-
PDF
- Split View
-
Views
-
Cite
Cite
Jonas Oldgren, Lars Wallentin, Rizwan Afzal, Jean-Pierre Bassand, Andrzej Budaj, Susan Chrolavicius, Keith A.A. Fox, Christopher B. Granger, Shamir R. Mehta, Prem Pais, Ron J.G. Peters, Denis Xavier, Jun Zhu, Salim Yusuf, for the OASIS-6 Investigators, Effects of fondaparinux in patients with ST-segment elevation acute myocardial infarction not receiving reperfusion treatment, European Heart Journal, Volume 29, Issue 3, February 2008, Pages 315–323, https://doi.org/10.1093/eurheartj/ehm578
Close - Share Icon Share
Abstract
At least one quarter of ST-segment elevation myocardial infarction (STEMI) patients do not receive reperfusion therapy, and these patients are at high risk for new ischaemic events. We evaluated fondaparinux treatment vs. usual care, i.e. placebo or unfractionated (UF) heparin, in a pre-specified subgroup of 2867 (out of 12 092) patients not receiving reperfusion treatment in the OASIS-6 trial.
In all, 1458 patients were randomized to fondaparinux 2.5 mg once daily subcutaneously up to 8 days and 1409 patients to usual care (control). Randomization was stratified by indication for UF heparin (stratum II, n = 1226) or not (stratum I, n = 1641) based on the investigator’s judgment.
The proportion of patients who suffered death or myocardial re-infarction at 30 days (primary outcome) was 12.2% in the fondaparinux vs. 15.1% in the control group, hazard ratio (HR) 0.80; 95% confidence interval (CI) 0.65–0.98. There was no increase in severe bleedings, HR 0.82; CI 0.44–1.55, or strokes, HR 0.62; CI 0.29–1.33. Consequently, the composite of death, myocardial re-infarction, or severe bleeding were significantly reduced at 30 days, HR 0.81; CI 0.67–0.99. Reductions in death or myocardial re-infarction at 30 days were consistent in stratum I with fondaparinux vs. placebo, HR 0.88; 95% CI 0.65–1.19, and in stratum II with fondaparinux vs. UF heparin infusion for 24–48 h (n = 806), HR 0.74; CI 95% 0.57–0.97, P = 0.41 for heterogeneity.
In STEMI patients not receiving reperfusion treatment, fondaparinux reduces the composite of death or myocardial re-infarction without an increase in severe bleedings or strokes as compared to placebo or UF heparin.
Introduction
Treatment of patients with acute myocardial infarction presenting with ST-segment elevation (STEMI) often includes antithrombotic treatment and, where suitable, reperfusion with either primary percutaneous coronary intervention (PCI)1 or thrombolytic therapy.2 Eligibility for reperfusion therapy may, however, be restricted by several factors including the presence of contraindications to thrombolytic therapy, mainly increased bleeding risk, late presentation (which is variably interpreted as presentation greater than 6 or 12 h after symptom onset), and limited access to primary PCI. Thus, at least one quarter of STEMI patients do not receive reperfusion therapy.3–5 This patient group is heterogeneous but includes many high-risk patients3–5 who have higher mortality rates, and therefore have an urgent need for improved treatment.
Platelet inhibition with aspirin has been shown to reduce mortality and re-infarction in patients with STEMI receiving a wide range of therapies, including those not receiving reperfusion therapy.6 A meta-analysis suggests benefit of unfractionated (UF) heparin in patients not receiving concomitant aspirin treatment, the vast majority of them not receiving fibrinolytic treatment.6 There are however very sparse data on the effects of UF heparin in aspirin treated STEMI patients not receiving reperfusion treatment. The low molecular weight (LMW) heparin enoxaparin has not been shown to be superior to UF heparin in STEMI patients not receiving reperfusion treatment.7 Only reviparin (another LMW heparin) has, as compared to placebo, been shown to reduce the composite of mortality, re-infarction, and stroke,8 but this drug is not widely available. Thus, in patients who are ineligible for reperfusion therapy, treatment with antithrombotic agents other than aspirin is generally left to the discretion of individual physicians.
The synthetic pentasaccharide fondaparinux selectively binds antithrombin and rapidly inhibits factor Xa.9 In patients with acute coronary syndromes presenting without ST-elevation, the OASIS-510 trial revealed similar short-term efficacy of fondaparinux and enoxaparin in preventing early ischaemic events but a large reduction in bleedings by fondaparinux treatment, leading to a reduction in deaths at 1 and 6 months.
The OASIS-6 trial11 evaluated the impact of a strategy of anticoagulant treatment with subcutaneous (SC) fondaparinux 2.5 mg once daily during hospital stay compared with standard approaches to anticoagulant therapy in a broad range of patients with STEMI treated with primary PCI, thrombolysis, or no reperfusion therapy. Briefly, the primary composite outcome of death or myocardial re-infarction at 30 days was significantly reduced by fondaparinux, from 11.2% in the control group to 9.7% in the fondaparinux group, with a non-significant trend towards fewer severe haemorrhages. Given that reperfusion therapy was not used in one quarter of patients, we conducted a pre-specified subgroup analysis in this subset of 2867 patients.
