrCl), and those with major bleeding risk (i.e. frequent and/or with high impact) requiring a default of ≥48 h cessation.
| . | Dabigatran . | Apixaban–Edoxaban–Rivaroxaban . | ||
|---|---|---|---|---|
| No important bleeding risk and/or adequate local haemostasis possible: perform at trough level (i.e. ≥12 or 24 h after last intake) . | ||||
| Low risk . | High risk . | Low risk . | High risk . | |
| CrCl ≥80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 50–80 mL/min | ≥36 h | ≥72 h | ≥24 h | ≥48 h |
| CrCl 30–50 mL/mina | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
| CrCl 15–30 mL/mina | Not indicated | Not indicated | ≥36 h | ≥48 h |
| CrCl <15 mL/min | No official indication for use | |||
| There is no need for pre-operative bridging with LMWH/UFH | ||||
| . | Dabigatran . | Apixaban–Edoxaban–Rivaroxaban . | ||
|---|---|---|---|---|
| No important bleeding risk and/or adequate local haemostasis possible: perform at trough level (i.e. ≥12 or 24 h after last intake) . | ||||
| Low risk . | High risk . | Low risk . | High risk . | |
| CrCl ≥80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 50–80 mL/min | ≥36 h | ≥72 h | ≥24 h | ≥48 h |
| CrCl 30–50 mL/mina | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
| CrCl 15–30 mL/mina | Not indicated | Not indicated | ≥36 h | ≥48 h |
| CrCl <15 mL/min | No official indication for use | |||
| There is no need for pre-operative bridging with LMWH/UFH | ||||
Bold values deviate from the common stopping rule of ≥24 h low risk, ≥48 h high risk. Low risk: with a low frequency of bleeding and/or minor impact of a bleeding; high risk with a high frequency of bleeding and/or important clinical impact.
CrCl, creatinine clearance.
aMany of these patients may be on the lower dose of dabigatran (i.e. 110 mg BID) or apixaban (i.e. 2.5 mg BID), or have to be on the lower dose of rivaroxaban (i.e. 15 mg OD) or edoxaban (i.e. 30 mg OD).
| . | Dabigatran . | Apixaban–Edoxaban–Rivaroxaban . | ||
|---|---|---|---|---|
| No important bleeding risk and/or adequate local haemostasis possible: perform at trough level (i.e. ≥12 or 24 h after last intake) . | ||||
| Low risk . | High risk . | Low risk . | High risk . | |
| CrCl ≥80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 50–80 mL/min | ≥36 h | ≥72 h | ≥24 h | ≥48 h |
| CrCl 30–50 mL/mina | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
| CrCl 15–30 mL/mina | Not indicated | Not indicated | ≥36 h | ≥48 h |
| CrCl <15 mL/min | No official indication for use | |||
| There is no need for pre-operative bridging with LMWH/UFH | ||||
| . | Dabigatran . | Apixaban–Edoxaban–Rivaroxaban . | ||
|---|---|---|---|---|
| No important bleeding risk and/or adequate local haemostasis possible: perform at trough level (i.e. ≥12 or 24 h after last intake) . | ||||
| Low risk . | High risk . | Low risk . | High risk . | |
| CrCl ≥80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 50–80 mL/min | ≥36 h | ≥72 h | ≥24 h | ≥48 h |
| CrCl 30–50 mL/mina | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
| CrCl 15–30 mL/mina | Not indicated | Not indicated | ≥36 h | ≥48 h |
| CrCl <15 mL/min | No official indication for use | |||
| There is no need for pre-operative bridging with LMWH/UFH | ||||
Bold values deviate from the common stopping rule of ≥24 h low risk, ≥48 h high risk. Low risk: with a low frequency of bleeding and/or minor impact of a bleeding; high risk with a high frequency of bleeding and/or important clinical impact.
CrCl, creatinine clearance.
aMany of these patients may be on the lower dose of dabigatran (i.e. 110 mg BID) or apixaban (i.e. 2.5 mg BID), or have to be on the lower dose of rivaroxaban (i.e. 15 mg OD) or edoxaban (i.e. 30 mg OD).
If emergency surgery is required that cannot be delayed, specific or aspecific reversal of the anticoagulant may be considered by the agents mentioned below under ‘Management of bleeding’.
