-
PDF
- Split View
-
Views
-
Cite
Cite
Joon S. Kim, Mohammed Z. Rizwan, Deborah J. Clegg, Greg M. Anderson, Leptin Signaling Is Not Required for Anorexigenic Estradiol Effects in Female Mice, Endocrinology, Volume 157, Issue 5, 1 May 2016, Pages 1991–2001, https://doi.org/10.1210/en.2015-1594
Close - Share Icon Share
Abstract
Estradiol and leptin are critical hormones in the regulation of body weight. The aim of this study was to determine whether this cross talk between leptin receptor (LepRb) and estrogen receptor-α (ERα) signaling is critical for estradiol's anorexigenic effects. Leprb-Cre mice were crossed with Cre-dependent Tau-green fluorescent protein (GFP) reporter, Stat3-flox or Erα-flox mice to generate female mice with GFP expression, signal transducer and activator of transcription 3 (STAT3) knockout (KO), or ERα KO, specifically in LepRb-expressing cells. The proportion of Leprb-GFP cells colocalizing ERα was high (∼80%) in the preoptic area but low (∼10%) in the mediobasal hypothalamus, suggesting that intracellular cross talk between these receptors is minimal for metabolic regulation. To test whether estradiol enhanced arcuate leptin sensitivity, ovarectomized mice received varying levels of estradiol replacement. Increasing estrogenic states did not increase the degree of leptin-induced STAT3 phosphorylation. LepRb-specific STAT3 KO mice and controls were ovarectomized and given either chronic estradiol or vehicle treatment to test whether STAT3 is required for estrogen-induced body weight suppression. Both groups of estradiol-treated mice showed an equivalent reduction in body weight and fat content compared with vehicle controls. Finally, mice lacking ERα specifically in LepRb-expressing neurons also showed no increase in body weight or impairments in metabolic function compared with controls, indicating that estradiol acts independently of leptin-responsive cells to regulate body weight. However, fecundity was impaired in in Leprb-ERα KO females. Contrary to the current dogma, we report that estradiol has minimal direct actions on LepRb cells in the mediodasal hypothalamus and that its anorexigenic effects can occur entirely independently of LepRb-STAT3 signaling in female mice.
Leptin and estradiol are both potent anorexigenic signals and critical regulators of energy balance. As with leptin-deficient and leptin receptor-deficient mice (1–5), estrogen deficiency caused by ovariectomy (OVX) (6, 7), aromatase knockout (KO) (8), or estrogen receptor-α (ERα) KO (9) consistently results in an obese phenotype. Obesity-related metabolic disorders are less common in premenopausal women than men but increase after menopause (10). The anorexigenic effects of estradiol are very similar to those of leptin; both hormones cause dose-dependent reductions in food intake (11, 12); both up-regulate and induce an excitatory tone on anorexigenic proopiomelanocortin and cocaine- and amphetamine-regulated transcript neurons (13–15), and both inhibit orexigenic agouti-related peptide (AgRP) and neuropeptide-Y (NPY) neurons in the arcuate nucleus (Arc) of the hypothalamus (16–19).
It is well described in the literature that leptin predominantly acts through its long-form receptor (LepRb) in the hypothalamus and induces the phosphorylation of signal transducer and activator of transcription (STAT)-3 (20). Deletion of STAT3 specifically from LepRb-expressing cells leads to obesity in male and female mice, whereas deletion of the leptin signaling molecule STAT5 from LepRb cells was without effect (21). Whereas it is well accepted that estradiol is required to act in the brain to regulate energy balance (10), the mechanism underlying this effect is not well understood. Estradiol acts to reduce body weight via ERα (22, 23) and remains efficacious in both leptin-deficient and leptin receptor-deficient mice but is unable to do so in a brain-specific STAT3 KO mouse (4). It was proposed that estradiol does not require leptin or its receptor but acts on the same signaling pathway, STAT3, to exert its anorexigenic effects (reviewed in reference 24). In agreement with this, STAT3 is a known nonclassical target of agonist-bound ERα (25, 26). Additionally, in one paper all cells immunoreactive for ERα were reported to coexpress LepRb immunoreactivity in the rat preoptic area (POA), Arc and ventromedial hypothalamus (VMH) (27); further evidence for potential cross-talk between the two hormones.
In this report, we explore the extent to which a direct cross talk between leptin and estradiol signaling is possible in the mouse hypothalamus using a transgenic approach to more definitively visualize LepRb. Second, we address whether STAT3 of the LepRb signaling pathway or ERα in neurons that coexpress LepRb are critical for mediating estradiol's antiobesity effects.
Materials and Methods
Animals
Lines of transgenic Leprb-Cre, Tau-green fluorescent protein (GFP), Stat3-flox, Erα-flox, and Npy-Tau-Sapphire-GFP mice were maintained on a C57BL/6 background. The Leprb-Cre line (see references 21 and 28–30 for the generation and characterization of this mouse) and the Npy-Tau-Sapphire-GFP line (see reference 14) were purchased from the Jackson Laboratories (B6.129-Leprtm2(cre)Rck/J and B6.Cg-Tg[Npy-MAPT/Sapphire]1Rck/J strains, respectively). When this Leprb-Cre mouse line is crossed with various fluorescent reporter lines, we and others have shown that the percentage of hemizygous Leprb-Cre reporter cells that coexpress leptin-induced pSTAT3 and pSTAT3 cells that coexpress the Leprb-Cre reporter, ranges from 60% to 95% across the arcuate, dorsomedial, lateral hypothalamic, and premamilliary nuclei (21, 30), although the percentage of pSTAT3-positive ventromedial hypothalamus cells coexpressing Leprb-Cre reporter ranges from 28% to 97%, depending on the duration between leptin treatment and perfusion (30). The Stat3-flox (see references 21 and 32), Erα-flox line (see reference 33), and Tau-GFP (ROSA26-CAGS-τGFP) lines were obtained from collaborators. Mice were genotyped from tail-tip DNA. They were housed under conditions of controlled lighting (lights on from 6:00 am to 6:00 pm) and temperature (2ºC 2 ± 1ºC), with ad libitum access to standard chow and water, and were 7–10 (experiments 1–3) or 4–20 (experiment 4) weeks old. All animal experimental protocols were approved by the University of Otago Animal Ethics Committee and the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center.