Methods
OASIS-611 was a randomized, double blind trial of a strategy of using fondaparinux as anticoagulant treatment during hospital stay vs. usual care, i.e. UF heparin or no anticoagulant treatment, in STEMI patients treated with fibrinolytics, primary PCI, or no reperfusion therapy. The study was approved by the respective ethics committees and regulatory bodies and involved 447 centers from 41 countries. After obtaining written informed consent, patients presenting with STEMI within 24 h of symptom onset were initially enrolled. This time window was shortened to <12 h after ∼4300 patients had been enrolled, based on the results of the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation (CREATE) trial8 indicating little benefit of anticoagulant treatment in patients randomized beyond 12 h. This decision was made without knowledge of any interim results from OASIS-6. Patients with contraindications to anticoagulation, including those at high risk of bleeding, receiving oral anticoagulants, or with creatinine levels >265 mmol/L (3.0 mg/dL), were excluded. Randomization was stratified by the indication for UF heparin (stratum II) or not (stratum I) based on the investigator’s judgment. The present analysis includes only patients deemed in-eligible for reperfusion therapy by the investigator, e.g. because of late presentation or absolute contraindications to reperfusion therapy, and they could also fall into either stratum I or II according to the protocol, thus reflecting the variability in current clinical practice.
In all, 1226 patients not receiving reperfusion therapy were enrolled in stratum I. These patients were allocated to receive either blinded fondaparinux 2.5 mg SC once daily or matching placebo for up to 8 days or hospital discharge, if earlier. In stratum II, 1641 patients not receiving reperfusion therapy were enrolled. These patients were allocated to receive either blinded fondaparinux or matching placebo (initial dose IV and subsequent doses SC) for up to 8 days or hospital discharge. Those in the control group of stratum II received UF heparin bolus injection of 60 IU/kg followed by an infusion of 12 IU/kg/h for 24–48 h. Equivalent placebo bolus injections and infusions were used in the fondaparinux group. The maximum dose of the UF heparin bolus was 4000 IU and maximum initial infusion rate of 1000 IU/h for patients >70 kg and adjusted to maintain aPTT within the therapeutic range of 1.5–2.0 times for control. To maintain the double-blind criteria, patients receiving UF heparin or placebo infusion (for >3 h) had regular activated partial thromboplastin time monitoring using a hemachron device. A central computerized system produced either real or sham activated partial thromboplastin time values, which were used to adjust the rate of infusion.
The primary and composite outcome of the OASIS-6 study was death or myocardial re-infarction at 30 days. These outcomes were also assessed at 9 days and at study end (minimum of 90 and maximum of 180 days). We decided a priori and without any knowledge of results to follow all patients randomized after 21 March 2005 for only 90 days; thus in the present subgroup 1033 patients were followed for 90 days and 1834 patients for 180 days. The primary purpose of the present sub-study was to evaluate the efficacy and safety of fondaparinux compared with control (placebo or UF heparin) in the subset of patients who were not receiving any type of reperfusion therapy. The analysis of the combined efficacy and safety outcomes, including death, myocardial re-infarction, and severe bleeding was pre-specified. The definition of re-infarction and the classification of deaths and haemorrhages have been previously published.11 All deaths, re-infarctions, strokes, and severe or major bleeds were centrally adjudicated using standardized definitions. In the main trial, two approaches for classification of bleeding complications were used. In the current analysis, we used one of these, i.e. the classification of bleeding that allows comparison to thrombolysis in myocardial infarction trials.12 We classified all bleeding episodes into ‘severe’ [fatal haemorrhage, intracranial haemorrhage, cardiac tamponade, or a clinically significant haemorrhage with a decrease in haemoglobin (Hb) of ≥5 g/dL, with each blood transfusion unit counting for 1.0 g/dL of Hb], ‘minor’ (clinically overt haemorrhage with decrease in Hb 3.0–5.0 g/dL that did not meet criteria for severe haemorrhage, with each blood transfusion unit counting as the equivalent of a 1 g/dL of Hb), and ‘other’.
Statistical analysis
Descriptive statistics (percentages for discrete variables and mean ± SD for continuous variables) were generated for baseline characteristics. Treatment effects are presented as hazard ratios (HR) and two-sided 95% confidence intervals (CI) derived with Cox’s proportional hazard model, and where appropriate tests for heterogeneity. Pre-specified subgroups included initial reperfusion strategy (thrombolytic, primary PCI, or not receiving reperfusion treatment), stratum (UF heparin use or not), gender, and time from symptom onset to start of treatment. Given the large number of subgroup analyses specified, we pre-specified a two-sided level test for interactions between subgroups of 0.01. The statistical analyses were performed by a statistician at the Population Health Research Institute. SAS version 9.1 was used for analysis (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics and antithrombotic treatment before randomization were well balanced between patients randomized to fondaparinux or control (UF heparin or placebo) and are summarized in Table 1. As compared to the patients receiving reperfusion treatment, the patients in the present analysis were older (mean 64.8 vs. 60.5 years) and the mean delay time from symptom onset to randomization was twice as long (mean 10.7 vs. 5.3 h). The proportions of patients with a medical history of previous myocardial infarction, stroke, hypertension, or heart failure were considerably higher in the group of patients not receiving reperfusion treatment.