EHRA/HRS/APHRS recently published an extensive consensus document on antithrombotic management in patients undergoing electrophysiological procedures.16 The Updated Practical Guide is in line with those recommendations. In patients undergoing device implantation, there is consensus about lower thromboembolic and bleeding rates with uninterrupted VKA, at least in patients with an increased embolic risk.17 For NOAC-treated patients, we do not see a reason to deviate from the overall scheme with timed cessation before intervention, without bridging (Table 3). Smaller studies did not show a benefit of uninterrupted NOAC (and even a trend for more bleeding).18,19
Best management of anticoagulation around pulmonary vein isolation (PVI) remains elusive given the heterogeneity of studies performed. Although associated with a risk for frequent or major bleeding, PVI is also associated with a high thromboembolic risk. There is international consensus that in VKA-treated patients PVI should be performed without VKA interruption.4,20,21 Whether such an approach is safe in patients on NOAC therapy is less clear. Non-vitamin-K antagonist oral anticoagulants have the advantage of predictable waning/onset of their anticoagulant effect, without need for bridging with LMWH which was the prime reason for the peri-procedural bleedings as seen in bridged VKA patients. A first randomized trial, Venture-AF (with rivaroxaban, of which the last dose was presumably given 12 h before the procedure in most patients; no exact data reported), showed similar bleeding and ischaemic event rates, although in a rather small population leading to an underpowered trial.22 Therefore, while awaiting data from other ongoing prospective trials, we recommend an institutional protocol for NOAC patients undergoing AF ablation. This may consist of changing patients to uninterrupted VKA, of uninterrupted NOAC therapy, or of well-planned cessation of NOAC. Meta-analysis data indicate that a last intake of NOAC 24 h before the procedure is a viable ‘default’ strategy. Continued intake until the evening before the procedure or even the morning of the procedure seems to be safe in experienced centres. A number of factors should be considered for the timing of last intake, like kidney function, CHA2DS2-VASc score of the patient, experience of the operator, type and extent of additional ablation beyond PVI, and the presence of peri-procedural imaging to guide transseptal puncture. When NOAC is last taken ≥36 h before the intervention, a transoesophageal echocardiography (TOE) should be considered before ablation. The same applies if adherence to correct NOAC intake in the weeks before ablation is doubtful.
Management of bleeding
Recent progress in the development of specific reversal agents is summarized in the Updated Guide. A specific reversal agent for dabigatran (idarucizumab, a humanized antibody fragment that specifically binds dabigatran)23 is close to approval by EMA and FDA. The REVERSE-AD trial showed a nearly complete reversal of the anticoagulant effects of dabigatran by idarucizumab within minutes.24 Similar agents for FXa inhibitors are under development, such as andexanet alfa (a recombinant human FXa analogue that competes for the FXa inhibitors with FXa) and aripazine, a small synthetic molecule that seems to have more generalized antagonistic effects.25,26 A graded approach to bleeding is presented in Figure 2. When idarucizumab would not be readily available during a major bleeding complication under dabigatran, or in case bleeding occurs in a patient treated with any of the FXa inhibitors, one can resort to nonspecific reversal strategies: many animal and healthy volunteer studies have confirmed the effects of prothrombin complex concentrate (PCC) or activated prothrombin complex concentrates (aPCC),27,28 but newer research has indicated that the dose of PCC needed for full reversal is higher than stated in the original Practical Guide, and thus has been updated accordingly (50 U/kg vs. 25 U/kg).27–29 The efficacy of PCC or aPCC in patients who are actively bleeding has not been firmly established (i.e. that they reduce blood loss and improve outcome),30 and one has to balance the potential pro-thrombotic effects against the potential anticoagulant benefits.31,32
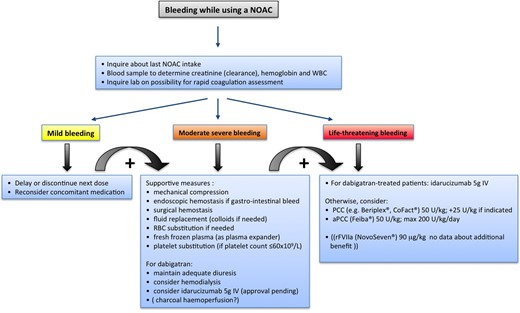
Management of bleeding in patients taking NOACs. Possible therapeutic measures in case of minor or severe bleeding in patients on NOAC therapy. Based on van Ryn et al.34
Cardioversion
A new flowchart has been added to depict different clinical scenarios related to electrical cardioversion (Figure 3). In patients on long-term NOAC therapy (i.e. ≥3 weeks), subgroup analyses from the different trials have shown that electrical cardioversion had a similar (and very low) thromboembolic risk as under warfarin.33–35 The recently published X-VeRT trial confirmed the low peri-cardioversion stroke risk in patients treated with rivaroxaban compared with warfarin in a prospective, controlled design, although with insufficient patient numbers to demonstrate statistically sound non-inferiority.36 As there is no coagulation assay available for any NOAC that provides information on effective anticoagulation over the past 3 weeks, it is mandatory to explicitly ask the patient about adherence over the last weeks and to document the answer in their file. If in doubt about adherence, a TOE should be performed prior to cardioversion.