Experiment 1
To quantify LepRb and ERα coexpression in hypothalamic regions, Leprb-expressing cells were visualized through Cre-dependent GFP expression as a result of cross-breeding the Leprb-Cre mouse line with the Tau-GFP reporter line, producing Leprb-GFP mice. We (21) and others (30) have previously shown that the Leprb-Cre mouse can be used to faithfully visualize most leptin-responsive cells in the hypothalamus. Adult male (n = 5), proestrous female (n = 5), and diestrous female (n = 5) Leprb-GFP mice were anesthetized with sodium pentobarbital (200 mg/kg, ip) and perfused transcardially with 4% paraformaldehyde. Collected brains were postfixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, sectioned into 30-μm coronal slices on a microtome with a freezing stage and immunostained for ERα. Free-floating sections were washed three times in 0.1 M Tris-buffered saline, incubated for 24 hours at 4ºC in polyclonal rabbit anti-ERα primary antibody (1:20 000; Santa Cruz Biotechnology) (Table 1) diluted in 0.1 M Tris-buffered saline, 0.1% Triton-X, 0.25% BSA, and 2% normal goat serum, washed as above then incubated in Alexa Fluor 568 goat antirabbit IgG (1:500; Molecular Probes, Life Technologies) for 2 hours. Mounted sections were viewed using fluorescent light microscopy (460–480 nm excitation wavelength range for Leprb-GFP and 540–580 nm excitation wavelength range for ERα) and photographed at ×200 magnification for analysis. Endogenous GFP expression in the median preoptic nucleus (MnPO), rostral preoptic area (rPOA), rostral (−1.2 mm to −1.7 mm relative to bregma), middle (−1.7 mm to −2.2 mm), and caudal (−2.2 mm to −2.7 mm) Arc, VMH, dorsomedial hypothalamus (DMH), and paraventricular nucleus was merged with ERα immunoreactivity. Cell counts from at least three sections per region per animal were averaged. ERα-coexpressing Leprb-GFP cells were identified if a red ERα-immunoractive nucleus was observed within the GFP-expressing cytosol. Archived neuron-specific ERα KO mouse brain sections (see reference 33 for details of these mice) served as an immunohistochemical negative control; no ERα immunoreactivity was observed when these tissues were subjected to the above immunohistochemistry procedure.
| Peptide/Protein Target . | Antigen Sequence (if Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised (Monoclonal or Polyclonal) . | Dilution Used . | DOI or Publication Data . |
|---|---|---|---|---|---|---|
| Synthetic phosphopeptide around Tyr705 of mouse STAT3 | 9131 | pSTAT3 (Thy705) | Cell Signaling | Rabbit | 1:3000 | Endocrinology. 2013; 154:2318–2330 |
| ERα | Epitope mapping at the C terminus of ERα of mouse origin | ERα (MC-20) antibody | Santa Cruz Biotechnology, sc-542 | Rabbit, polyclonal | 1:20 000 | EN-14-1226 |
| GFP | Anti-GFP antibody | Millipore, AB3080 | Chicken | 1:2000 | ||
| Rabbit IgG | Alexa Fluor 568 conjugate IgG | Molecular Probes | Goat | 1:500 | 10.1210/en.2013–2042 | |
| Chicken IgG | Alexa Fluor 488 conjugate IgG | Molecular Probes | Goat | 1:500 | Endocrinology. 2013; 154:2410–2420 | |
| Anitrabbit IgG | Biotinylated goat antirabbit IgG | Vector Labs, catalog number BA-1000 | Goat, polyclonal | 1:500 | Endocrinology. 2013; 154:3817–3825 |
| Peptide/Protein Target . | Antigen Sequence (if Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised (Monoclonal or Polyclonal) . | Dilution Used . | DOI or Publication Data . |
|---|---|---|---|---|---|---|
| Synthetic phosphopeptide around Tyr705 of mouse STAT3 | 9131 | pSTAT3 (Thy705) | Cell Signaling | Rabbit | 1:3000 | Endocrinology. 2013; 154:2318–2330 |
| ERα | Epitope mapping at the C terminus of ERα of mouse origin | ERα (MC-20) antibody | Santa Cruz Biotechnology, sc-542 | Rabbit, polyclonal | 1:20 000 | EN-14-1226 |
| GFP | Anti-GFP antibody | Millipore, AB3080 | Chicken | 1:2000 | ||
| Rabbit IgG | Alexa Fluor 568 conjugate IgG | Molecular Probes | Goat | 1:500 | 10.1210/en.2013–2042 | |
| Chicken IgG | Alexa Fluor 488 conjugate IgG | Molecular Probes | Goat | 1:500 | Endocrinology. 2013; 154:2410–2420 | |
| Anitrabbit IgG | Biotinylated goat antirabbit IgG | Vector Labs, catalog number BA-1000 | Goat, polyclonal | 1:500 | Endocrinology. 2013; 154:3817–3825 |
| Peptide/Protein Target . | Antigen Sequence (if Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised (Monoclonal or Polyclonal) . | Dilution Used . | DOI or Publication Data . |
|---|---|---|---|---|---|---|
| Synthetic phosphopeptide around Tyr705 of mouse STAT3 | 9131 | pSTAT3 (Thy705) | Cell Signaling | Rabbit | 1:3000 | Endocrinology. 2013; 154:2318–2330 |
| ERα | Epitope mapping at the C terminus of ERα of mouse origin | ERα (MC-20) antibody | Santa Cruz Biotechnology, sc-542 | Rabbit, polyclonal | 1:20 000 | EN-14-1226 |
| GFP | Anti-GFP antibody | Millipore, AB3080 | Chicken | 1:2000 | ||
| Rabbit IgG | Alexa Fluor 568 conjugate IgG | Molecular Probes | Goat | 1:500 | 10.1210/en.2013–2042 | |
| Chicken IgG | Alexa Fluor 488 conjugate IgG | Molecular Probes | Goat | 1:500 | Endocrinology. 2013; 154:2410–2420 | |
