-
PDF
- Split View
-
Views
-
Cite
Cite
Bryan A. Jones, Lydia S. Wagner, Neil V. Watson, The Effects of Bisphenol A Exposure at Different Developmental Time Points in an Androgen-Sensitive Neuromuscular System in Male Rats, Endocrinology, Volume 157, Issue 8, 1 August 2016, Pages 2972–2977, https://doi.org/10.1210/en.2015-1574
Close - Share Icon Share
The industrial plasticizer bisphenol A (BPA) is a ubiquitous endocrine disruptor to which the general human population is routinely exposed. Although BPA is well known as an estrogenic mimic, there have been some suggestions that this compound may also alter activity at the androgen receptor. To determine whether BPA does have antiandrogenic properties, we evaluated BPA effects in the spinal nucleus of the bulbocavernosus and dorsolateral nucleus, sexually dimorphic groups of motor neurons in the lumbar spinal cord that are critically dependent on androgens for survival and maintenance, as well as the monomorphic retrodorsolateral nucleus. In experiment 1, we administered varying concentrations of BPA to juvenile rats pre- and postnatally and examined both the number and size of motor neurons in adulthood. In experiment 2, different doses of BPA were given to adult rats for 28 days, after which the soma size of motor neurons were measured. Although no effect of BPA on neural survival or soma size was noted after perinatal BPA exposure, BPA exposure did result in a decrease in soma size in all motor neuron pools after chronic exposure in adulthood. These findings are discussed with regard to putative antiandrogenic effects of BPA; we argue that BPA is not antiandrogenic but is acting through nonandrogen receptor-dependent mechanisms.
Bisphenol A (BPA) is an endocrine-disrupting compound known to have weak estrogenic activity (1). This ubiquitous compound, found in food tins, polyvinyl chloride, dental sealants, and some plastic water bottles, can also affect other signaling systems. For example, BPA can antagonize thyroid (2) and aryl-hydrocarbon receptors (3), activate glucocorticoid receptors (4) and the membrane-bound estrogen receptor GPR30 (5), and bind with high affinity at the estrogen-related receptor-γ (6). A number of in vitro studies have also noted that BPA can act as an antagonist at the androgen receptor (AR) (7–10). The evidence for AR antagonism in vivo has been less convincing. One group found that BPA can inhibit synaptogenesis in intact adult male rats and prevent testosterone (T)-induced increases of synaptogenesis in gonadectomised males (8). Furthermore, Xu et al reported that perinatal BPA can inhibit performance in an androgen-sensitive spatial-learning task in adults (9) and prepubescent male rats (10), but other studies have not (11, 12). Accordingly, the National Toxicology Program concluded that BPA does not antagonize AR (13).
To further examine any AR-blocking effects of BPA, we tested this compound on an androgen-sensitive pool of motoneurons within the lumbar spinal cord (L5 and L6). This group of neurons, the spinal nucleus of the bulbocavernosus (SNB), innervates the bulbocavernosus/levator ani muscles of the penis (14). During gestation, the SNB of both male and female rats develop about 300 neurons, but later this is pared down to approximately 200 in males and 60 in females, which innervate the external anal sphincter (15), and this process evidently requires the activity of AR for male-typical survival of these neurons (16). Two other pools of motoneurons are found in L5 and L6. The sexually dimorphic dorsolateral nucleus (DLN) innervates the ischiocavernosus muscle of the penis and requires androgens for survival, whereas the retrodorsolateral nucleus (RDLN) innervates the flexor digitorum brevis muscle and is not androgen dependent (17). In adulthood, motoneurons in the SNB maintain androgen-dependent plasticity (18), whereas castration reduces the soma size of neurons in the SNB but not RDLN (19). We thus used these differing parameters to examine any antiandrogenic effects of BPA after either perinatal exposure to BPA or chronic exposure in adulthood. Specifically, we looked to see whether motoneuron survival is altered after perinatal exposure to BPA or whether neuronal soma size is decreased after chronic exposure to BPA in adulthood.
Materials and Methods
Experimental design 1: Perinatal BPA exposure
Fifteen 60-day old Long-Evans (LE) females from Charles River Laboratories served as dams. They were maintained in the Animal Resource Centre (ARC) at Simon Fraser University (SFU) and had access to standard rat chow (Purina) and water ad libitum. All procedures conformed to the guidelines of the Canadian Council on Animal Care and were subject to previous approval by the SFU Animal Care Committee.