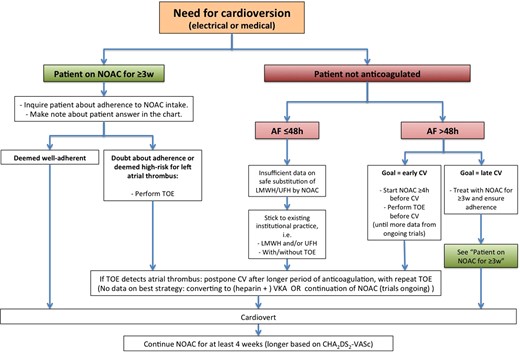
Cardioversion work-flow in AF patients treated with NOACs, depending on the duration of the arrhythmia and prior anticoagulation.
In patients with new-onset AF present for >48 h in whom early cardioversion is preferred without ≥3 weeks pre-treatment with NOACs, a strategy with at least a single NOAC dose ≥4 h before cardioversion is safe and effective, provided that a TOE is performed prior to cardioversion. This strategy has been evaluated in X-VeRT36 and is under evaluation in ongoing trials with other NOACs.
Whether intake of at least 1 pill of NOAC is a feasible strategy in patients with AF of ≤48 h duration, who are currently often cardioverted after a single dose of LMWH or start of unfractionated heparin (with continuation of anticoagulation for ≥4 weeks later on) needs further study. Some of these patients are being included in ongoing trials. In the absence of such data, we recommend adherence to current institutional practice with/without heparin/LMWH and with/without TOE in these patients.
Atrial fibrillation patients with coronary artery disease and in need of (concomitant) antiplatelet therapy
One of the most complex clinical settings comprises the antithrombotic management of AF patients with an acute coronary syndrome (ACS), in need of an elective coronary intervention, or with chronic vascular disease with an indication for single or dual antiplatelet therapy. The number of scenarios, the number of available drugs, and their combinations are so extensive that there is only a paucity of specific data that can guide the clinician in an individual setting. The Updated guide gives a background overview of ‘key scientific’ data in this field, before providing guidance on acute and long-term clinical scenarios. We recognize that institutions and physicians have their own strategies: in the light of all the knowledge gaps, they should not consider our guidance as a rule written in stone, but rather as a foundation (‘default strategy’) to base their own approach. For each scenario, we have highlighted the evidence gaps, and provided patient factors that have to be weighed in the balance to shift away from the default scenario into different directions, in line with other ESC consensus statements.37 These default scenarios are summarized in two completely new flowcharts, one on ‘acute management of elective revascularization or ACS in AF patients treated with NOAC’ (Figure 4) and another on ‘default scenarios and criteria for adaptation for long-term treatment of patients on NOAC therapy after revascularization or ACS’ (Figure 5).
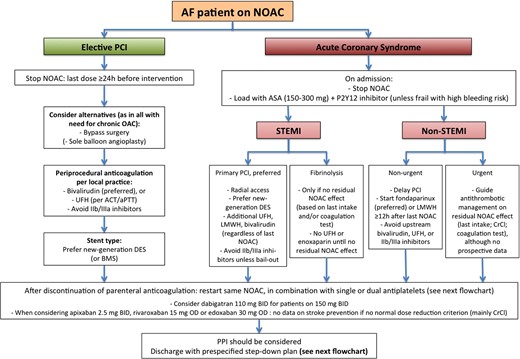
Acute management of revascularization or acute coronary syndrome in AF patients treated with NOACs. See text for further discussion.
