-
PDF
- Split View
-
Views
-
Cite
Cite
Ron J.G. Peters, Campbell Joyner, Jean-Pierre Bassand, Rizwan Afzal, Susan Chrolavicius, Shamir R. Mehta, Jonas Oldgren, Lars Wallentin, Andrzej Budaj, Keith A. Fox, Salim Yusuf, for the OASIS-6 investigators, The role of fondaparinux as an adjunct to thrombolytic therapy in acute myocardial infarction: a subgroup analysis of the OASIS-6 trial, European Heart Journal, Volume 29, Issue 3, February 2008, Pages 324–331, https://doi.org/10.1093/eurheartj/ehm616
Close - Share Icon Share
Abstract
No antithrombotic therapy has been shown to reduce mortality when used with thrombolytics in acute myocardial infarction (AMI). In the OASIS-6 trial, fondaparinux significantly reduced mortality and reinfarction without increasing bleeding in 12 092 patients with acute ST elevation MI.
We report the results of a subgroup analysis in the 5436 patients (45%) receiving thrombolytics. According to local practice, 4415 patients did not have an indication for unfractionated heparin (stratum 1) and 1021 did (stratum 2). Fondaparinux reduced the primary study outcome of death or MI at 30 days [Hazard ratio (HR) 0.79, 95% confidence interval (CI) 0.68–0.92] with consistent reductions in both mortality (HR and CI) and reinfarction (HR and CI). There was a non-significantly lower rate of stroke (HR 0.77, CI 0.48–1.25). The risk of severe bleeding was significantly reduced (HR 0.62, CI 0.40–0.94), and thus the balance of benefit and risk (death, MI and severe haemorrhage) was clearly reduced by fondaparinux (HR 0.77, 95% CI 0.67–0.90). Results were consistent in the two strata, by the different types of thrombolytics and across various time intervals from symptom onset to treatment.
In STEMI patients treated with thrombolytic agents (predominantly streptokinase), fondaparinux significantly reduced the risk of death, re-MI and severe bleeds.
Introduction
Thrombolytic therapy for acute myocardial infarction (AMI) presenting with ST segment elevations (STEMI) routinely includes aspirin and more recently clopidogrel. The role of antithrombin agents in this setting remains unclear. With non-fibrin specific (NFS) thrombolytic agents, administration of unfractionated heparin (UFH) provides no clinical benefit but increases the risk of bleeding complications.1,2 Although contemporary guidelines recommend the administration of UFH with fibrin specific (FS) thrombolytic therapy, there are no data that such therapy reduces mortality or morbidity, and that the benefits outweigh the increased risk of bleeding.3–5 A recent trial in patients treated with FS agents indicated that enoxaparin reduces the risk of reinfarction as compared to UFH, without a significant reduction in mortality, but with an increased risk of major bleeding, including intracranial haemorrhage (ICH) and other fatal bleeds.6 A large trial of reviparin compared with placebo indicates a reduction in mortality, and reinfarction, but with an excess in intracranial and other major bleeding.7 However, reviparin is not available in many countries, and the company has not sought regulatory approval for AMI. Thus, for patients treated with thrombolytic therapy, in most countries, there is no anticoagulant available that has demonstrated a reduction in mortality, with an acceptable safety profile. Therefore, there is a need for antithrombotic agents that reduce death and recurrent MI without increasing the risk of bleeding or strokes.
The recent OASIS (Organization for the Assessment of Strategies for Ischaemic Syndromes) 6 trial demonstrated that fondaparinux, a synthetic pentasaccharide, when compared with standard therapy (either placebo or UFH), reduced the incidence of death and MI in STEMI patients treated with either primary percutaneous coronary intervention (PCI), thrombolysis, or no reperfusion thereapy. Since the balance between benefit and risk is likely dependent on the type of reperfusion therapy, we explored the efficacy and safety of fondaparinux in the subgroup of patients treated with thrombolytic therapy.
Methods
OASIS-6 was a randomized, double blind trial of fondaparinux vs. usual care in 12 092 patients with STEMI, enrolled within 24 h of symptom onset.8 This time window was reduced to <12 h after ∼4300 patients had been enrolled, based on the results of the CREATE trial which indicated little benefit in those randomized beyond 12 h.5 At the time of this decision, we had no knowledge of any interim results from OASIS-6. Patients with contraindications to anticoagulation, including those at high risk of bleeding, receiving oral anticoagulants, or with creatinine levels >265.2 mg/dL (3.0 mmol/L), were excluded.
Randomization was stratified by indication for the use of UFH based on the investigator's judgment. Thus, the trial reflects the uncertainty on the value of UFH, and consequently the variability of its use in clinical practice. Overall, in stratum 1 (no indication for UFH) 5658 patients were enrolled, whereas 6434 patients were enrolled in stratum 2 (indication for UFH, e.g. intended use of FS thrombolytic, patients not eligible for fibrinolytics but eligible for antithrombotics, or those scheduled for primary PCI). Patients in stratum 1 were assigned to receive blinded fondaparinux 2.5 mg (or 5.0 mg in case of PCI without the use of a glycoprotein 2b/3a inhibitor) initially intravenously, and then subcutaneously once daily or matching placebo on subsequent days for up to 8 days or hospital discharge, if earlier. Patients in stratum 2 were assigned to receive either blinded fondaparinux or matching placebo (initial dose intravenous and subsequent doses subcutaneously) for up to 8 days or hospital discharge. Those in the control group received UFH bolus injection of 60 IU/kg followed by an infusion of 12 IU/kg per hour for 24–48 h. Equivalent placebo bolus and injections were used in the fondaparinux group. Thus, in stratum 2 a comparison is made of two active treatments. The maximum dose of the bolus was 4000 IU and maximum initial infusion rate of 1000 IU/h for patients weighing more than 70 kg and adjusted to maintain activated partial thromboplastin time within the therapeutic range of 1.5–2.0 times control. Higher doses could be used during PCI.