The females were trained to drink corn oil from a syringe, to ensure proper administration of the vehicle and also to reduce stress associated with treatment. All BPA or vehicle solutions were administered via syringe by ARC staff. Females were first paired with LE stud males and then moved to single housing in polysulfone cages with BPA-free water sacks. Pregnant females were randomly assigned to 1 of 5 BPA (>99% pure; Sigma) groups: oil vehicle, 5-μg/kg bw/d, 50-μg/kg bw/d, 500-μg/kg bw/d, or 5000-μg/kg bw/d, with 3 females in each group except the 500-μg/kg group, where 1 female did not become pregnant despite the presence of a vaginal plug. The day that plugs were found was considered gestational day 1, and treatments went from gestational day 7 through postnatal day (P)14, with parturition being considered P0. This time frame was chosen as it most closely resembles the likely exposure pattern in human embryo and fetus. Litters were culled to 4 males and 4 females each, except in the 500-μg/kg bw/d group, in which litters were culled to 6 males and 6 females each; thus, each group had 12 males and 12 females, yielding a total sample size of 120 animals. Upon weaning (P21), pups were group housed in polysulfone cages with same-sex littermates and access to tap water and standard Purina rat chow ad libitum. A number of behavioral tests were conducted between the ages of 90 and 150 days, as described previously (12, 20). For the analysis of motoneurons, only the spinal cords of the males were removed and investigated.
After testing, animals were killed by carbon dioxide inhalation and then perfused transcardially with PBS followed by 4% paraformaldehyde. Spinal cords were removed and placed in paraformaldehyde for 2 hours and then transferred to 30% sucrose in PBS as cryoprotectant. The tissue was then frozen and sliced with a sliding microtome into 3 parallel series of sections at a thickness of 50 μm and then transferred to de Olmos solution for storage at −20°C (21). The spinal cords were mounted on slides, stained with thionin, put through a series of alcohols and xylene, and then coverslipped immediately. Some tissue was unusable, due to technical difficulties. However, final group size was 9 or above in every case.
Slides were examined on a Nikon Eclipse E600 microscope with proper Kohler illumination. Pictures were taken at ×20 using a Nikon DS-Fi1 digital camera (Nikon Canada) and Nikon Elements F software (Nikon Canada) on a PC running Windows 7 (Microsoft). Subsequently, using ImageJ software (National Institutes of Health), all motoneurons within the SNB, DLN, and RDLN were counted and traced by an observer blind to condition, and soma sizes were calculated. Only darkly stained neurons with a clear nucleus and clearly observable nucleolus were included in both the counts and the soma size calculations.
Experimental design 2: Adult BPA exposure
Fifty adult LE males were maintained in a colony room in the ARC at SFU, with access to rat chow (Purina) and water ad libitum. All procedures conformed to the guidelines of the Canadian Council on Animal Care and were subject to previous approval by the University Animal Care Committee before the study.
BPA was dissolved in water and each male was randomly assigned to 1 of 5 BPA groups (same doses as above) and group housed in polysulfone cages with access to rat chow ad libitum. Animals were administered BPA-containing water for 28 days. BPA concentrations in water for each group were determined in accordance with a previous pilot study in which daily BPA-containing water consumption was tracked in age-matched male LE rats. Animals averaged 400 g at the beginning of the study, and based on our observations of 50-mL water/d being consumed, we created a stock solution of 40 mg/L, which was administered to our highest dose group (5000-μg/kg bw/d). This concentration is well below the maximum H2O solubility of BPA of 100 mg/L. This stock solution was serially diluted in 10-fold steps to produce the treatment for each group, such that the 500-μg/kg bw/d group received 4 mg/L, the 50-μg/kg bw/d group received 0.4 mg/L, and the 5-μg/kg bw/d group received 0.04 mg/L in water. Fluid consumption throughout the study was continuously monitored to ensure that animals were exposed to their assigned doses, as described above.
After 28 days, animals were killed and perfused as described earlier, and their spinal cords were removed. Tissue was frozen and sliced into transverse sections 50 μm thick and then stained and mounted as described in experiment 1. Tracing of soma size within the SNB, DLN, and RDLN was then performed as previously described.