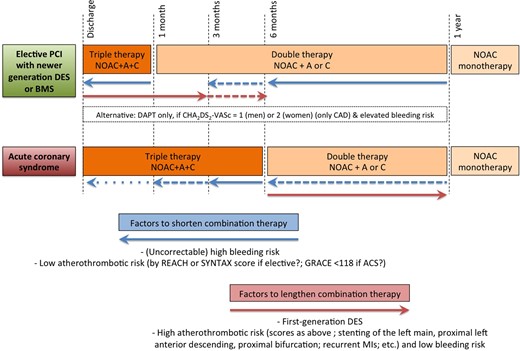
Default scenarios and criteria for adaptation for long-term treatment of patients on NOAC therapy after revascularization or acute coronary syndrome. There are innumerable possible variations on this global theme, as discussed in the text. Patient characteristics and institutional practices should be taken into account to individualize the approach. This figure wants to create a ‘backbone’ as guidance for such tailored approaches. A: aspirin 75–100 mg OD; C: clopidogrel 75 mg OD.
The acute scenario (Figure 4) constitutes coronary interventions in AF patients already on NOACs. Whereas guidelines recommend to maintain VKA patients uninterrupted on their treatment, both during elective or urgent percutaneous coronary intervention (PCI), NOACs should preferably be temporarily discontinued for elective interventions and upon presentation with ACS. This allows safe initiation of antiplatelet therapy and standard local anticoagulation practices peri-procedurally. In stabilized patients (i.e. no recurrent ischaemia or need for other invasive treatment), anticoagulation can be restarted after parenteral anticoagulation is stopped. It is reasonable to restart the NOAC that the patient was taking before the ACS or elective procedure. There are no data to recommend switching to VKA (which may even be associated with higher bleeding and thromboembolic risks, especially in VKA-naïve patients in whom the correct VKA dose is unknown), or to one particular NOAC. The same applies for AF patients after coronary bypass grafting. Whereas it has been shown that the lower dose of dabigatran (110 mg BID) is non-inferior to VKA for stroke prevention but has a lower risk of major bleeding compared with VKA and dabigatran 150 mg BID, also in patients receiving antiplatelet treatment, this dose can be considered during the combination phase.38 The benefits in stroke prevention in patients with a normal renal function is uncertain in patients with the lower dosages of the other NOACs, and such lower-dose choice can therefore not be recommended for those other agents.
Concerning long-term management (Figure 5), patients after revascularization and/or ACS need to be discharged with a prespecified downgrade schedule of antithrombotic agents (i.e. from triple to double therapy, and from double therapy to anticoagulation in monotherapy) to reduce the risk of bleeding while protecting against coronary events. After elective PCI or ACS, we propose a default time of triple therapy of 1 month and 6 months for a bare metal stent or newer DES stent, respectively, thereafter stepping down to double therapy (with OAC and either aspirin or clopidogrel) until 1 year. Factors that weigh in to lengthen or shorten the periods on triple and double therapy are indicated in the flowchart. In a small subset of patients with a low stroke risk (CHA2DS2-VASc of 1 in males or 2 in females, i.e. only CAD) and elevated bleeding risk, one could opt to treat with only dual antiplatelet therapy, without anticoagulants, although in ACTIVE-W there were numerically more myocardial infarctions (MIs) with aspirin plus clopidogrel compared with warfarin.39
For all coronary artery disease (CAD) patients with AF, the default is to step down to anticoagulation in monotherapy after 1 year, except for those with a very high risk for coronary events and an acceptably low bleeding risk. There is no indication that the advantages of NOACs (in monotherapy) over VKAs are not preserved in CAD patients with AF. Lacking direct comparative data, there is also no strong argument for preferring one NOAC over others in this setting.
Neurological situations
In analogy with the different clinical circumstances with CAD, both acute and chronic neurological situations are considered in the Practical Guide. Acute intracerebral haemorrhage (ICH) under NOAC therapy constitutes a particular subform of acute bleeding. Until the new antidotes for NOACs become available, nonspecific procoagulants such as PCC or aPCC can be considered, although their impact on prognosis is unknown. Patients presenting with acute ischaemic stroke under (N)OAC therapy present an even greater clinical conundrum. Until there are reliable and sensitive rapid (point-of-care) tests for the individual NOAC, we would discourage the use of thrombolytics in situations with uncertainty about the anticoagulation status or when NOACs have been administered within the last 24(−48) h. Mechanical recanalization of occluded vessels with stent retrievers may be considered as an alternative treatment option, although no prospectively collected data exist in patients under NOAC therapy.