The primary outcome of the study was death or MI at 30 days. All patients were followed for a minimum of 3 and a maximum of 6 months. Consequently, ‘study end’ in this report indicates a follow-up of 3–6 months. The definition of reinfarction and the classification of deaths and haemorrhages have been previously published.6 In the main trial, two approaches for classifying bleeding complications were used. In the current analysis, we use the classification of bleeding that allows comparison with the thrombolysis in myocardial infarction trials.9 We subdivided all bleeding episodes into ‘severe’ [fatal haemorrhage, ICH, cardiac tamponade, or a clinically significant haemorrhage with a decrease in haemoglobin (Hb) of ≥5 g/dL, with each blood transfusion unit counting for 1.0 g/dL of Hb], ‘minor’ (clinically overt haemorrhage with decrease in Hb 3.0–5.0 g/dL that did not meet criteria for severe haemorrhage, with each blood transfusion unit counting as the equivalent of a 1 g/dL of Hb), and ‘other’.
Statistical analysis
Treatment effects, as measured by hazard ratio (HR) and two-sided 95% confidence interval (CI), were derived with Cox's proportional hazard model, stratified by the indication for UFH. No P-values for comparisons of active treatment vs. controls are presented in this paper, as these represent subgroup results. Instead HRs and their CIs are presented, and where appropriate tests for heterogeneity. Because several subgroup analyses were conducted, the pre-specified two-sided level to test for interactions between subgroups was 0.01.
The primary interest of the present analysis was the efficacy and safety of fondaparinux compared with control (placebo or UFH) in the subset of patients who were treated with thrombolytic therapy. Therefore, the primary outcomes of the main trial were used, i.e. the rates of death, MI, or severe bleeding at 30 days.
In addition to the two strata by indication for use of UFH, pre-specified subgroups included age (above and below the median), sex, initial reperfusion strategy (thrombolytic, primary PCI, or neither), time to reperfusion therapy from symptom onset, GRACE (Global Registry of Acute Coronary Events) risk score16 (above or below the median), and pre-randomization heparin use. This report is confined to patients who were treated with thrombolytic therapy. The analysis of the combined efficacy and safety outcomes, including death, MI, and severe bleeding, was pre-specified, as well as an analysis of the combined efficacy outcome of death, MI, and stroke.
The statistical analyses were performed at the Population Health Research Institute SAS version 9.1 was used for analysis (SAS Institute Inc., Cary, NC, USA).
Results
Overall results
The results of the OASIS-6 trial, including the randomization procedure and a flow diagram of the selection of patients, have been previously published and are briefly summarized here. Overall, the primary outcome of death or reinfarction at 30 days was significantly reduced by fondaparinux, from 11.2% in the control group to 9.7% in the fondaparinux group (HR, 0.86; 95% CI, 0.77–0.96; P = 0.008). Of the12 092 patients included, 2867 (23.7%) did not receive any reperfusion therapy. Primary PCI was performed in 3789 patients (31.3%), whereas thrombolytic therapy was used in 5436 patients (45%).
Patients receiving thrombolytic agents
Of the 5436 patients who received thrombolytic therapy, 4415 patients did not have an indication for heparin therapy (stratum 1) and 1021 did (stratum 2) (Tables 1 and 2). Streptokinase was the most commonly used thrombolytic agent (73%). In stratum 1, 4395 patients (99.6%) received a NFS thrombolytic agent (streptokinase n = 3829, urokinase n = 566). In stratum 2, 855 patients (83.7%) received a FS thrombolytic agent (tPA n = 214, rPA n = 259 and tNK n = 380). Consequently, control treatment with NFS thrombolytics was placebo in 96.4%, with FS thrombolytics it was heparin in 97.7%.
| OASIS-6 . | Stratum I . | Stratum II . | Total . | ||
|---|---|---|---|---|---|
| Placebo . | Fonda . | UFH . | Fonda . | ||
| 2835 . | 2823 . | 3221 . | 3213 . | 12 092 . | |
| Non-fibrin specific thrombolytics | 2216 | 2179 | 83 | 83 | 4561 |
| Fibrin specific thrombolytics | 9 | 11 | 436 | 419 | 875 |
| Any thrombolytic | 2225 | 2190 | 519 | 502 | 5436 |
| OASIS-6 . | Stratum I . | Stratum II . | Total . | ||
|---|---|---|---|---|---|
| Placebo . | Fonda . | UFH . | Fonda . | ||
| 2835 . | 2823 . | 3221 . | 3213 . | 12 092 . | |
| Non-fibrin specific thrombolytics | 2216 | 2179 | 83 | 83 | 4561 |
| Fibrin specific thrombolytics | 9 | 11 | 436 | 419 | 875 |
| Any thrombolytic | 2225 | 2190 | 519 | 502 | 5436 |
UFH, unfractionated heparin.
| OASIS-6 . | Stratum I . | Stratum II . | Total . | ||
|---|---|---|---|---|---|
| Placebo . | Fonda . | UFH . | Fonda . | ||
| 2835 . | 2823 . | 3221 . | 3213 . | 12 092 . | |
| Non-fibrin specific thrombolytics | 2216 | 2179 | 83 | 83 | 4561 |
| Fibrin specific thrombolytics | 9 | 11 | 436 | 419 | 875 |
| Any thrombolytic | 2225 | 2190 | 519 | 502 | 5436 |
| OASIS-6 . | Stratum I . | Stratum II . | Total . | ||
|---|---|---|---|---|---|
| Placebo . | Fonda . | UFH . | Fonda . | ||
| 2835 . | 2823 . | 3221 . | 3213 . | 12 092 . | |
| Non-fibrin specific thrombolytics | 2216 | 2179 | 83 | 83 | 4561 |
| Fibrin specific thrombolytics | 9 | 11 | 436 | 419 | 875 |
| Any thrombolytic | 2225 | 2190 | 519 | 502 | 5436 |
UFH, unfractionated heparin.