Baseline characteristics and medications within 7 days prior to randomization
| . | Control (n = 1409) . | Fondaparinux (n = 1458) . | OASIS-6 receiving reperfusion treatment (n = 9225) . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Mean . | SD . |
| Age (years) | 65.0 | 12.7 | 64.6 | 12.4 | 60.5 | 12.0 |
| Body mass index (kg/m2) | 26.5 | 4.5 | 26.7 | 4.5 | 26.5 | 4.3 |
| Onset to randomization (h) | 10.7 | 6.5 | 10.7 | 6.4 | 5.3 | 4.2 |
| Heart rate (beats/min) | 77.6 | 14.1 | 77.4 | 14.2 | 75.8 | 14.5 |
| Diastolic blood pressure (mm Hg) | 80.9 | 14.0 | 81.5 | 13.9 | 81.4 | 14.5 |
| Systolic blood pressure (mm Hg) | 134.4 | 23.6 | 135.3 | 23.3 | 133.9 | 23.3 |
| n | % | n | % | |||
| Men | 903 | 64.1 | 955 | 65.5 | 6888 | 74.7 |
| Previous myocardial infarction | 231 | 16.4 | 240 | 16.5 | 1047 | 11.3 |
| Previous stroke | 146 | 10.4 | 147 | 10.1 | 508 | 5.5 |
| Hypertension | 899 | 63.8 | 969 | 66.5 | 4713 | 51.1 |
| Heart failure | 387 | 27.5 | 376 | 25.8 | 921 | 10.0 |
| Diabetes | 250 | 17.7 | 253 | 17.4 | 1649 | 17.9 |
| Anterior myocardial infarction | 730 | 51.8 | 711 | 48.8 | 4162 | 45.1 |
| New left bundle branch block | 37 | 2.6 | 31 | 2.1 | 50 | 0.5 |
| Aspirin | 815 | 57.8 | 851 | 58.4 | 5803 | 62.9 |
| Clopidogrel | 123 | 8.7 | 112 | 7.7 | 1566 | 17.0 |
| UH heparin | 150 | 10.6 | 179 | 12.3 | 1458 | 15.8 |
| LMW heparin | 44 | 3.1 | 46 | 3.2 | 131 | 1.4 |
| Oral anticoagulants | 2 | 0.1 | 4 | 0.3 | 23 | 0.2 |
| . | Control (n = 1409) . | Fondaparinux (n = 1458) . | OASIS-6 receiving reperfusion treatment (n = 9225) . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Mean . | SD . |
| Age (years) | 65.0 | 12.7 | 64.6 | 12.4 | 60.5 | 12.0 |
| Body mass index (kg/m2) | 26.5 | 4.5 | 26.7 | 4.5 | 26.5 | 4.3 |
| Onset to randomization (h) | 10.7 | 6.5 | 10.7 | 6.4 | 5.3 | 4.2 |
| Heart rate (beats/min) | 77.6 | 14.1 | 77.4 | 14.2 | 75.8 | 14.5 |
| Diastolic blood pressure (mm Hg) | 80.9 | 14.0 | 81.5 | 13.9 | 81.4 | 14.5 |
| Systolic blood pressure (mm Hg) | 134.4 | 23.6 | 135.3 | 23.3 | 133.9 | 23.3 |
| n | % | n | % | |||
| Men | 903 | 64.1 | 955 | 65.5 | 6888 | 74.7 |
| Previous myocardial infarction | 231 | 16.4 | 240 | 16.5 | 1047 | 11.3 |
| Previous stroke | 146 | 10.4 | 147 | 10.1 | 508 | 5.5 |
| Hypertension | 899 | 63.8 | 969 | 66.5 | 4713 | 51.1 |
| Heart failure | 387 | 27.5 | 376 | 25.8 | 921 | 10.0 |
| Diabetes | 250 | 17.7 | 253 | 17.4 | 1649 | 17.9 |
| Anterior myocardial infarction | 730 | 51.8 | 711 | 48.8 | 4162 | 45.1 |
| New left bundle branch block | 37 | 2.6 | 31 | 2.1 | 50 | 0.5 |
| Aspirin | 815 | 57.8 | 851 | 58.4 | 5803 | 62.9 |
| Clopidogrel | 123 | 8.7 | 112 | 7.7 | 1566 | 17.0 |
| UH heparin | 150 | 10.6 | 179 | 12.3 | 1458 | 15.8 |
| LMW heparin | 44 | 3.1 | 46 | 3.2 | 131 | 1.4 |
| Oral anticoagulants | 2 | 0.1 | 4 | 0.3 | 23 | 0.2 |
Baseline characteristics and medications within 7 days prior to randomization
| . | Control (n = 1409) . | Fondaparinux (n = 1458) . | OASIS-6 receiving reperfusion treatment (n = 9225) . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Mean . | SD . |
| Age (years) | 65.0 | 12.7 | 64.6 | 12.4 | 60.5 | 12.0 |
| Body mass index (kg/m2) | 26.5 | 4.5 | 26.7 | 4.5 | 26.5 | 4.3 |
| Onset to randomization (h) | 10.7 | 6.5 | 10.7 | 6.4 | 5.3 | 4.2 |
| Heart rate (beats/min) | 77.6 | 14.1 | 77.4 | 14.2 | 75.8 | 14.5 |
| Diastolic blood pressure (mm Hg) | 80.9 | 14.0 | 81.5 | 13.9 | 81.4 | 14.5 |
| Systolic blood pressure (mm Hg) | 134.4 | 23.6 | 135.3 | 23.3 | 133.9 | 23.3 |
| n | % | n | % | |||