Although a history of a spontaneous ICH constitutes a contraindication against anticoagulation, patients with a prior ICH have higher ischaemic stroke and mortality rates, partly due to the cessation of anticoagulation after the ICH.40,41 The Guide summarizes considerations related to different types of intracranial bleeding about the potential to restart NOAC therapy. It also provides a flowchart on the timing for restart of anticoagulation after an ischaemic stroke, depending on its size and/or additional imaging (Figure 6). This scheme currently undergoes prospective validation in ongoing clinical trials.
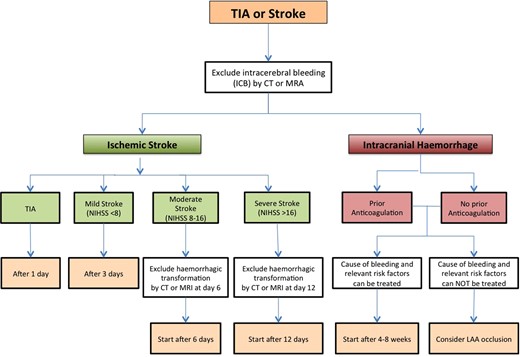
Flowchart for the initiation or re-initiation of anticoagulation after transient ischaemic attack (TIA)/stroke or intracerebral haemorrhage.
Patients with AF and known carotid athorothrombosis with mild-to-moderate asymptomatic stenosis can be treated with NOACs only, without the need for additional antiplatelet therapy, in analogy with stable CAD patients as described above. Patients with AF and symptomatic high-degree stenosis of the internal carotid artery should be operated and not stented. This avoids prolonged triple therapy with high risk of major bleeding in stented patients. In patients undergoing endarterectomy, addition of aspirin is recommended immediately prior to and for 10 days after surgery.42
Other practical considerations
The need for proper patient education and a well-structured follow-up have been reiterated from the original Practical Guide. Before prescribing anticoagulation and considering an NOAC to a patient with AF, kidney function (expressed by a Cockcroft-Gault estimate of glomerular filtration rate, GFR) is required, since NOACs have exclusions and dose recommendations based on GFR. The Guide lists online free GFR and frailty calculators that may assist in decision-making. In the absence of clinical data or experience, NOAC therapy should be avoided in AF patients on haemodialysis or pre-terminal chronic kidney disease (CrCl ≤15 mL/min), although even the benefit of VKAs in such patients is not unequivocally proven.
The uniform EHRA NOAC Anticoagulation Card, proposed in 2013 and available for download in 16 languages, has been slightly modified: there is a dedicated box to state the rationale and planned cessation date of any concomitant antiplatelet therapy; the card acknowledges the participation of pharmacists during follow-up; and the schedule for laboratory checks (especially kidney function) has been slightly modified. A simple ‘rule’ is to specify a recheck interval in ‘number of months = CrCl/10’. The card and text emphasize the need for education, both of the patient and other caregivers in order to improve adherence. Plasma level monitoring cannot be considered as a tool for adherence monitoring. Attention for adherence during regular follow-up visits, technological aids (like smartphone reminders; electronic pill boxes, and a centralized pharmacy dispensing database) may be other tools in a system-wide approach to improve adherence. The Guide calls for prospective, methodologically sound studies on NOAC adherence, including comparing once and twice daily NOACs since the pharmacodynamic and clinical impact of suboptimal adherence may be different.43
Conclusions
New tools create new responsibilities. Non-vitamin-K antagonist oral anticoagulants have been shown to be an attractive alternative for VKA therapy in AF patients, offering net clinical benefit in a wide array of patients. Physicians should make themselves feel confident on using NOAC therapy in clinical practice, and recognize limitations in current knowledge about these drugs. We hope that the Updated Practical Guide is a valuable resource in that regard.
Funding
This article and derived educational materials (slide set, web site, booklet, and NOAC card) were produced by and under the sole responsibility of EHRA, the European Heart Rhythm Association, and supported by Bayer Pharma AG, Boehringer-Ingelheim, Bristol-Myers-Squibb and Pfizer Alliance, and Daiichi-Sankyo Europe GmbH in the form of an Unrestricted Educational Grant. The EHRA writing committee collaborated with medical advisors from the different companies to assure data accuracy and completeness.