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 318 (13.8) | 241 (10.7) | 0.76 | 0.64 0.90 | 0.175 |
| FS lytics | 875 | 54 (12.1) | 52 (12.1) | 1.01 | 0.69–1.48 | |
| Severe haemorrhage | ||||||
| NFS lytics | 4561 | 45 (2.0) | 27 (1.2) | 0.60 | 0.37–0.97 | 0.846 |
| FS lytics | 875 | 11 (2.5) | 7 (1.7) | 0.67 | 0.26–1.73 |
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 318 (13.8) | 241 (10.7) | 0.76 | 0.64 0.90 | 0.175 |
| FS lytics | 875 | 54 (12.1) | 52 (12.1) | 1.01 | 0.69–1.48 | |
| Severe haemorrhage | ||||||
| NFS lytics | 4561 | 45 (2.0) | 27 (1.2) | 0.60 | 0.37–0.97 | 0.846 |
| FS lytics | 875 | 11 (2.5) | 7 (1.7) | 0.67 | 0.26–1.73 |
FS, fibrin specific thrombolytic agents; NFS, non-fibrin specific thrombolytic agents; HR, hazard ratio; 95% CI, 95% confidence interval.
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 318 (13.8) | 241 (10.7) | 0.76 | 0.64 0.90 | 0.175 |
| FS lytics | 875 | 54 (12.1) | 52 (12.1) | 1.01 | 0.69–1.48 | |
| Severe haemorrhage | ||||||
| NFS lytics | 4561 | 45 (2.0) | 27 (1.2) | 0.60 | 0.37–0.97 | 0.846 |
| FS lytics | 875 | 11 (2.5) | 7 (1.7) | 0.67 | 0.26–1.73 |
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 318 (13.8) | 241 (10.7) | 0.76 | 0.64 0.90 | 0.175 |
| FS lytics | 875 | 54 (12.1) | 52 (12.1) | 1.01 | 0.69–1.48 | |
| Severe haemorrhage | ||||||
| NFS lytics | 4561 | 45 (2.0) | 27 (1.2) | 0.60 | 0.37–0.97 | 0.846 |
| FS lytics | 875 | 11 (2.5) | 7 (1.7) | 0.67 | 0.26–1.73 |
FS, fibrin specific thrombolytic agents; NFS, non-fibrin specific thrombolytic agents; HR, hazard ratio; 95% CI, 95% confidence interval.
Baseline characteristics of all patients and those treated with thrombolytics are presented in Tables 3 and 4. Medications used after randomization included beta-blockers (83.6%), ACE inhibitors (78.4%), and statins (72.2%), with no differences between subgroups. Antithrombotic therapies started after randomization are presented in Tables 5 and 6. Rescue PCI was performed in 46 patients (2.1%) in stratum 1 and 59 patients (2.8%) in stratum 2. Outcomes at 30 days (primary study outcome) are presented in Table 7 and in Figures 1–2. In patients treated with thrombolytic agents, lower event rates were observed with fondaparinux for each of the individual and composite outcomes, including stroke, with HRs varying between 0.74 and 0.80. The primary outcome (death and MI at 30 days) was reduced from 13.6 to 10.9% (HR 0.79, 95% CI 0.68–0.92). In stratum 1, the primary endpoint was reduced from 13.8 to 10.8% (HR 0.77, 95% CI 0.65–0.91), in stratum 2, it was reduced from 12.3 to 11.2% (HR 0.91, 95% CI 0.63–1.30). All CIs include the overall result, and there was no statistical heterogeneity among these subgroups. Results in the two strata at 30 days are presented in Figure 2. There was consistent benefit whether the control treatment was placebo or UFH. The results in the subgroup of patients who received thrombolytics were consistent with patients who received no reperfusion therapy, and consequently with the combined subgroups of patients who were not treated with primary PCI (Figure 2). An analysis by type of thrombolytic agent (FS vs. NFS) showed consistency of efficacy and safety outcomes in the two second order subgroups (Figure 3). In NFS thrombolytics: death or MI 10.7% with fondaparinux vs. 13.8% in the control group (receiving placebo in 96.4%) (HR 0.76, 95% CI 0.64–0.90); in FS thrombolytics: death or MI 12.1% with fondaparinux vs. 12.1% in the control group (receiving UFH in 97.9%) (HR 1.01, 95% CI 0.69–1.48).