| Anitrabbit IgG | Biotinylated goat antirabbit IgG | Vector Labs, catalog number BA-1000 | Goat, polyclonal | 1:500 | Endocrinology. 2013; 154:3817–3825 |
| Peptide/Protein Target . | Antigen Sequence (if Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised (Monoclonal or Polyclonal) . | Dilution Used . | DOI or Publication Data . |
|---|---|---|---|---|---|---|
| Synthetic phosphopeptide around Tyr705 of mouse STAT3 | 9131 | pSTAT3 (Thy705) | Cell Signaling | Rabbit | 1:3000 | Endocrinology. 2013; 154:2318–2330 |
| ERα | Epitope mapping at the C terminus of ERα of mouse origin | ERα (MC-20) antibody | Santa Cruz Biotechnology, sc-542 | Rabbit, polyclonal | 1:20 000 | EN-14-1226 |
| GFP | Anti-GFP antibody | Millipore, AB3080 | Chicken | 1:2000 | ||
| Rabbit IgG | Alexa Fluor 568 conjugate IgG | Molecular Probes | Goat | 1:500 | 10.1210/en.2013–2042 | |
| Chicken IgG | Alexa Fluor 488 conjugate IgG | Molecular Probes | Goat | 1:500 | Endocrinology. 2013; 154:2410–2420 | |
| Anitrabbit IgG | Biotinylated goat antirabbit IgG | Vector Labs, catalog number BA-1000 | Goat, polyclonal | 1:500 | Endocrinology. 2013; 154:3817–3825 |
Experiment 2
To test whether hypothalamic leptin responsiveness is enhanced by estradiol, we evaluated Leprb-STAT3 signaling under different estrogenic states. Adult female wild-type mice were ovariectomized and given a sc slow-release, estradiol-filled silicone rubber capsule at varying concentrations. Low estradiol (n = 6; 0.05 mg/kg dose, made from 10 mm length, 1.02 mm inner diameter, and 2.16 mm outer diameter silicone rubber tubing filled with 0.1 mg/mL β-estradiol/silicone rubber adhesive), high estradiol (n = 5; 0.5 mg/kg dose, made from identical tubing filled with 1.0 mg/mL β-estradiol/silicone rubber adhesive), or vehicle (n = 5, containing silicone rubber adhesive only) capsules were implanted for 7 days.
A further group (n = 4) received low-estradiol implants plus a single 0.05 mg/kg sc injection of estradiol benzoate in sesame oil 4 hours prior to perfusion, This represents a commonly used model of the increasing circulating concentration estradiol that occurs in the follicular phase in females, inducing a switch from negative to positive estrogenic feedback and a preovulatory-like gonadotrophin surge (eg, reference 33). After 7 days of implant treatment, all mice were given a physiological dose of leptin (0.02 mg/kg sc; National Hormone and Peptide Program, Torrance, CA) to induce STAT3 phosphorylation (pSTAT3) in the Arc. A pilot study using intact females mice (n = 5) comparing two different leptin doses (1 mg/kg and 0.02 mg/kg) showed that the latter dose induces a robust response but does not saturate the level of Arc pSTAT3, therefore avoiding any potential ceiling effect on leptin responsiveness (see Results). Mice were perfused as above 2 hours after the leptin injection, and brains were collected for pSTAT3 immunohistochemistry. Uteri were weighed to confirm the efficacy of the estradiol treatments. Arc sections were washed as above (washes also separated each of the following steps), incubated in 1 mM EDTA (pH 8.0) at 90ºC for 15 minutes in 0.3% hydrogen peroxide for 10 minutes and then in rabbit anti-pSTAT3 (tyr705) primary antibody for 24 hours at 4ºC (1:3000; Cell Signaling Technology) (Table 1). This was followed by a 1-hour incubation in biotinylated goat antirabbit IgG secondary antibody (1:500; Vector Laboratories), Vector Elite ABC solution (1:100; Vector Laboratories), and finally, nickel-enhanced diaminobenzidine solution (Sigma-Aldrich). Immunoreactive cells were visualized and photographed under bright-field microscopy at ×200 magnification.
Experiment 3
To test whether estradiol's antiobesity effects are dependent on LepRb-STAT3 signaling, we generated mice in which STAT3 was conditionally deleted from Leprb-expressing cells. Homozygous Leprb-Cre mice were crossed with homozygous Stat3-flox (Stat3fl/fl) mice; breeding the heterozygous offspring back to Stat3fl/fl mice resulted in the homozygous deletion of STAT3 specifically in cells that express Leprb (henceforth referred to as Leprb-STAT3 KO mice). We have previously verified and reported the widespread reduction in leptin-induced hypothalamic STAT3 signaling in this mouse line (21). Female Leprb-STAT3 KO mice and Stat3fl/fl controls were OVX and given either empty (controls, n = 7; KOs, n = 4) or estradiol (2 mg/mL) filled (controls, n = 7; KOs, n = 4) sc implants. Body weights were recorded daily and implants replaced weekly. After 14 days, all mice were decapitated and fresh brains were collected for quantitative PCR analysis. Coronal brain sections (300 μm thick) were cut in a cryostat and punches of Arc tissues were collected using a sterile 21-gauge micropunch needle (internal diameter 500 μm). Total RNA from punched tissues was extracted from samples using RNeasy Plus microspin columns (QIAGEN). RNA quality was assessed by spectrophotometry and agarose gel electrophoresis, and 70 ng of total RNA was subjected to deoxyribonuclease I treatment (Promega Corp). Total RNA was then reverse transcribed with SuperScript III reverse transcriptase (Life Technologies) using random hexamers as primers.