| Men | 903 | 64.1 | 955 | 65.5 | 6888 | 74.7 |
| Previous myocardial infarction | 231 | 16.4 | 240 | 16.5 | 1047 | 11.3 |
| Previous stroke | 146 | 10.4 | 147 | 10.1 | 508 | 5.5 |
| Hypertension | 899 | 63.8 | 969 | 66.5 | 4713 | 51.1 |
| Heart failure | 387 | 27.5 | 376 | 25.8 | 921 | 10.0 |
| Diabetes | 250 | 17.7 | 253 | 17.4 | 1649 | 17.9 |
| Anterior myocardial infarction | 730 | 51.8 | 711 | 48.8 | 4162 | 45.1 |
| New left bundle branch block | 37 | 2.6 | 31 | 2.1 | 50 | 0.5 |
| Aspirin | 815 | 57.8 | 851 | 58.4 | 5803 | 62.9 |
| Clopidogrel | 123 | 8.7 | 112 | 7.7 | 1566 | 17.0 |
| UH heparin | 150 | 10.6 | 179 | 12.3 | 1458 | 15.8 |
| LMW heparin | 44 | 3.1 | 46 | 3.2 | 131 | 1.4 |
| Oral anticoagulants | 2 | 0.1 | 4 | 0.3 | 23 | 0.2 |
| . | Control (n = 1409) . | Fondaparinux (n = 1458) . | OASIS-6 receiving reperfusion treatment (n = 9225) . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Mean . | SD . |
| Age (years) | 65.0 | 12.7 | 64.6 | 12.4 | 60.5 | 12.0 |
| Body mass index (kg/m2) | 26.5 | 4.5 | 26.7 | 4.5 | 26.5 | 4.3 |
| Onset to randomization (h) | 10.7 | 6.5 | 10.7 | 6.4 | 5.3 | 4.2 |
| Heart rate (beats/min) | 77.6 | 14.1 | 77.4 | 14.2 | 75.8 | 14.5 |
| Diastolic blood pressure (mm Hg) | 80.9 | 14.0 | 81.5 | 13.9 | 81.4 | 14.5 |
| Systolic blood pressure (mm Hg) | 134.4 | 23.6 | 135.3 | 23.3 | 133.9 | 23.3 |
| n | % | n | % | |||
| Men | 903 | 64.1 | 955 | 65.5 | 6888 | 74.7 |
| Previous myocardial infarction | 231 | 16.4 | 240 | 16.5 | 1047 | 11.3 |
| Previous stroke | 146 | 10.4 | 147 | 10.1 | 508 | 5.5 |
| Hypertension | 899 | 63.8 | 969 | 66.5 | 4713 | 51.1 |
| Heart failure | 387 | 27.5 | 376 | 25.8 | 921 | 10.0 |
| Diabetes | 250 | 17.7 | 253 | 17.4 | 1649 | 17.9 |
| Anterior myocardial infarction | 730 | 51.8 | 711 | 48.8 | 4162 | 45.1 |
| New left bundle branch block | 37 | 2.6 | 31 | 2.1 | 50 | 0.5 |
| Aspirin | 815 | 57.8 | 851 | 58.4 | 5803 | 62.9 |
| Clopidogrel | 123 | 8.7 | 112 | 7.7 | 1566 | 17.0 |
| UH heparin | 150 | 10.6 | 179 | 12.3 | 1458 | 15.8 |
| LMW heparin | 44 | 3.1 | 46 | 3.2 | 131 | 1.4 |
| Oral anticoagulants | 2 | 0.1 | 4 | 0.3 | 23 | 0.2 |
Almost all patients received aspirin, and the use of beta-blockers, angiotensin converting enzyme (ACE) inhibitors and lipid lowering agents after randomization was also high (Table 2). Post randomization non-study UF heparin was used in 7.7% of the patients, LWM heparin in 5.8%, and glycoprotein (GP) IIB/IIIA receptor antagonists in 4.2% of the patients, with similar proportions in the control and fondaparinux groups but slightly lower than in patients receiving reperfusion treatment. Approximately 26% of the patients were also treated with clopidogrel, as compared to a more than twice as high proportion in patients receiving reperfusion treatment. Study treatment with fondaparinux was administered for 8 (5; 8) days (median, first and third quartiles) and corresponding placebo for 8 (6; 8) days.
| . | Control . | Fondaparinux . | OASIS-6 receiving reperfusion treatment . | |||
|---|---|---|---|---|---|---|
| . | n = 1409 . | % . | n = 1458 . | % . | n = 9225 . | % . |
| Aspirin | 1334 | 94.7 | 1390 | 95.4 | 8956 | 97.1 |
| Non-study UF heparin | 119 | 8.4 | 103 | 7.1 | 1110 | 12.0 |
| LMW heparin | 86 | 6.1 | 79 | 5.4 | 541 | 5.9 |
| GP IIb/IIIa | 57 | 4.0 | 62 | 4.3 | 1773 | 19.2 |
| Clopidogrel | 360 | 25.6 | 375 | 25.7 | 5765 | 62.5 |
| Ticlopidine | 63 | 4.5 | 60 | 4.1 | 914 | 9.9 |
| Oral anticoagulants | 37 | 2.6 | 35 | 2.4 | 216 | 2.3 |
| Beta blockers | 1136 | 80.6 | 1190 | 81.7 | 7840 | 85.0 |