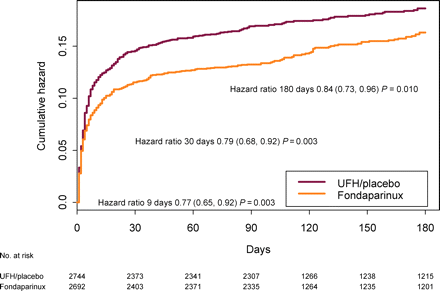
Kaplan–Meier curve: incidence of death and myocardial infarction in patients treated with thrombolytics
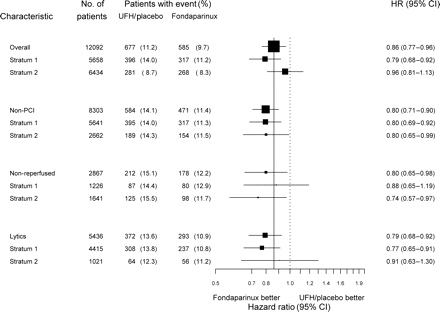
Primary efficacy outcome (death and myocardial infarction at 30 days) in the overall population and in the subgroups of non-primary percutaneous coronary intervention treated patients
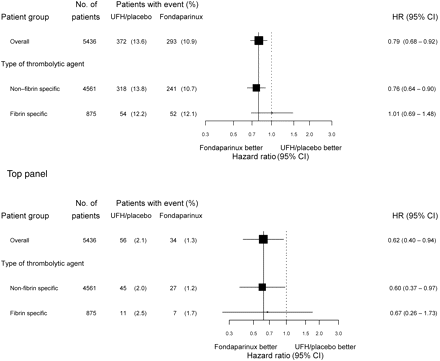
Thirty day efficacy (death and myocardial infarction, top) and safety (severe haemorrhage, bottom) outcome in patients who received thrombolytic therapy
Baseline characteristics and medications within 7 days prior to randomization
| . | All patients, n = 12 092 . | Thrombolytic therapy, n = 5436 . |
|---|---|---|
| Age (years, mean, SD) | 61.5 (12.2) | 60.1 (12.1) |
| Male (%) | 72.3 | 74.9 |
| Previous MI (%) | 12.6 | 12.3 |
| PCI (%) | 3.0 | 2.0 |
| CABG (%) | 1.2 | 0.9 |
| Other CAD (%) | 20.5 | 20.9 |
| Fam. hx. of CAD (%) | 13.0 | 11.2 |
| Stroke (%) | 6.6 | 5.9 |
| Hypertension (%) | 54.4 | 51.0 |
| Heart failure (%) | 13.9 | 14.6 |
| Diabetes (%) | 17.8 | 17.9 |
| Aspirin (%) | 61.8 | 53.2 |
| Clopidogrel/ticlopidine (%) | 46.5 | 8.4 |
| Non-study UFH (%) | 14.8 | 6.8 |
| LMWH (%) | 1.8 | 1.6 |
| GPIIb/IIIa (%) | 1.9 | 0.3 |
| Oral anticoagulants (%) | 0.2 | 0.2 |
| Mean ± SD | Mean ± SD | |
| Heart rate (b.p.m.) | 76 ± 14.5 | 76.5 ± 14.6 |
| Diastolic BP (mmHg) | 81.4 ± 14.4 | 81.2 ± 14.3 |
| Systolic BP (mmHg) | 134.1 ± 23.4 | 132.5 ± 23.0 |
| Weight (Kg) | 75.4 ± 14.5 | 73.4 ± 14.0 |
| BMI (Kg/mm2) | 26.5 ± 4.3 | 26.1 ± 4.3 |
| Onset to randomization (h) | 6.6 ± 5.3 | 5.7 ± 4.3 |
| . | All patients, n = 12 092 . | Thrombolytic therapy, n = 5436 . |
|---|---|---|
| Age (years, mean, SD) | 61.5 (12.2) | 60.1 (12.1) |
| Male (%) | 72.3 | 74.9 |
| Previous MI (%) | 12.6 | 12.3 |
| PCI (%) | 3.0 | 2.0 |
| CABG (%) | 1.2 | 0.9 |
| Other CAD (%) | 20.5 | 20.9 |
| Fam. hx. of CAD (%) | 13.0 | 11.2 |
| Stroke (%) | 6.6 | 5.9 |
| Hypertension (%) | 54.4 | 51.0 |
| Heart failure (%) | 13.9 | 14.6 |
| Diabetes (%) | 17.8 | 17.9 |
| Aspirin (%) | 61.8 | 53.2 |
| Clopidogrel/ticlopidine (%) | 46.5 | 8.4 |
| Non-study UFH (%) | 14.8 | 6.8 |
| LMWH (%) | 1.8 | 1.6 |
| GPIIb/IIIa (%) | 1.9 | 0.3 |
| Oral anticoagulants (%) | 0.2 | 0.2 |
| Mean ± SD | Mean ± SD | |
| Heart rate (b.p.m.) | 76 ± 14.5 | 76.5 ± 14.6 |
| Diastolic BP (mmHg) | 81.4 ± 14.4 | 81.2 ± 14.3 |
| Systolic BP (mmHg) | 134.1 ± 23.4 | 132.5 ± 23.0 |
| Weight (Kg) | 75.4 ± 14.5 | 73.4 ± 14.0 |
| BMI (Kg/mm2) | 26.5 ± 4.3 | 26.1 ± 4.3 |
| Onset to randomization (h) | 6.6 ± 5.3 | 5.7 ± 4.3 |
UFH, unfractionated heparin; LMWH: low-molecular weight heparin; GPIIb/IIIa: glycoprotein IIb/IIIa; MI, myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; BMI: body mass index; BP: blood pressure.