Duplicate uniplex reactions for measurement of Npy mRNA levels were carried out on cDNA samples using the following probe/primer mix: probe, 56-FAM/AAGGAA AGC/ZEN/ACAGAAAACGCCCC/3IABkFQ (final concentration 0.25 μM); forward primer, AGCCCTGAGACACTGATTTC; reverse primer, TGGAAAAGTCGGGAGAACAAG (final concentrations 0.5 μM; product length 91 bp; amplification efficiency 0.99; National Center for Biotechnology Information accession number NM_023456). Probes and primers specific for three reference genes were tested over our experimental samples: β-actin, polymerase (RNA) II (DNA directed) polypeptide A (Polr2a), and tata-box binding protein (Tbp). The reference gene Polr2a was chosen in this experiment due to its minimal variation across experimental groups: probe, 56-FAM/ATTATCGGC/ZEN/ATTTGGCGCTCCTGTGT/3IABkFQ (final concentration 0.25 μM); forward primer, GCACCACGTCCAATGATAT; reverse primer, GTGCTGCTGCTTCCATAA (final concentration 0.9 and 0.3 μM, respectively; product length 266 bp; amplification efficiency 0.95; National Center for Biotechnology Information reference sequence NM_009089.2). Reactions (20 μL) were prepared in 96-well white Roche LightCycler 480 multiwell plates, with Roche optical adhesive covers (Roche Applied Science) using Roche PCR master mix (Roche Applied Science). Using the Roche Light Cycler 480 machine, samples were heated to 50ºC for 2 minutes and then 95ºC for 10 minutes before 40 cycles of 95ºC for 15 seconds and 60ºC for 1 minute as per the manufacturer's instructions. Data were analyzed using the comparative Cq method in which Cq is the quantification cycle number at which the fluorescence reading is first recorded above background levels. Subtracting the average Cq value for the gene of interest from the average Cq value for the reference gene gave the δCq. Subtracting the mean δCq value from the δCq for each sample gave the relative change (δδCq). Finally, the arithmetic formula, 2−δδCq, was used to achieve relative quantification.
To test whether estradiol acts directly on NPY neurons, intact adult male (n = 5) and female (n = 7) Npy-Tau-Sapphire-GFP mice were used to visualize NPY neurons. These mice express Sapphire-GFP, a blue-shifted GFP variant, under the endogenous transcriptional control of the Npy promoter (14). The endogenous Sapphire-GFP was immunolabeled using chicken anti-GFP primary antibody (1:2000; Millipore) (Table 1) and visualized by Alexa Fluor 488 goat antichicken IgG (1:500; Molecular Probes, Life Technologies). ERα immunohistochemistry was performed as described in experiment 1, and sections were visualized on an LSM 710 upright confocal laser scanning microscope (Carl Zeiss) using 488 nm (for Npy-GFP) and 543 nm (for ERα) laser excitation lines and filters.
Experiment 4
To test whether direct estrogenic actions are required downstream of LepRb signaling for body weight regulation, the Leprb-Cre mouse line was crossed with the Erα-flox line and the heterozygous progeny either bred together or backcrossed to Erαfl/fl mice to generate female mice homozygous for ERα deletion specifically in cells that express LepRb (Leprb-ERα KO). Body weights of these mice (n = 7) and ERαfl/fl controls (n = 10) were measured weekly from weaning until 16 weeks of age. The ERα KO was confirmed using ERα immunohistochemistry (as described in experiment 1) in MnPO sections to detect the reduction in total ERα-immunoreactive cell counts. A separate cohort of female Leprb-ERα KO (n = 8) and ERαfl/fl control (n = 7) mice (18–20 wk old) was used for oral glucose tolerance and metabolic cage measurements. For the oral glucose tolerance test (OGTT), mice were fasted for 3 hours and glucose (2.5 g/kg) was administered by oral gavage. Blood samples were collected from the tail vein and glucose concentration was measured using glucometer. Mice were analyzed for respiratory exchange ratio (RER), heat production, food intake, and ambulatory movement using a combined indirect calorimetry system (TSE Systems GmbH) as described previously (34). Mice were habituated to the metabolic chambers for 6 days prior to testing, and total testing was conducted over a 24-hour period. RER was calculated as the molar ratio between CO2 delivered and O2 consumed. Changes in RER values reveal alterations in the main substrates undergoing global oxidative metabolism; oxidation of glucose leads to RER values equal to 1, whereas the oxidation of a fatty acid molecule leads to an RER value of 0.7. For analysis of ambulatory movement, mice were placed in a cage containing an invisible laser beam grid. The frequency of beam breaks was used to measure locomotor activity.
Another cohort of female Leprb-ERα KO and ERαfl/fl or Leprb-Cre control (n = 8–11) mice were used to test whether direct estrogenic actions are required on LepRb neurons for puberty onset and reproductive function. They were monitored daily for indicators of pubertal onset, namely vaginal opening and then estrous cyclicity onset (first estrous, detected by vaginal lavage and cytology) until puberty had occurred in all animals. One week later, estrous cycles were monitored continuously for 10–12 days and the proportion of time spent in proestrous, estrous, metestrous, and diestrous phases determined. Adult fecundity was assessed by pairing with wild-type males for 3 months and recording the number of litters obtained.
Statistical analysis
All data are presented as means ± SEM. Where results of a single treatment were compared with a control group, data were analyzed using a Student's t test. Where more than two groups were compared, data were analyzed using a one-way or two-way ANOVA, with repeated measures as appropriate, followed by Holm-Sidak's multiple comparisons test to determine where significant differences occurred. P < .05 was considered statistically significant.
Results
Experiment 1: colocalization of Leprb-GFP and ERα
Representative examples of nuclear ERα immunoreactivity and its colocalization with Leprb-GFP in different hypothalamic regions are shown in Figure 1, A–D. The percentage of Leprb-GFP cells coexpressing ERα was not different between male, diestrous female or proestrous female mice in any region (Figure 1F). Colocalization was highest (70%–90%) in the MnPO and rPOA, from which the gonadal axis is regulated. Elsewhere in the hypothalamus, however, including the mediobasal hypothalamic regions that are important for body weight regulation, we observed very low (5%–15%) amounts of colocalization. The distribution of ERα immunoreactivity relative to the Leprb-GFP expression was not uniform across the Arc, with the medial and caudal Arc showing virtually no ERα in the medioventral portion, in which most Leprb-GFP expression was found (Figure 1, B and D). In the rostral Arc, Leprb and ERα tended to be more uniformly distributed (Figure 1C). ERα staining was essentially absent in negative control tissue from a neuronal ERα KO mouse line (Figure 1E).