| Calcium channel blockers | 203 | 14.4 | 211 | 14.5 | 849 | 9.2 |
| ACE inhibitors | 1075 | 76.3 | 1149 | 78.9 | 7201 | 78.1 |
| ARB agents | 55 | 3.9 | 55 | 3.8 | 292 | 3.2 |
| Spironolactone/eplerenone | 181 | 12.8 | 153 | 10.5 | 873 | 9.5 |
| Loop diuretics | 554 | 39.3 | 555 | 38.1 | 2547 | 27.6 |
| Statins | 805 | 57.1 | 830 | 57.0 | 7291 | 79.0 |
| . | Control . | Fondaparinux . | OASIS-6 receiving reperfusion treatment . | |||
|---|---|---|---|---|---|---|
| . | n = 1409 . | % . | n = 1458 . | % . | n = 9225 . | % . |
| Aspirin | 1334 | 94.7 | 1390 | 95.4 | 8956 | 97.1 |
| Non-study UF heparin | 119 | 8.4 | 103 | 7.1 | 1110 | 12.0 |
| LMW heparin | 86 | 6.1 | 79 | 5.4 | 541 | 5.9 |
| GP IIb/IIIa | 57 | 4.0 | 62 | 4.3 | 1773 | 19.2 |
| Clopidogrel | 360 | 25.6 | 375 | 25.7 | 5765 | 62.5 |
| Ticlopidine | 63 | 4.5 | 60 | 4.1 | 914 | 9.9 |
| Oral anticoagulants | 37 | 2.6 | 35 | 2.4 | 216 | 2.3 |
| Beta blockers | 1136 | 80.6 | 1190 | 81.7 | 7840 | 85.0 |
| Calcium channel blockers | 203 | 14.4 | 211 | 14.5 | 849 | 9.2 |
| ACE inhibitors | 1075 | 76.3 | 1149 | 78.9 | 7201 | 78.1 |
| ARB agents | 55 | 3.9 | 55 | 3.8 | 292 | 3.2 |
| Spironolactone/eplerenone | 181 | 12.8 | 153 | 10.5 | 873 | 9.5 |
| Loop diuretics | 554 | 39.3 | 555 | 38.1 | 2547 | 27.6 |
| Statins | 805 | 57.1 | 830 | 57.0 | 7291 | 79.0 |
UF, unfractionated; LMW, low molecular weight; GP, glycoprotein; ACE, angiotensin converting enzyme.
ARB, angiotensin II receptor blocker.
| . | Control . | Fondaparinux . | OASIS-6 receiving reperfusion treatment . | |||
|---|---|---|---|---|---|---|
| . | n = 1409 . | % . | n = 1458 . | % . | n = 9225 . | % . |
| Aspirin | 1334 | 94.7 | 1390 | 95.4 | 8956 | 97.1 |
| Non-study UF heparin | 119 | 8.4 | 103 | 7.1 | 1110 | 12.0 |
| LMW heparin | 86 | 6.1 | 79 | 5.4 | 541 | 5.9 |
| GP IIb/IIIa | 57 | 4.0 | 62 | 4.3 | 1773 | 19.2 |
| Clopidogrel | 360 | 25.6 | 375 | 25.7 | 5765 | 62.5 |
| Ticlopidine | 63 | 4.5 | 60 | 4.1 | 914 | 9.9 |
| Oral anticoagulants | 37 | 2.6 | 35 | 2.4 | 216 | 2.3 |
| Beta blockers | 1136 | 80.6 | 1190 | 81.7 | 7840 | 85.0 |
| Calcium channel blockers | 203 | 14.4 | 211 | 14.5 | 849 | 9.2 |
| ACE inhibitors | 1075 | 76.3 | 1149 | 78.9 | 7201 | 78.1 |
| ARB agents | 55 | 3.9 | 55 | 3.8 | 292 | 3.2 |
| Spironolactone/eplerenone | 181 | 12.8 | 153 | 10.5 | 873 | 9.5 |
| Loop diuretics | 554 | 39.3 | 555 | 38.1 | 2547 | 27.6 |
| Statins | 805 | 57.1 | 830 | 57.0 | 7291 | 79.0 |
| . | Control . | Fondaparinux . | OASIS-6 receiving reperfusion treatment . | |||
|---|---|---|---|---|---|---|
| . | n = 1409 . | % . | n = 1458 . | % . | n = 9225 . | % . |
| Aspirin | 1334 | 94.7 | 1390 | 95.4 | 8956 | 97.1 |
| Non-study UF heparin | 119 | 8.4 | 103 | 7.1 | 1110 | 12.0 |
| LMW heparin | 86 | 6.1 | 79 | 5.4 | 541 | 5.9 |
| GP IIb/IIIa | 57 | 4.0 | 62 | 4.3 | 1773 | 19.2 |
| Clopidogrel | 360 | 25.6 | 375 | 25.7 | 5765 | 62.5 |
| Ticlopidine | 63 | 4.5 | 60 | 4.1 | 914 | 9.9 |
| Oral anticoagulants | 37 | 2.6 | 35 | 2.4 | 216 | 2.3 |
| Beta blockers | 1136 | 80.6 | 1190 | 81.7 | 7840 | 85.0 |
| Calcium channel blockers | 203 | 14.4 | 211 | 14.5 | 849 | 9.2 |
| ACE inhibitors | 1075 | 76.3 | 1149 | 78.9 | 7201 | 78.1 |
| ARB agents | 55 | 3.9 | 55 | 3.8 | 292 | 3.2 |
| Spironolactone/eplerenone | 181 | 12.8 | 153 | 10.5 | 873 | 9.5 |
| Loop diuretics | 554 | 39.3 | 555 | 38.1 | 2547 | 27.6 |
| Statins | 805 | 57.1 | 830 | 57.0 | 7291 | 79.0 |
UF, unfractionated; LMW, low molecular weight; GP, glycoprotein; ACE, angiotensin converting enzyme.
ARB, angiotensin II receptor blocker.