Baseline characteristics and medications within 7 days prior to randomization
| . | All patients, n = 12 092 . | Thrombolytic therapy, n = 5436 . |
|---|---|---|
| Age (years, mean, SD) | 61.5 (12.2) | 60.1 (12.1) |
| Male (%) | 72.3 | 74.9 |
| Previous MI (%) | 12.6 | 12.3 |
| PCI (%) | 3.0 | 2.0 |
| CABG (%) | 1.2 | 0.9 |
| Other CAD (%) | 20.5 | 20.9 |
| Fam. hx. of CAD (%) | 13.0 | 11.2 |
| Stroke (%) | 6.6 | 5.9 |
| Hypertension (%) | 54.4 | 51.0 |
| Heart failure (%) | 13.9 | 14.6 |
| Diabetes (%) | 17.8 | 17.9 |
| Aspirin (%) | 61.8 | 53.2 |
| Clopidogrel/ticlopidine (%) | 46.5 | 8.4 |
| Non-study UFH (%) | 14.8 | 6.8 |
| LMWH (%) | 1.8 | 1.6 |
| GPIIb/IIIa (%) | 1.9 | 0.3 |
| Oral anticoagulants (%) | 0.2 | 0.2 |
| Mean ± SD | Mean ± SD | |
| Heart rate (b.p.m.) | 76 ± 14.5 | 76.5 ± 14.6 |
| Diastolic BP (mmHg) | 81.4 ± 14.4 | 81.2 ± 14.3 |
| Systolic BP (mmHg) | 134.1 ± 23.4 | 132.5 ± 23.0 |
| Weight (Kg) | 75.4 ± 14.5 | 73.4 ± 14.0 |
| BMI (Kg/mm2) | 26.5 ± 4.3 | 26.1 ± 4.3 |
| Onset to randomization (h) | 6.6 ± 5.3 | 5.7 ± 4.3 |
| . | All patients, n = 12 092 . | Thrombolytic therapy, n = 5436 . |
|---|---|---|
| Age (years, mean, SD) | 61.5 (12.2) | 60.1 (12.1) |
| Male (%) | 72.3 | 74.9 |
| Previous MI (%) | 12.6 | 12.3 |
| PCI (%) | 3.0 | 2.0 |
| CABG (%) | 1.2 | 0.9 |
| Other CAD (%) | 20.5 | 20.9 |
| Fam. hx. of CAD (%) | 13.0 | 11.2 |
| Stroke (%) | 6.6 | 5.9 |
| Hypertension (%) | 54.4 | 51.0 |
| Heart failure (%) | 13.9 | 14.6 |
| Diabetes (%) | 17.8 | 17.9 |
| Aspirin (%) | 61.8 | 53.2 |
| Clopidogrel/ticlopidine (%) | 46.5 | 8.4 |
| Non-study UFH (%) | 14.8 | 6.8 |
| LMWH (%) | 1.8 | 1.6 |
| GPIIb/IIIa (%) | 1.9 | 0.3 |
| Oral anticoagulants (%) | 0.2 | 0.2 |
| Mean ± SD | Mean ± SD | |
| Heart rate (b.p.m.) | 76 ± 14.5 | 76.5 ± 14.6 |
| Diastolic BP (mmHg) | 81.4 ± 14.4 | 81.2 ± 14.3 |
| Systolic BP (mmHg) | 134.1 ± 23.4 | 132.5 ± 23.0 |
| Weight (Kg) | 75.4 ± 14.5 | 73.4 ± 14.0 |
| BMI (Kg/mm2) | 26.5 ± 4.3 | 26.1 ± 4.3 |
| Onset to randomization (h) | 6.6 ± 5.3 | 5.7 ± 4.3 |
UFH, unfractionated heparin; LMWH: low-molecular weight heparin; GPIIb/IIIa: glycoprotein IIb/IIIa; MI, myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; BMI: body mass index; BP: blood pressure.
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 260 (11.3) | 194 (8.6) | 0.75 | 0.62–0.90 | 0.046 |
| FS lytics | 875 | 33 (7.4) | 39 (9.1) | 1.24 | 0.78–1.97 | |
| MI | ||||||
| NFS lytics | 4561 | 71 (3.3) | 57 (2.6) | 0.80 | 0.56–1.13 | 0.359 |
| FS lytics | 875 | 26 (6.1) | 14 (3.5) | 0.57 | 0.30–1.08 |
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 260 (11.3) | 194 (8.6) | 0.75 | 0.62–0.90 | 0.046 |
| FS lytics | 875 | 33 (7.4) | 39 (9.1) | 1.24 | 0.78–1.97 | |
| MI | ||||||
| NFS lytics | 4561 | 71 (3.3) | 57 (2.6) | 0.80 | 0.56–1.13 | 0.359 |
| FS lytics | 875 | 26 (6.1) | 14 (3.5) | 0.57 | 0.30–1.08 |
FS: fibrin specific thrombolytic agents, NFS: non-fibrin specific thrombolytic agents, HR: hazard ratio, 95% CI: 95% confidence interval.
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 260 (11.3) | 194 (8.6) | 0.75 | 0.62–0.90 | 0.046 |
| FS lytics | 875 | 33 (7.4) | 39 (9.1) | 1.24 | 0.78–1.97 | |
| MI | ||||||
| NFS lytics | 4561 | 71 (3.3) | 57 (2.6) | 0.80 | 0.56–1.13 | 0.359 |
| FS lytics | 875 | 26 (6.1) | 14 (3.5) | 0.57 | 0.30–1.08 |
| Death/MI . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| NFS lytics | 4561 | 260 (11.3) | 194 (8.6) | 0.75 | 0.62–0.90 | 0.046 |
| FS lytics | 875 | 33 (7.4) | 39 (9.1) | 1.24 | 0.78–1.97 | |
| MI | ||||||
| NFS lytics | 4561 | 71 (3.3) | 57 (2.6) | 0.80 | 0.56–1.13 | 0.359 |
| FS lytics | 875 | 26 (6.1) | 14 (3.5) | 0.57 | 0.30–1.08 |
FS: fibrin specific thrombolytic agents, NFS: non-fibrin specific thrombolytic agents, HR: hazard ratio, 95% CI: 95% confidence interval.