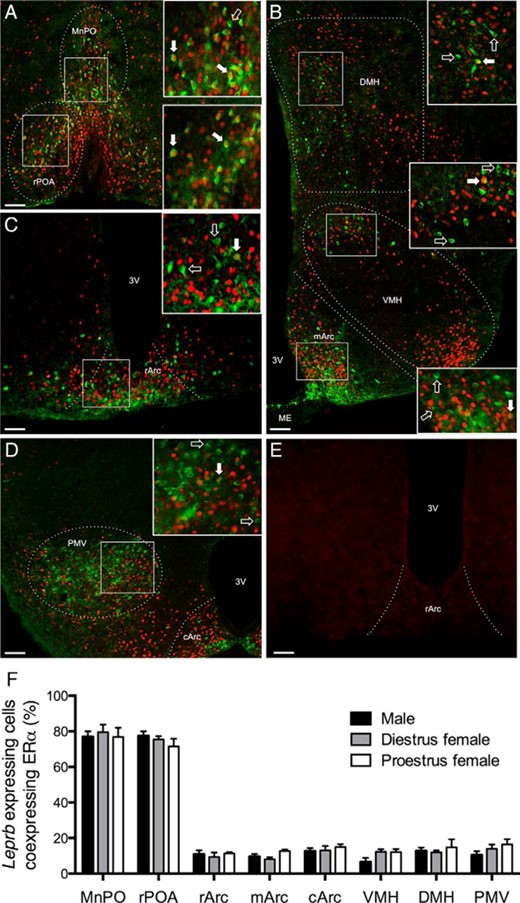
Extensive hypothalamic ERα coexpression with LepRb is limited to the preoptic region in male and female mice. Representative examples of ERα immunoreactivity (red nuclear staining) and LepRb-Cre-GFP (green cytosol and fiber staining) in the rPOA and MnPO (A), DMH, VMH, and medial arcuate nucleus (mArc) (B), rostral arcuate nucleus (rArc) (C), and the caudal arcuate nucleus (cArc) and ventral premammilary nucleus (PMV) (D) are shown. Within each panel, the boxed regions are enlarged on the left. Filled arrows indicate colocalized cells; unfilled arrows indicate noncolocalized LepRb-Cre-GFP cells. E, Absence of staining in neuronal ERα KO mouse tissue. Scale bars, 50 μm. ME, median eminence; 3V, third ventricle. F, Percentage of LepRb-Cre-GFP cells that coexpress ERα within each region in male, diestrous females, and proestrous females (n = 5 per group). Error bars denote SEM.
Experiment 2: estradiol does not increase leptin-induced pSTAT3 expression
A submaximal leptin dose for induction of pSTAT3 immunoreactivity was selected based on a preliminary comparison of 1-mg/kg (previously shown to produce a maximal response) and 0.02-mg/kg doses (Figure 2A). The lower dose yielded a robust but significantly lower response than 1 mg/kg (t[7] = 3.72, P < .01) and was selected for the experiment examining estradiol's effects on Arc leptin responsiveness. The leptin-induced pSTAT3 response in varying estrogenic states was measured in the Arc. The results did not support the idea that estrogen exposure enhances leptin sensitivity; rather, it was found that OVX+low estradiol and OVX+high estradiol mice displayed slightly less pSTAT3-immunoreactive cells in the Arc compared with OVX controls (F[3, 16] = 6.83, P < .05) (Figure 2C). Numbers of arcuate pSTAT3-immunoreactive cells were not significantly affected by acute estradiol benzoate treatment (Figure 2C). To confirm the efficacy of the estradiol treatments used in this experiment, uteri were weighed as an index of circulating estradiol concentration. Estradiol caused a dose dependent increase in uterine wet weight (F[3, 19] = 35.18, P < .001) such that at the high dose uterine weights were 3 times that of OVX untreated controls (Figure 2B).
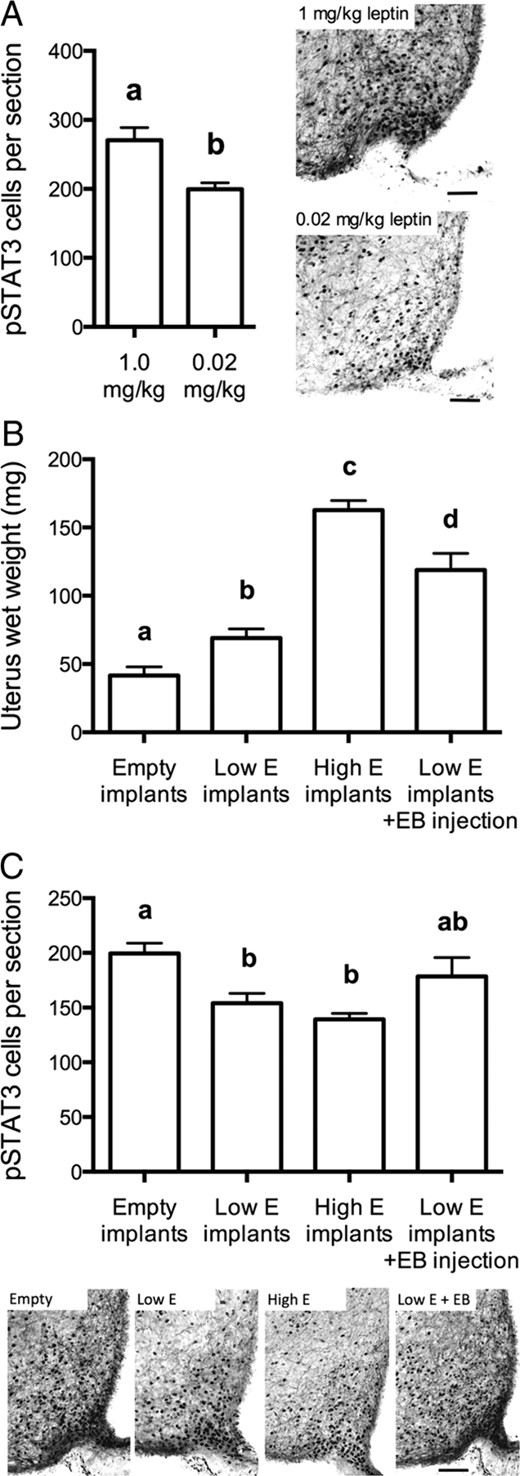
Estradiol does not enhance leptin responsiveness in the Arc in female mice. A, left panel, Number of pSTAT3-immunoreactive cells per arcuate section in response to 1 or 0.02 mg/kg leptin. The latter dose was chosen a submaximal dose. Right panels show representative examples of pSTAT3 staining at each leptin dose. B, Chronic (1 wk) estradiol (E) implant treatment (low dose: 0.05 μg/kg; high dose: 0.5 μg/kg) and an acute (4 h) EB (0.05 μg/kg) injection increased uterine weight in proportion to the dose and duration of treatment. C, Estradiol treatments did not enhance Arc leptin responsiveness; instead leptin-induced arcuate pSTAT3 cell counts were slightly reduced by the estradiol implants. Lower panels show representative examples of pSTAT3 staining at each estradiol dose. Scale bars, 50 μm. Treatment groups that do not have common letters are significantly different (P < .05). Error bars denote SEM.