In the subgroup of patients not receiving reperfusion therapy, the composite of death or myocardial re-infarction at 30 days (primary study outcome) was significantly reduced in the fondaparinux group as compared to UF heparin/placebo (Figure 1). There was a consistent trend towards reduction in the individual components of the primary endpoint (Table 3). The proportions of patients with severe bleedings or strokes were not significantly different between the fondaparinux group compared with the UF heparin/placebo group. Overall, fondaparinux treatment favourably affected the clinical benefit–risk balance, i.e. the composite of death, myocardial re-infarction, and severe bleedings, and the composite of death, myocardial re-infarction, and stroke (Table 3).
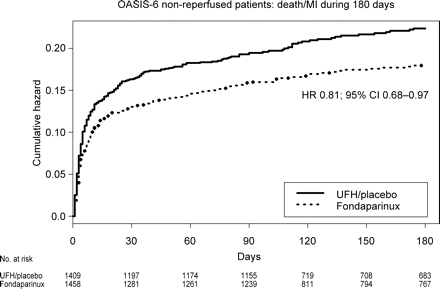
Kaplan–Meier curves with comparison of the cumulative hazard of death or myocardial re-infarction in fondaparinux (solid line) and control (dashed line) groups. HR and 95% CI were derived with Cox’s proportional hazard model. In all, 1033 patients were followed for 90 days and 1834 patients for 180 days
| . | Control (n = 1409) . | Fondaparinux (n = 1409) . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | . | . |
| Death | 176 | 12.5 | 153 | 10.5 | 0.83 | 0.67–1.04 |
| Myocardial re-infarction (MI) | 49 | 3.7 | 34 | 2.5 | 0.66 | 0.43–1.02 |
| Stroke | 17 | 1.3 | 11 | 0.8 | 0.62 | 0.29–1.33 |
| Severe haemorrhage | 21 | 1.5 | 18 | 1.3 | 0.82 | 0.44–1.55 |
| Death/MI | 212 | 15.1 | 178 | 12.2 | 0.80 | 0.65–0.98 |
| Death, MI, stroke | 225 | 16.0 | 185 | 12.7 | 0.78 | 0.64–0.95 |
| Death/MI/severe haemorrhage | 215 | 15.3 | 183 | 12.6 | 0.81 | 0.67–0.99 |
| . | Control (n = 1409) . | Fondaparinux (n = 1409) . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | . | . |
| Death | 176 | 12.5 | 153 | 10.5 | 0.83 | 0.67–1.04 |
| Myocardial re-infarction (MI) | 49 | 3.7 | 34 | 2.5 | 0.66 | 0.43–1.02 |
| Stroke | 17 | 1.3 | 11 | 0.8 | 0.62 | 0.29–1.33 |
| Severe haemorrhage | 21 | 1.5 | 18 | 1.3 | 0.82 | 0.44–1.55 |
| Death/MI | 212 | 15.1 | 178 | 12.2 | 0.80 | 0.65–0.98 |
| Death, MI, stroke | 225 | 16.0 | 185 | 12.7 | 0.78 | 0.64–0.95 |
| Death/MI/severe haemorrhage | 215 | 15.3 | 183 | 12.6 | 0.81 | 0.67–0.99 |
| . | Control (n = 1409) . | Fondaparinux (n = 1409) . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | . | . |
| Death | 176 | 12.5 | 153 | 10.5 | 0.83 | 0.67–1.04 |
| Myocardial re-infarction (MI) | 49 | 3.7 | 34 | 2.5 | 0.66 | 0.43–1.02 |
| Stroke | 17 | 1.3 | 11 | 0.8 | 0.62 | 0.29–1.33 |
| Severe haemorrhage | 21 | 1.5 | 18 | 1.3 | 0.82 | 0.44–1.55 |
| Death/MI | 212 | 15.1 | 178 | 12.2 | 0.80 | 0.65–0.98 |
| Death, MI, stroke | 225 | 16.0 | 185 | 12.7 | 0.78 | 0.64–0.95 |
| Death/MI/severe haemorrhage | 215 | 15.3 | 183 | 12.6 | 0.81 | 0.67–0.99 |
| . | Control (n = 1409) . | Fondaparinux (n = 1409) . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | . | . |
| Death | 176 | 12.5 | 153 | 10.5 | 0.83 | 0.67–1.04 |
| Myocardial re-infarction (MI) | 49 | 3.7 | 34 | 2.5 | 0.66 | 0.43–1.02 |
| Stroke | 17 | 1.3 | 11 | 0.8 | 0.62 | 0.29–1.33 |
| Severe haemorrhage | 21 | 1.5 | 18 | 1.3 | 0.82 | 0.44–1.55 |
| Death/MI | 212 | 15.1 | 178 | 12.2 | 0.80 | 0.65–0.98 |
| Death, MI, stroke | 225 | 16.0 | 185 | 12.7 | 0.78 | 0.64–0.95 |
| Death/MI/severe haemorrhage | 215 | 15.3 | 183 | 12.6 | 0.81 | 0.67–0.99 |
The benefits of fondaparinux treatment on the composite of death or myocardial re-infarction were apparent already at 3 days, HR 0.77; 95% CI 0.58–1.04, and sustained to study end after 3–6 months (Figure 1). The clinical benefit–risk composite outcome of death, myocardial re-infarction, and severe bleeds was also reduced by fondaparinux at study end, HR 0.82; 95% CI 0.69–0.97.
Stratum I and II
According to the judgment of their physicians, 1226 patients did not (stratum I) and 1641 patients did have an indication for UF heparin treatment (stratum II). Fondaparinux treatment was administered for 8 (6; 8) days in stratum I and 8 (5; 8) days in stratum II. UF heparin infusion was given for 42 (25; 48) hours in stratum II.