| . | Overall . | Thrombolytic . | ||
|---|---|---|---|---|
| . | n . | Per cent . | n . | Per cent . |
| Randomized | 12 092 | 100% | 5436 | 100% |
| Aspirin | 11680 | 96.6% | 5248 | 96.5% |
| Non-study UFH | 1332 | 11.0% | 565 | 10.4% |
| LMWH | 706 | 5.8% | 261 | 4.8% |
| GPIIb/IIIa inhibitors | 1892 | 15.6% | 183 | 3.4% |
| Bivalirudin or hirudin | 12 | 0.1% | 3 | 0.1% |
| Clopidogrel | 6500 | 53.8% | 2352 | 43.3% |
| Ticlopidine | 1037 | 8.6% | 214 | 3.9% |
| Oral anticoagulants | 288 | 2.4% | 137 | 2.5% |
| . | Overall . | Thrombolytic . | ||
|---|---|---|---|---|
| . | n . | Per cent . | n . | Per cent . |
| Randomized | 12 092 | 100% | 5436 | 100% |
| Aspirin | 11680 | 96.6% | 5248 | 96.5% |
| Non-study UFH | 1332 | 11.0% | 565 | 10.4% |
| LMWH | 706 | 5.8% | 261 | 4.8% |
| GPIIb/IIIa inhibitors | 1892 | 15.6% | 183 | 3.4% |
| Bivalirudin or hirudin | 12 | 0.1% | 3 | 0.1% |
| Clopidogrel | 6500 | 53.8% | 2352 | 43.3% |
| Ticlopidine | 1037 | 8.6% | 214 | 3.9% |
| Oral anticoagulants | 288 | 2.4% | 137 | 2.5% |
UFH, unfractionated heparin; LMWH: low-molecular weight heparin; GPIIb/IIIa: glycoprotein IIb/IIIa.
| . | Overall . | Thrombolytic . | ||
|---|---|---|---|---|
| . | n . | Per cent . | n . | Per cent . |
| Randomized | 12 092 | 100% | 5436 | 100% |
| Aspirin | 11680 | 96.6% | 5248 | 96.5% |
| Non-study UFH | 1332 | 11.0% | 565 | 10.4% |
| LMWH | 706 | 5.8% | 261 | 4.8% |
| GPIIb/IIIa inhibitors | 1892 | 15.6% | 183 | 3.4% |
| Bivalirudin or hirudin | 12 | 0.1% | 3 | 0.1% |
| Clopidogrel | 6500 | 53.8% | 2352 | 43.3% |
| Ticlopidine | 1037 | 8.6% | 214 | 3.9% |
| Oral anticoagulants | 288 | 2.4% | 137 | 2.5% |
| . | Overall . | Thrombolytic . | ||
|---|---|---|---|---|
| . | n . | Per cent . | n . | Per cent . |
| Randomized | 12 092 | 100% | 5436 | 100% |
| Aspirin | 11680 | 96.6% | 5248 | 96.5% |
| Non-study UFH | 1332 | 11.0% | 565 | 10.4% |
| LMWH | 706 | 5.8% | 261 | 4.8% |
| GPIIb/IIIa inhibitors | 1892 | 15.6% | 183 | 3.4% |
| Bivalirudin or hirudin | 12 | 0.1% | 3 | 0.1% |
| Clopidogrel | 6500 | 53.8% | 2352 | 43.3% |
| Ticlopidine | 1037 | 8.6% | 214 | 3.9% |
| Oral anticoagulants | 288 | 2.4% | 137 | 2.5% |
UFH, unfractionated heparin; LMWH: low-molecular weight heparin; GPIIb/IIIa: glycoprotein IIb/IIIa.
| . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| Stratum 1 | 4415 | 308 (13.8) | 237 (10.8) | 0.77 | 0.65–0.91 | 0.414 |
| Stratum 2 | 1021 | 64 (12.3) | 56 (11.2) | 0.91 | 0.63–1.30 | |
| Severe haemorrhage | ||||||
| Stratum 1 | 4415 | 42 (2.0) | 27 (1.3) | 0.65 | 0.40–1.05 | 0.680 |
| Stratum 2 | 1021 | 14 (2.7) | 7 (1.4) | 0.52 | 0.21–1.29 |
| . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| Stratum 1 | 4415 | 308 (13.8) | 237 (10.8) | 0.77 | 0.65–0.91 | 0.414 |
| Stratum 2 | 1021 | 64 (12.3) | 56 (11.2) | 0.91 | 0.63–1.30 | |
| Severe haemorrhage | ||||||
| Stratum 1 | 4415 | 42 (2.0) | 27 (1.3) | 0.65 | 0.40–1.05 | 0.680 |
| Stratum 2 | 1021 | 14 (2.7) | 7 (1.4) | 0.52 | 0.21–1.29 |
FS: fibrin specific thrombolytic agents, NFS: non-fibrin specific thrombolytic agents, HR: hazard ratio, 95% CI: 95% confidence interval.
| . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| Stratum 1 | 4415 | 308 (13.8) | 237 (10.8) | 0.77 | 0.65–0.91 | 0.414 |
| Stratum 2 | 1021 | 64 (12.3) | 56 (11.2) | 0.91 | 0.63–1.30 | |
| Severe haemorrhage | ||||||
| Stratum 1 | 4415 | 42 (2.0) | 27 (1.3) | 0.65 | 0.40–1.05 | 0.680 |
| Stratum 2 | 1021 | 14 (2.7) | 7 (1.4) | 0.52 | 0.21–1.29 |
| . | n . | Control, n (%) . | Fondaparinux, n (%) . | HR . | 95% CI . | P-value for interaction . |
|---|---|---|---|---|---|---|
| Death/MI | ||||||
| Stratum 1 | 4415 | 308 (13.8) | 237 (10.8) | 0.77 | 0.65–0.91 | 0.414 |
| Stratum 2 | 1021 | 64 (12.3) | 56 (11.2) | 0.91 | 0.63–1.30 | |
| Severe haemorrhage | ||||||
| Stratum 1 | 4415 | 42 (2.0) | 27 (1.3) | 0.65 | 0.40–1.05 | 0.680 |
| Stratum 2 | 1021 | 14 (2.7) | 7 (1.4) | 0.52 | 0.21–1.29 |
FS: fibrin specific thrombolytic agents, NFS: non-fibrin specific thrombolytic agents, HR: hazard ratio, 95% CI: 95% confidence interval.