Experiment 3: anorexigenic effect of estradiol is retained in Leprb-STAT3fl/fl mice
Confirmation of deletion of functional STAT3 in Leprb-STAT3 KO mice using immunohistochemistry for pSTAT3 has been reported elsewhere for this cohort of mice (21). In the absence of estradiol treatment, Leprb-STAT3 KO mice tended to be heavier than Stat3fl/fl controls, but this difference did not reach statistical significance over the study period. Estradiol replacement suppressed body weight gain in both genotypes from just after the first week of treatment, so that by day 14, they were 15% lighter on average than vehicle-treated animals (F[1, 18] = 12.28, P < .01, Figure 3, A and B). In Leprb-STAT3 KO mice, the body weight-suppressing effect of estradiol was undiminished, with no significant differences between genotypes of comparable estrogenic status. The main effect of estradiol treatment was significant from 10 days after surgery until the end of the experiment (Figure 3A). Carcass analysis at day 14 revealed that estradiol treatment of both Leprb-STAT3 KO and Stat3fl/fl controls also resulted in a significantly lower abdominal fat content (F[1, 12] = 61.86, P < .001, Figure 3C) compared with the respective untreated controls, with no significant difference between genotypes except for a 57% increase in abdominal fat content in untreated Leprb-STAT3 KO mice compared with their respective Stat3fl/fl controls (t[12] = 3.49, P < .01, Figure 3C). Thus, estradiol clearly retained the ability to normalize the metabolic effects of ovariectomy, even in the absence of STAT3 signaling in LepRb cells.
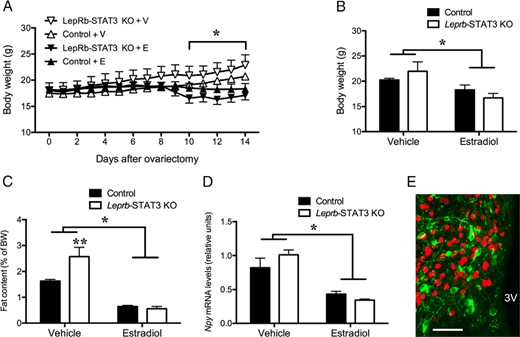
Mice with absent STAT3 signaling in LepRb neurons have normal metabolic responses to estradiol. Chronic estradiol replacement prevented the body weight gain (A and B), abdominal fat accumulation (C), and elevated arcuate Npy mRNA levels (D) in response to ovariectomy in both LepR-STAT3 KO and littermate control mice. E, estradiol; V, vehicle. E, Virtually no colocalization was observed between NPY-GFP (green cytosol and fiber staining) and ERα immunoreactivity (red nuclear staining), suggesting that estrogenic effects on NPY neurons are likely to be indirect. 3V, third ventricle; *, Main effect of estradiol, P < .05; **, effect of LepRb-STAT3 KO, P < .01. Error bars denote SEM.
Estradiol indirectly inhibits NPY expression in Leprb-STAT3 KO mice
Consistent with the pronounced metabolic effects of estradiol in the Leprb-STAT3 KO mice reported above, quantitative PCR analysis after the 2-week body weight experiment revealed that both the estradiol-treated Leprb-STAT3 KO and Stat3fl/fl mice had markedly lower Npy gene expression in the Arc compared with their vehicle-treated counterparts (F[1, 12] = 41.60, P < .001, Figure 3D), with no significant difference between genotypes. Because 50%–70% of arcuate NPY neurons are leptin responsive (35, 36) and we have shown in experiment 1 that less than 15% of arcuate LepRb neurons contain ERα, the likelihood of strong arcuate NPY and ERα colocalization is low. In confirmation of this, only a very minimal proportion (3%) of NPY-GFP neurons were observed to coexpress ERα immunoreactivity in male and female mice (see Figure 3E for a representative image). Therefore, the effect of estradiol on NPY neurons appears to be indirectly mediated.
Experiment 4: LepRb-ERα KO mice exhibit normal body weight and energy homeostasis but decreased fecundity
Confirmation of deletion of ERα using immunohistochemistry revealed a significant reduction in the number of ERα-stained cells in the MnPO in Leprb-ERα KO mice compared with ERαfl/fl controls (t[13] = 2.16, P < .05, Figure 4A). In all mediobasal hypothalamic regions, in which we have shown that less than 15% of LepRb neurons contain ERα (Figure 1F), no significant differences in numbers of ERα-immunoreactive cells were observed (data not shown). Leprb-ERα KO mice did not show any differences in body weight compared with Erαfl/fl controls over the monitoring period (4–16 wk of age) (Figure 4B). Leprb-ERα KO mice also displayed normal whole body lean and fat mass (Figure 4C), ambulatory movement level (Figure 4E), heat production (Figure 4F), and RER (Figure 4G) relative to controls. Cumulative food intake was not different between the groups during the dark phase but was sufficiently increased during the light phase (t[13] = 2.35, P < .05) to cause the overall 24-hour food intake to be significantly increased in Leprb-ERα KO mice compared with controls (t[13] = 2.61, P < .05, Figure 4D). Glucose clearance was unchanged between groups after an OGTT (Figure 4H).