The benefits of fondaparinux treatment on the composite of death or myocardial re-infarction were apparent already at 3 days, both in stratum I, fondaparinux vs. placebo HR 0.76; 95% CI 0.48–1.20, and in stratum II, fondaparinux vs. UF heparin HR 0.79, 95% CI 0.53–1.16, P = 0.91 for heterogeneity. There were directionally consistent results on the primary composite of death or myocardial re-infarction at 30 days in the two strata, fondaparinux vs. placebo, HR 0.88; 95% CI 0.65–1.19, and fondaparinux vs. UF heparin, HR 0.74; 95% CI 0.57–0.97 (Figure 2).
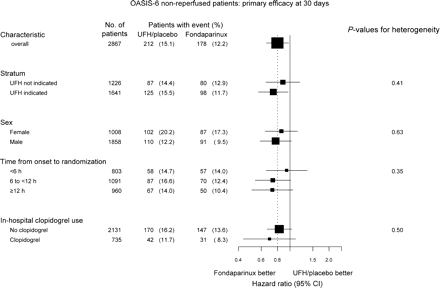
Comparison of fondaparinux vs. UF heparin or placebo in subgroups. The dashed vertical line represents the overall effect. The sizes of the boxes are proportional to the numbers of patients. In some patients information used to characterize patients into subgroups is missing. UFH, unfractionated heparin
Severe bleedings at 3 days were not different between fondaparinux and placebo, HR 0.39; 95% CI 0.08–2.00, or fondaparinux and UF heparin, HR 0.72; 95% CI 0.30–1.71, P = 0.51 for heterogeneity. Neither were there differences in severe bleedings at 30 days, fondaparinux vs. placebo, HR 0.78; 95% CI 0.21–2.89, and fondaparinux vs. UF heparin, HR 0.84; 95% CI 0.41–1.72 (Figure 3).
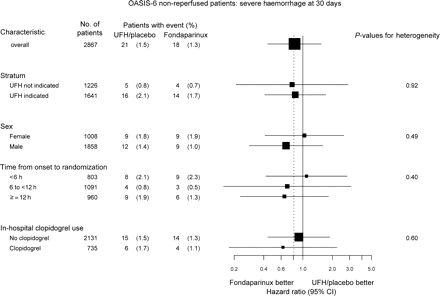
Comparison of fondaparinux vs. UF heparin or placebo in subgroups. The dashed vertical line represents the overall effect. The sizes of the boxes are proportional to the numbers of patients. In some patients information used to characterize patients into subgroups is missing. UFH, unfractionated heparin
Time from symptom onset to randomization and gender
Twenty eight per cent of the patients not treated with reperfusion therapy were randomized within 6 h from symptom onset, 38% between 6 and 12 h, and 34% at 12 or more hours from symptom onset. There was no significant heterogeneity in the effect of fondaparinux vs. control on the composite of death or myocardial re-infarction at 30 days in relation to time from symptom onset to randomization (Figure 2). Similar trends for a reduction by fondaparinux treatment in the composite of death or myocardial re-infarction at 30 days were found in women and men (Figure 2). Neither were there any significant differences in the risk of severe bleedings by fondaparinux nor control in these subgroups, i.e. time from symptom onset to randomization or gender (Figure 3).
Concomitant clopidogrel treatment
In all, 735 (26%) of the patients were treated with clopidogrel post randomization, 709 (96.5%) of them in addition to aspirin. There was no significant heterogeneity in the effect of fondaparinux vs. control in patients with or without concomitant clopidogrel treatment (Figure 2). The rates of severe bleedings were low in both the fondaparinux and control groups, with and without clopidogrel, respectively, with no significant heterogeneity (Figure 3).
Discussion
In acute STEMI patients not receiving reperfusion treatment, a treatment strategy with fondaparinux up to 8 days significantly reduced the composite of mortality and myocardial re-infarction at 30 days compared with placebo or UF heparin infusion given for a median of 42 h. The majority of patients also received aspirin, beta-blockers, and ACE inhibitors, all of which are known to improve prognosis in STEMI patients.6,13,14 The risk of bleeding or strokes was not increased and these results were consistent with the findings in both OASIS-510 and the main OASIS-611 trial. There was no significant heterogeneity concerning the effects of fondaparinux between patients with or without UF heparin treatment in the control group, with benefits of fondaparinux apparent already after 3 days.
Although early reperfusion therapy is strongly recommended in STEMI treatment guidelines, the proportion of STEMI patients not receiving reperfusion treatment varies from 21 to 46% in recent clinical trials,8,11,15 both reflecting differences in clinical practice but also depending on the criteria for inclusion (e.g. time from symptom onset) in the trials. In the American National Registry of Myocardial Infarction (NRMI) 2 and 3,16 ∼30% of the 376 753 STEMI patients presenting within 12 h from symptom onset did not receive reperfusion treatment, without major changes over time from 1994–1999. Similarly, in the Global Registry of Acute Coronary Events (GRACE),3 30% of the 1763 patients presenting within 12 h of symptom onset did not receive reperfusion therapy. Reasons for underutilization of reperfusion therapy include late presentation, limited access to reperfusion therapy, and lack of economic resources in developing countries, but may also reflect absolute or perceived contraindications such as increased bleeding risk, advanced age, and female gender. The patients not receiving reperfusion treatment in the OASIS-6 study were older and included a higher proportion of patients with hypertension, heart failure, previous myocardial infarction, and stroke, probably at least in part explaining the higher ischaemic event rate in the present analysis as compared to the patients receiving reperfusion treatment. High-risk patients were also less likely to receive reperfusion therapy in the NRMI17 and GRACE3 registries, i.e. elderly and patients with a history of congestive heart failure, previous coronary bypass surgery, history of diabetes, and those presenting without chest pain. Thus, the subgroup of STEMI patients not receiving reperfusion therapy are at substantial risk, and so even modest reductions in mortality or morbidity in this population would be clinically important.