Conflict of interest: H.H. is coordinating clinical investigator for the Biotronik-sponsored EuroEco study on health-economics of remote device monitoring. H.H. is a member of the scientific advisory board of Boehringer-Ingelheim, Bayer, BMS-Pfizer, Daiichi-Sankyo, and Sanofi-Aventis, received lecturing fees from these same companies and from Merck, Cardiome, Biotronik, St Jude Medical, and received unconditional research grants through the University of Leuven from St Jude Medical, Medtronic, Biotronik, and Boston Scientific Inc.
P.V. has received research funding through the University of Leuven from Boehringer-Ingelheim, Bayer HealthCare, Daiichi-Sankyo, and ThromboGenics. P.V. has received speaker honoraria from Boehringer-Ingelheim, Bayer Healthcare, Daiichi-Sankyo, Pfizer and Sanofi-Aventis.
M.A. has received advisory board fees from Bayer, Boehringer-Ingelheim, Bristol-Meyer-Squib, Pfizer, and Daiichi-Sankyo, fees for development of educational presentations from Boehringer-Ingelheim and travel support by St Jude Medical.
M.A. has received consulting fees and speaker honoraria from Biosense Webster, Bayer HealthCare, Boehringer-Ingelheim, Sanofi-Aventis, Bristol-Myers-Squibb, Daichii-Sankyo, Pfizer, as well as speaker honoraria from Boston Scientific and Pioneer Medical Devices.
H.C.D. received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer-Ingelheim, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, MSD, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth and Yamanouchi. Financial support for research projects was provided by AstraZeneca, GSK, Boehringer-Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi-Aventis, Syngis, and Talecris. Within the past year HCD served as editor of Aktuelle Neurologie, Arzneimittelthera-pie, Kopfschmerznews, Stroke News and the Treatment Guidelines of the German Neurological Society, as co-editor of Cephalalgia and on the editorial board of Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. HCD has no ownership interest and does not own stocks of any pharmaceutical company.
W.H. received grants for clinical research from Boehringer-Ingelheim Pharmaceuticals.
J.O. received institutional research grant from Boehringer-Ingelheim and has received consulting and speaker fees from Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, and Pfizer.
P.S. has received research funding through the University of Leuven from AstraZeneca and GSK. P.S. has received speaker and/or consulting honoraria from Boehringer-Ingelheim, Bayer Healthcare, Daiichi-Sankyo, Pfizer, Sanofi-Aventis, Bristol-Meyer-Squib, and Abbott J.C. received grants for clinical research from Bristol-Myers-Squibb, Daiichi-Sankyo, Sanofi-Aventis, and Servier. J.C. served as an advisor, speaker and/or, consultant for Actelion Pharmaceuticals, ARYx Therapeutics, Bristol-Myers-Squibb, Cardiome Pharma, CV Therapeutics, Daiichi-Sankyo, Menarini Group, Merck, Novartis Pharmaceuticals, Pfizer, Sanofi-Aventis, Servier, and Xention. He served as a member of the data and safety monitoring board for Bristol-Myers-Squibb, Novartis Pharmaceuticals and Servier. He served as an expert witness for Johnson & Johnson, Sanofi-Aventis and Servier.
P.K. received consulting fees and honoraria from 3 M Medica, MEDA Pharma, AstraZeneca, Bayer Healthcare, Biosense Webster, Boehringer-Ingelheim, Daiichi-Sankyo, German Cardiac Society, MEDA Pharma, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer/BMS, sanofi, Servier, Siemens, TAKEDA, and support for research from 3 M Medica/MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, SANOFI, St Jude Medical, German Federal Ministry for Education and Research (BMBF), Fondation Leducq, German Research Foundation (DFG), and the European Union (EU).
Acknowledgements
EHRA Scientific Documents Committee: Gregory Y.H. Lip (EHRA Scientific Documents Committee Chair), Bulent Gorenek (EHRA Scientific Documents Committee Co-Chair), Christian Sticherling, Laurent Fauchier, Hein Heidbuchel, Angel Moya Mitjans, Mark A. Vos, Michele Brignole, Gheorghe-Andrei Dan, Michele Gulizia, Francisco Marin, Giuseppe Boriani, Deirdre Lane, and Irene Savelieva.
References
Author notes
This is an abridged version of an article published in Europace: Heidbuchel H, Verhamme P, Alings M, Antz M, Diener H-C, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17:1467–1507.