Event rates by 30 days in patients who received thrombolytic therapy. Kaplan–Meier and Cox PH hazard ratios
| . | Control, n = 2744 . | Fonda, n = 2692 . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | ||
| Death/MI | 372 | 13.6 | 293 | 10.9 | 0.79 | 0.68–-0.92 |
| Death | 293 | 10.7 | 233 | 8.7 | 0.80 | 0.68–0.95 |
| MI | 97 | 3.8 | 71 | 2.8 | 0.74 | 0.54–1.00 |
| Stroke | 38 | 1.5 | 29 | 1.1 | 0.77 | 0.48–1.25 |
| Death/MI/stroke | 393 | 14.3 | 306 | 11.4 | 0.78 | 0.67–0.91 |
| Severe haemorrhage | 56 | 2.1 | 34 | 1.3 | 0.62 | 0.40–0.94 |
| Death/MI/severe haemorrhage | 386 | 14.1 | 297 | 11.0 | 0.77 | 0.67–0.90 |
| . | Control, n = 2744 . | Fonda, n = 2692 . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | ||
| Death/MI | 372 | 13.6 | 293 | 10.9 | 0.79 | 0.68–-0.92 |
| Death | 293 | 10.7 | 233 | 8.7 | 0.80 | 0.68–0.95 |
| MI | 97 | 3.8 | 71 | 2.8 | 0.74 | 0.54–1.00 |
| Stroke | 38 | 1.5 | 29 | 1.1 | 0.77 | 0.48–1.25 |
| Death/MI/stroke | 393 | 14.3 | 306 | 11.4 | 0.78 | 0.67–0.91 |
| Severe haemorrhage | 56 | 2.1 | 34 | 1.3 | 0.62 | 0.40–0.94 |
| Death/MI/severe haemorrhage | 386 | 14.1 | 297 | 11.0 | 0.77 | 0.67–0.90 |
MI, myocardial infarction.
Event rates by 30 days in patients who received thrombolytic therapy. Kaplan–Meier and Cox PH hazard ratios
| . | Control, n = 2744 . | Fonda, n = 2692 . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | ||
| Death/MI | 372 | 13.6 | 293 | 10.9 | 0.79 | 0.68–-0.92 |
| Death | 293 | 10.7 | 233 | 8.7 | 0.80 | 0.68–0.95 |
| MI | 97 | 3.8 | 71 | 2.8 | 0.74 | 0.54–1.00 |
| Stroke | 38 | 1.5 | 29 | 1.1 | 0.77 | 0.48–1.25 |
| Death/MI/stroke | 393 | 14.3 | 306 | 11.4 | 0.78 | 0.67–0.91 |
| Severe haemorrhage | 56 | 2.1 | 34 | 1.3 | 0.62 | 0.40–0.94 |
| Death/MI/severe haemorrhage | 386 | 14.1 | 297 | 11.0 | 0.77 | 0.67–0.90 |
| . | Control, n = 2744 . | Fonda, n = 2692 . | HR . | 95% CI . | ||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | ||
| Death/MI | 372 | 13.6 | 293 | 10.9 | 0.79 | 0.68–-0.92 |
| Death | 293 | 10.7 | 233 | 8.7 | 0.80 | 0.68–0.95 |
| MI | 97 | 3.8 | 71 | 2.8 | 0.74 | 0.54–1.00 |
| Stroke | 38 | 1.5 | 29 | 1.1 | 0.77 | 0.48–1.25 |
| Death/MI/stroke | 393 | 14.3 | 306 | 11.4 | 0.78 | 0.67–0.91 |
| Severe haemorrhage | 56 | 2.1 | 34 | 1.3 | 0.62 | 0.40–0.94 |
| Death/MI/severe haemorrhage | 386 | 14.1 | 297 | 11.0 | 0.77 | 0.67–0.90 |
MI, myocardial infarction.
The risk of severe bleeding was also reduced by fondaparinux (1.3% vs. 2.1%, HR 0.62, 95% CI 0.40–0.94), and thus the benefit-risk balance of death, MI and severe haemorrhage was also clearly reduced by fondaparinux (HR 0.77, 95% CI 0.67–0.90, Table 7). Outcomes at study end (3–6 months) show a similar pattern and the reduction in death and MI persisted to study end (Figure 1). Results were consistently observed at different times from onset to randomization (data not shown). Similarly, the risk of severe haemorrhage was reduced by fondaparinux independent of the time from onset to randomization (data not shown).
Discussion
Our data demonstrate that fondaparinux significantly reduced the risk of death and MI, with a trend towards fewer strokes in STEMI patients treated with thrombolytic therapy. In addition, there was a reduction in severe bleeding, so that the pre-specified outcome balancing efficacy and safety was clearly reduced by fondaparinux. Treating a 1000 patients will prevent 32 deaths, MI, strokes, or a severe bleeding complication compared with standard treatment. Of note, these benefits led to a reduction in mortality. Thus, fondaparinux is the only antithrombotic that has been demonstrated to reduce mortality in patients receiving thrombolytic therapy without an increase in bleeding or strokes.
Further subdivision of patients according to heparin use or type of thrombolytic agent demonstrates consistency of effects. However, the number of patients in stratum 2 and in the subgroup with FS are relatively modest and the CIs for the HRs for both efficacy and safety are wide (Figures 2–3). However, the point estimates are consistent with those observed in the overall trial (internal consistency), and CIs of all subgroup results include the overall trial result (Figure 2).10 In addition, the results are consistent with the findings in the OASIS-5 study where fondaparinux was superior to enoxaparin (which is likely superior to UFH) in reducing death and strokes and was associated with less bleeding (external replication). Furthermore, the reductions in the risk of bleeding in those receiving thrombolytics, and the various subgroups defined here would favour the use of fondaparinux since bleeding is associated with increased mortality. Therefore, our findings suggest that fondaparinux improves the results of thrombolytic therapy, compared with either placebo or UFH, and irrespective of the type of thrombolytic used. To be certain, however, a large trial would be required, evaluating fondaparinux and UFH in patients treated with FS agents.