An overview of randomized clinical trials evaluating UF heparin in patients with STEMI performed before the reperfusion era reported a 17% risk reduction in mortality and 22% reduction in re-infarction.18 However, aspirin was not commonly used in these patients, among whom there was no evidence of benefit. In the present study, with high rates of aspirin use, treatment with fondaparinux was superior to control, with consistent results in those receiving placebo or UF heparin in the control group.
LMW heparins in addition to aspirin have been evaluated in STEMI patients non-eligible for reperfusion treatment. The TETAMI (Treatment with Enoxaparin and Tirofiban in Acute Myocardial Infarction) trial7 evaluated 1224 STEMI patients who were not eligible for reperfusion therapy. Enoxaparin SC twice daily for 2–8 days was compared with UF heparin treatment, with and without the GPIIB/IIIA receptor antagonist tirofiban, in a 2 × 2 factorial design. There were no significant differences between the enoxaparin and UF heparin groups in the combined incidence of death, re-infarction, or recurrent angina at 30 days, and no significant differences in safety were observed. Additional therapy with tirofiban was not beneficial in this population.
In a pre-specified subgroup analysis of the CREATE trail,8 evaluating the LMW heparin reviparin vs. placebo in STEMI patients, 3325 (21%) out of the 15 570 patients did not receive any reperfusion therapy. Reviparin SC twice daily for 7 days reduced the composite of death, myocardial re-infarction, and stroke at 7 days as compared to placebo in non-reperfused patients, HR 0.79; 95% CI, 0.65–0.95. However, there was a significant increase in the rates of life-threatening or major bleeding (including intracranial haemorrhage) with reviparin, although the rates of bleedings in the subgroup of patients not receiving reperfusion therapy were very low. Reviparin might thereby be an alternative antithrombotic for STEMI patients not receiving reperfusion therapy, however, this drug is not widely available. Newer antithrombotic agents, i.e. direct thrombin inhibitors and intravenous platelet inhibitors, have not been evaluated in STEMI patients not receiving reperfusion therapy.
Platelet inhibition with aspirin has been shown to reduce mortality, with or without concomitant reperfusion therapy6 and is the cornerstone of antithrombotic treatment in STEMI. The recent Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT)15 evaluated short-term addition of clopidogrel to aspirin which significantly reduced mortality and the composite of death, re-infarction, or stroke without increased risk of major bleeding compared with aspirin alone in 45 852 STEMI patients. As the results of COMMIT were not available until halfway during this trial only ∼25% of patients in the present study received combined treatment with clopidogrel and aspirin. The effects of fondaparinux on both safety and efficacy appeared to be consistent in this subgroup compared with the remaining patients.
The unique aspect of fondaparinux is that at the doses used there is a clinically important reduction in the composite of mortality and re-infarction without an increase in severe bleeding in STEMI patients without reperfusion treatment as well as in patients treated with thrombolysis. This contrasts with the experiences with other antithrombotic agents in acute myocardial infarction such as UF heparin,6 LMW heparins (both enoxaparin and reviparin),8,19 direct thrombin inhibitors,20 or IV GPIIb/IIIa inhibitors.21 The results in the present analysis as well as the main OASIS-6 study are consistent with the markedly lower rates of bleeding with fondaparinux compared with enoxaparin in OASIS-5. This is clinically relevant since several studies in acute coronary syndromes have shown that bleeding is strongly associated with increased mortality,22,23 emphasizing the need for therapies that are both effective and safe.
Limitations
The separate analysis of the main types of reperfusion therapy (primary PCI, thrombolytic, and no reperfusion therapy), UF heparin use or not, gender, and time from symptom onset to start of treatment were pre-specified. However, further subgroup analyses, such as the post-hoc analysis of patients receiving clopidogrel treatment, should be regarded as exploratory and interpreted with considerable caution due to the large number of analyses performed. Nevertheless, the lack of statistical heterogeneity between the various subgroups not undergoing primary PCI, provides reassurance that the benefits of fondaparinux are not likely to differ in the various subgroups examined in this analysis. The reasons for not receiving reperfusion treatment were not recorded in individual patients and this is a limitation to the present analysis. Those receiving no reperfusion presented to hospital much later and were ∼5 years older, suggesting that the main reasons for not using reperfusion therapy were likely related to delays in presentation and advanced age, which is consistent with previous studies.
Conclusions
In STEMI patients not receiving reperfusion therapy, a treatment strategy with SC injections of fondaparinux as compared to usual care (UF heparin infusion or placebo) reduced the composite of death or myocardial re-infarction without increasing severe bleedings or strokes. This unique combination of results emphasizes that incremental benefit in reducing ischaemic events may be achieved without compromising safety.
Funding
The OASIS-6 study was jointly funded by Sanofi-Aventis, Organon, and GlaxoSmithKline.
Acknowledgements
The trial was conducted, data collected, and analyzed independently by the Population Health Research Institute and the steering committee. D.X. is supported by the Canadian Institutes of Health Research Canada-HOPE scholarship award. S.Y. hold a research chair of the Heart and Stroke Foundation of Ontario.
Conflict of interest: none declared.