Statistical analyses
One-way ANOVAs using SPSS 17 with post hoc Tukey tests were used to evaluate group differences in motoneuron survival, for animals in experiment 1, and, in soma size, for animals in both experiments.
Results
Experiment 1
Perinatal exposure to BPA had no effect on long term survival of motoneurons in any of the 3 motoneuron pools examined (SNB: F4,50 = 0.713, P = .587; DLN: F4,50 = 0.243, P = .912; and RDLN: F4,50 = 0.529, P = .715) nor did we see any differences in adult soma size (SNB, F4,50 = 0.292, P = .882; DLN, F4,50 = 0.505, P = .732; and RDLN, F4,50 = 0.18, P = .948) after perinatal exposure to BPA.
Experiment 2
Chronic exposure to BPA in adulthood had significant effect on the size of motoneurons in each of the 3 pools quantified here (SNB: F4,50 = 13.9, P < .001; DLN: F4,50 = 7.476, P < .001; and RDLN: F4,50 = 9.802, P < .001) (Figures 1–3, respectively). The post hoc Tukey's analysis revealed that all 4 BPA-dosed groups had significantly smaller SNB motoneuron soma sizes than did the control group (P < .001 for each comparison), whereas the 4 BPA dose groups did not differ from one another (data not shown). The same pattern of results was evident in the DLN, where motoneuron soma sizes in the BPA-free controls were significantly different from somata all BPA-treated groups (P = .006 vs 5 μg/kg; P = .002 vs 50 μg/kg; P = .006 vs 500 μg/kg; P < .001 vs 5000 μg/kg); again, no differences emerged among the 4 dose groups (data not shown). For the RDLN, somata of controls were also larger than for all of the BPA groups (P = .055 vs 5 μg/kg; P = .005 vs 50 μg/kg; P < .001 vs 500 and 5000 μg/kg); furthermore, somata of the 5000-μg/kg group were significantly smaller than the 5-μg/kg group (P = .029).
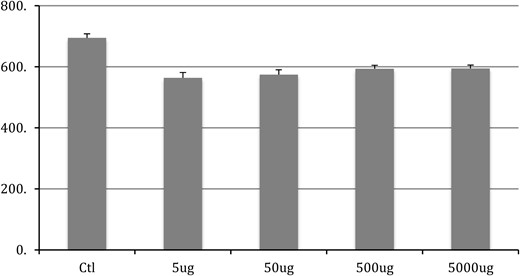
SNB soma size after chronic BPA exposure in adulthood. Control group is significantly (P < .05) different from all BPA groups. No difference exists between any other groups.
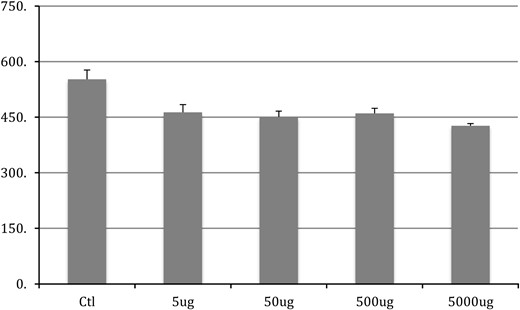
DLN soma size after chronic BPA exposure in adulthood. Control group is significantly (P < .05) different from all BPA groups. No difference exists between any other groups.
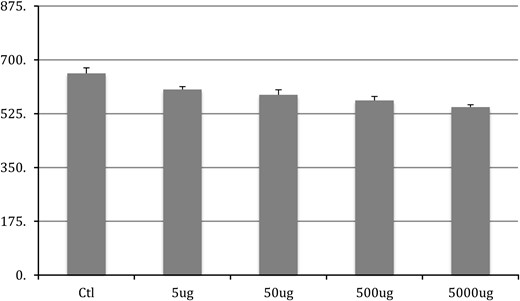
RDLN soma size after chronic BPA exposure in adulthood. Control group is significantly (P < .05) different from all BPA groups. The 5000 μg was also significantly smaller than the 5-μg group. No other differences were noted.
Discussion
We found that the administration of BPA during perinatal development had no effect on known AR-dependent parameters in the SNB or the DLN. In contrast, BPA significantly affected neuronal soma size after chronic exposure in adulthood. The pattern of results obtained is not consistent with a purely antiandrogenic account of BPA's action.