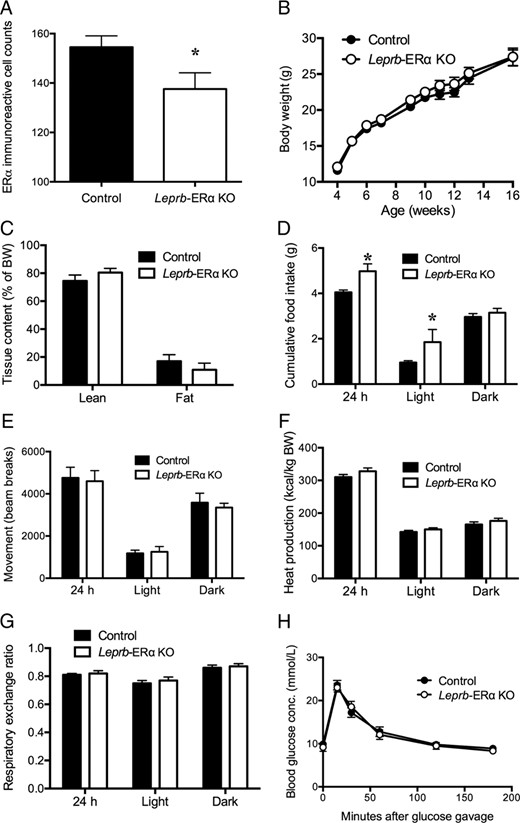
A, Confirmation of the ERα KO using immunohistochemistry revealed a significant reduction in ERα-immunoreactive cells in the MnPO in LepRb-ERα KO mice compared with littermate controls. Mice with ERα deletion from LepRb neurons do not show altered body weight gain (B), whole-body lean or fat mass (C), ambulatory movement (E), heat production (F), RER (G), or glucose disposal after an OGTT (H). D, Cumulative food intake was not different between the groups during the dark phase but was increased during the light phase in Leprb-ERα KO mice compared with controls. *, P < .05. Error bars denote SEM.
Because the number of ERα-stained cells was reduced in the MnPO, a region important for control of reproductive function, we generated additional Leprb-ERα KO and control females to test whether direct estrogenic actions on LepRb cells are required for puberty onset and fertility. Female Leprb-ERα KO mice exhibited normal puberty onset (age at vaginal opening and first estrus) and occurrence of estrous cycles compared with controls (Figure 5). However, when paired with wild-type males for 3 months, only three of eight Leprb-ERα KO females were able to produce litters compared with 10 of 11 control females (χ2[1,N=20] = 6.11, P = .01, Figure 5). The fertile control females littered every 3–4 weeks over the 3-month breeding period, whereas the three fertile Leprb-ERα KO females produced only a single litter each, and two of these litters were found dead on the day of birth. In contrast to females, the two Leprb-ERα KO males tested remained fertile in our breeding colonies (data not shown).
![Female mice with ERα deletion from LepRb neurons do not show altered puberty onset (vaginal opening [VO] and first estrus [first E]) or estrous cycles (percentage of time in metestrus or diestrus, [%MD], proestrus, [%P], and estrus [%E]) but have reduced adult fecundity compared with controls when paired with wild-type males. A single litter was produced in only three of eight Leprb-ERα KO females, compared with 10 of 11 control females that produced multiple litters over the 3-month breeding period. **, P = .01 vs control mice. Error bars denote SEM.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/endo/157/5/10.1210_en.2015-1594/3/m_zee9991684240005.jpeg?Expires=1748025946&Signature=fM-mWD1RCUK83iDc5pHJyLs45Yg1bv8tRgI0rSN~2nNQfFk6nOiq6O2ovdkqWMX1bZR8h6s8iRtMjVouJVOmm8MAu5ckU-1B7ZUOM3ho9uXJkvaQGi7pGNlM-u3Ty0JpsGdKLhAs6ec6HNSDAI4htGMXbXiStRQ36BpAIASav6HWMMPErfCuvtxjY6aFjHnBUqYQ7iiBHAJ9SxzFJUIrn7sbmk5OA4~KOi-D53Rh6DquO67X0mTarikNe6QNWJwLyHOS~mAoo0~mJpRl9kqEg13X9GhXAkCfvyRxIORcKRgbJW9lswc3QsYJCcsqmgo0boOjLXr~LgaC0aLBD0KyaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Female mice with ERα deletion from LepRb neurons do not show altered puberty onset (vaginal opening [VO] and first estrus [first E]) or estrous cycles (percentage of time in metestrus or diestrus, [%MD], proestrus, [%P], and estrus [%E]) but have reduced adult fecundity compared with controls when paired with wild-type males. A single litter was produced in only three of eight Leprb-ERα KO females, compared with 10 of 11 control females that produced multiple litters over the 3-month breeding period. **, P = .01 vs control mice. Error bars denote SEM.
To investigate whether the minimal metabolic effects of STAT3 and ERα KO from LepRb neurons were due to insufficient levels of Cre, Leprb-ERα KO mice with either hemizygous or homozygous Leprb-Cre were generated by crossing female mice hemizygous for Leprb-Cre and heterozygous for Erα-flox with male mice homozygous for both Leprb-Cre and Erα-flox. Body weight, food intake, ambulatory movement, and RER at 20 weeks of age were compared in these mice and in non-Cre controls. In both males and females, these metabolic parameters were similar to controls; there were no effects of Leprb-Cre zygosity except for a small increase in food intake during the light phase in female homozygous Leprb-Cre mice and in movement during the dark phase in male hemizygous Leprb-Cre mice compared with controls (both P < .05, Supplemental Figure 1). Furthermore, there were no effects of Leprb-Cre zygosity on date of puberty onset of estrous cycles (Supplemental Figure 2). Adult fecundity was not assessed for effects of Leprb-Cre zygosity.