The reduction in bleeding when compared with placebo (25 vs. 39 cases in stratum 1) was unexpected. Analysis by location of bleeding revealed a reduction in several types of serious bleeding complications including gastrointestinal bleeds, surgical bleeds, and cases of cardiac tamponade. By preventing ischaemic events, fondaparinux may have prevented invasive procedures with their associated risk of bleeding. The apparent reduction may also be due to the play of chance or, if real, due to a mechanism that is yet to be defined. In any case, our data, when interpreted conservatively confirm that fondaparinux does not increase the risk of bleeding, a position that is consistent with the markedly lower rates of bleeding with fondaparinux compared with enoxaparin seen in OASIS-5.
Contemporary guidelines recommend the routine administration of UFH in STEMI patients treated with FS thrombolytic agents.' This is largely based on three small trials indicating improved patency of the infarct-related coronary artery when intravenous UFH is added to FS thrombolytic agents.11–13 However, in two of these trials, patients treated with intravenous UFH did not receive aspirin10, whereas in the third study, in which all patients received aspirin, there was only a modest impact of intravenous UFH on patency. All trials were too small to reliably assess the impact on clinical outcomes and on safety, and recent trials of GP 2b/3a inhibitors have indicated that improved coronary patency need not result in a reduction in mortality, especially if bleeding or strokes are increased.14
A meta analysis of all available trials on a total of about 1450 patients evaluating the use of UFH with a FS thrombolytic agent indicates no reduction in mortality or morbidity, but a clear excess in bleeding as compared to placebo.15
The use of an anticoagulant in patients who have received streptokinase has been evaluated in several large trials. In the ISIS 3 and GISSI 2 trials, there was no reduction in mortality, a small reduction in recurrent MI in hospital (which was not sustained at 30 days), a tendency to increased ICH and a clear excess in major bleeds.1,2 The GUSTO trial demonstrated that IV UFH was not superior to subcutaneous UFH, but further increased bleeding.16 Thus, the currently available evidence for the use of UFH in patients receiving a FS thrombolytic and aspirin is weak, and the data evaluating UFH in patients receiving streptokinase clearly demonstrates no clinically important benefits, but an excess of major bleeds, including intracranial bleeds. Comparison of a new agent against UFH may therefore underestimate the risk of bleeding complications, whereas the demonstration of efficacy would be relatively unaffected. The above data have led to variability of UFH use after thrombolytic therapy, and reflects the uncertainty of the available data.
A recent meta analysis of trials comparing low-molecular weight heparin (LMWH) and placebo (completed after the initiation of OASIS-5 and -6), including the recently published CREATE study, has shown a reduction in death (by 10%, 14 deaths per 1000) and re-MI (by 25%, 6 per 1000) at the cost of seven major bleeding complications (including ICH) per 1000 (all 30 days).11
A meta analysis of trials of LMWH vs. UFH (in over 5000 patients) has shown a 6% relative risk reduction in death at 30 days and a 35% relative risk reduction in re-MI (19 per 1000 patients treated) at 30 days, at the cost of three strokes per 1000 patients treated.11 Results from the more recent ExTRACT study (n = 20,506) are consistent with this meta analysis.4 However, LMWH is associated with a substantial increase in severe bleeding complications as compared to UFH, including ICH and fatal bleeds.4,11
Our findings in this analysis of OASIS-6, of a non-significantly lower rate of bleeding and strokes, and of a significantly lower mortality with fondaparinux in patients receiving thrombolytic therapy, as well as benefit in the subgroup not receiving reperfusion therapy in the accompanying paper by Oldgren et al.,17 are consistent with the findings of the OASIS-5 trial in patients with non-ST elevation acute coronary syndrome (ACS), where fondaparinux was as effective as enoxaparin in the short term, but was substantially safer.18 Therefore the two trials together provide strong evidence of the benefits and safety of fondaparinux in a broad group of patients with ACSs.
Limitations
The trial was not designed to have sufficient power to independently examine the impact of fondaparinux in various subgroups but, like most trials, was designed to have adequate power to detect clinically important results based on the overall study population. The separate analysis of the main types of reperfusion therapy (primary PCI, thrombolytic and no reperfusion) was pre-specified in our statistical analysis plan. However, further subgroup analyses of those receiving thrombolytic agents should be interpreted with considerable caution, as the numbers of patients for some of the analyses tend to become very small and potentially unreliable, as the data are further or repeatedly subdivided in different ways.19–22 The influence of the play of chance is exemplified in Figure 2, where in non-reperfused patients the treatment effect of fondaparinux compared with heparin appears larger than compared with placebo. Therefore, the consistency of the results in those receiving thrombolytic therapy with those of the overall results should provide confidence in the robustness of the findings, but any further subgroup analyses should be viewed with caution.
Conclusion
The results of OASIS-6 demonstrate that overall, there is evidence of benefit for fondaparinux without evidence of an increase in bleeding risk. Subgroup analysis must be interpreted with caution. However, in STEMI patients treated with thrombolytic agents (predominantly streptokinase), fondaparinux significantly improved overall outcome, both in short term and in long term, with no evidence of heterogeneity by whether or not heparin was used in the control arm. Fondaparinux was associated with a unique safety profile, with no increase (and perhaps a decrease) in severe haemorrhage, irrespective of the type of thrombolytic, and compared with both heparin and placebo.
Acknowledgement
However, the trial was conducted, data collected, and analysed independently by the Population Health Research Institute and the steering committee.
Conflict of interest: All authors have received honoraria and/or consulting fees from the sponsors. None of the authors are shareholders for any of these companies.
Funding
The OASIS-6 study was jointly funded by Sanofi-Aventis, Organon, and GlaxoSmithKline.