Studies showing an antiandrogenic effect of BPA have typically examined human AR transfected into either yeast (7) or monkey kidney cells (22, 23), or the AR found in LNCaP cells (24). However, in vivo studies of BPA have found little evidence of androgenic effects (but see Ref. 25); in fact, BPA's effects in vivo are more consistent with estrogenic activity. Specifically, this has been noted in the Hershberger assay, a test of androgenic physiological parameters used for decades to assess compounds, including environmental endocrine disruptors (26). For example, vinclozolin, the antiandrogenic fungicide, was comparable with flutamide in preventing the development of genitalia and the urogenital tract (27), whereas BPA did not (28). Other research found that BPA exposure during the prenatal period leads to an enlarged prostate as well as gene expression changes akin to diethylstilbestrol or estradiol treatment (29). Conversely, flutamide treatment during this period prevents prostate formation, leading instead to a vaginal pouch (30) and, when paired with castration, leads to a dramatic reduction in the number of SNB motoneurons in males in adulthood (31). Alternatively, T proprionate administered to females during the perinatal period spared the loss of these neurons (15).
Neurologic and behavioral studies examining any antiandrogenic mechanism of BPA activity are also inconclusive. For example, administration of 300-μg/kg BPA prevented T-induced synaptogenesis in the hippocampus and prefrontal cortex of castrated rats while reducing spine density in intact animals (8), which may be mediated by estrogenic activity. Likewise low doses of BPA caused rapid spinogenesis in hippocampal slices from adult rat, likely due to the activity of estrogen-related receptor-γ (32). Furthermore, tests of BPA in the Morris water maze have yielded inconsistent results as described above. Regardless, complete masculinization of this behavior is not dependent solely on the AR, because estrogens have been shown to play a role (33, 34). Indeed, Xu et al (10) found that the HPC of adult mice exposed perinatally to BPA had decreased ERβ, which has mnemonic effect in male rats (35).
The literature, present data included, do not support a purely androgenic effect of BPA either. One study (19) found that 28 days after castration, motoneurons in the SNB and DLN, but not RDLN, decreased in size, whereas another (36) found that monomorphic motoneurons did not show decreased soma size after castration. Our finding that BPA exhibits a nonselective effect on motoneuron somatic morphology suggests that this involves some nonandrogenic mechanism. For example, thyroidectomy decreases motoneuron size in adult females (37), whereas decreased thyroid hormones in utero leads to smaller motoneurons before puberty (38). Dexamethasone, the corticosteroid agonist, has also been found to reduce motoneuron size (39), although basal levels of corticosteroid in adult rats have not been found to alter SNB somatic morphology (40). Finally, ample evidence has accumulated to suggest that estrogens do not participate in the maintenance of SNB motoneuron soma size in adulthood (14, 41). This prompts us to hypothesize that BPA mediates its effects on motoneuron soma size through nonandrogenic pathways.
Evidence from studies in humans seems to agree with our data. In humans, AR playa a strong role in the masculinization of the brain, because XY individuals with complete androgen insensitivity syndrome have female peripheral phenotype, female-typical sexual identity and sexual orientation (42), and have a female-typical pattern of cognitive abilities (43). Given this, we would expect BPA to alter the behavior of boys if it was in fact acting through the AR. However, BPA exposure in utero increased incidences of externalizing behaviors in 2-year-old girls (44), and increased aggression as well as internalizing problems in 3-year-old girls (45), effects not seen in boys. An inverse relationship has been found between serum BPA levels of adult males and sperm motility (46), perhaps due to oxidative stress (47) or epigenetic effects (48), as well as with levels of estradiol, TSH, and bioavailable androgens (49) further supporting our assertion here.
In sum, our results add to the growing body of data indicating that BPA, in vivo, cannot be considered an antiandrogen. We suggest that this non-AR-dependent effect on spinal motoneurons is likely to be harmful in some way. Furthermore, its ability to alter the neuromuscular physiology of reproductive systems in rats at environmentally relevant doses, as well as the documented association with decreased sperm motility and HPG activity in humans, is cause for concern.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- AR
androgen receptor
- ARC
Animal Resource Centre
- BPA
bisphenol A
- DLN
dorsolateral nucleus
- LE
Long-Evans
- P
postnatal day
- RDLN
retrodorsolateral nucleus
- SFU
Simon Fraser University
- SNB
spinal nucleus of the bulbocavernosus
- T
testosterone.