Discussion
In this study we assessed the extent to which LepRb and ERα are coexpressed in hypothalamic cells and then tested whether cross talk between the signaling of these receptors is required for estradiol's anorexigenic effects. Despite speculation that estradiol acts to enhance LepRb-STAT3 signaling, direct evidence of estrogenic interactions with the LepRb-STAT3 pathway is lacking in the literature. Our data do not support the widespread existence of such an interaction for metabolic function in female mice because LepRb and ERα coexpression was very limited in the mediobasal hypothalamus, and transgenic deletion of either STAT3 or ERα from LepRb cells did not compromise the ability of estradiol to suppress body weight. In contrast, LepRb and ERα were strongly coexpressed in the preoptic area and fecundity was impaired in in Leprb-ERα KO animals.
Bianco-Borges et al (37) in 2010 reported at least some colocalization of LepRb and ERα immunoreactivity in the mouse preoptic area and DMH; however, this was not quantified. Here we report a detailed characterization of LepRb and ERα colocalization throughout the murine hypothalamus. Surprisingly, strong coexpression was absent in all regions except the preoptic area. Very recently, da Silva et al (38) reported similarly low LepRb and ERα coexpression in the mediobasal hypothalamus and higher levels in the medial preoptic area and also showed that this coexpression tended to decline still further 1 month after ovariectomy. Our results and those of da Silva et al are in contrast to a previous report in rats, which reported a remarkably extensive coexpression of LepRb immunoreactivity in all ERα-expressing cells in the mPOA, Arc, and VMH (27). Given the technical difficulty in detecting LepRb protein on brain slices, an important point of difference is that we and da Silva et al used Leprb-Cre-dependent GFP mice instead of immunohistochemistry to map hypothalamic LepRb. Fluorescent reporting of Cre recombinase expression in this mouse has been previously validated against leptin-induced pSTAT3 (21, 28, 30). Using this Leprb-Cre-GFP mouse, the only region in which significant Leprb and ERα colocalization was observed was the preoptic area (rPOA and MnPO) in which estradiol acts to control the preovulatory gonadotrophin surge in females (33) and sexual behavior in males and females (39). Thus, colocalization in this region would be expected to have little bearing on metabolic and satiety effects of leptin and estradiol, both of which have been shown to occur in the mediobasal hypothalamus (40, 41).
The antiobesity effects of leptin are enhanced in the presence of high circulating estrogens (42), supporting the idea that gonadal steroids regulate leptin sensitivity (43). Indeed, a higher pSTAT3 response to leptin was observed in cells coexpressing both LepRb and ERα than those that express LepRb alone in breast cancer cell lines (44). With the low colocalization of Leprb-GFP and ERα reported in the Arc and VMH, in which most of leptin's antiobesity actions occur, physiologically important direct interactions between agonist-bound ERα and leptin-STAT3 signaling seem unlikely. This was supported in our second experiment since, in contrast to the data from breast cancer cells (44), leptin-induced pSTAT3 immunoreactive cell numbers in the Arc showed no increase with increasing doses of chronic estradiol treatment. In fact, we observed a slight dose-responsive reduction of pSTAT3 response with chronic estradiol treatment. Reports of estrogenic effects on leptin sensitivity are variable in the literature (see reference 10 for review). In one study, ip-injected estradiol was itself able to induce STAT3 phosphorylation 1 hour after injection (4). In this regard, it is worth noting that we observed a small but significant increase in arcuate pSTAT3 staining in acute estradiol benzoate (EB) treated mice compared with their equivalent non-EB-treated counterparts. Therefore, estradiol may acutely activate STAT3, perhaps independently of LepRb, whereas chronic estradiol may influence STAT3 activation differently.
Collectively our results suggest that estradiol does not directly act on the leptin-STAT3 signaling pathway in the regulation of body weight. This conclusion is supported by the fact that estradiol was able to significantly reduce body weight in OVX Leprb-STAT3 KO mice compared with untreated controls, thereby providing evidence that STAT3 of the LepRb signaling pathway is not required for estradiol's effect in energy homeostasis. Furthermore, the deletion of ERα specifically from Leprb-expressing cells also did not result in an obvious metabolic phenotype. These data suggest that ERα, the critical receptor for estradiol's regulation of body weight (22, 23), need not directly interact with LepRb signaling for body weight regulation. Although we do report an increased food intake in mice lacking ERα from the Leprb expressing cells, no other disruptions to energy homeostasis were observed. Furthermore, the increased food intake did not reflect on the overall body weight because the Leprb-ERα mice were not overweight compared with controls. This mild phenotype could potentially be attributable to low levels of Leprb-Cre expression, despite the fact that Cre recombinase from this mouse line has been shown to effectively drive fluorescence expression in numerous reporter mice (21, 28, 30, 45), and deletion of genes encoding insulin receptor, suppressor of cytokine signaling 3, phosphoinositide 3 kinase catalytic subunits and STAT3 from Leprb-Cre cells all caused significant metabolic phenotypes (21, 45–47). Nevertheless, we tested whether Leprb-Cre homozygosity in Leprb-ERα KO animals would lead to a more pronounced metabolic phenotype than Leprb-Cre hemizygosity. As shown in Supplemental Figure 1, body weight, food intake, ambulatory movement, and RER in homozygous Leprb-Cre mice were all similar to controls; there were virtually no effects of Leprb-Cre zygosity. This was also the case for timing of puberty onset and estrous cycles (Supplemental Figure 2).
Although high levels of circulating estrogens are known to enhance the antiobesity effects of leptin in vivo (42), it is likely that the two hormones act independently to reduce body weight and/or that estradiol increases leptin sensitivity indirectly. Our data suggest the latter to be the case in regard to the NPY/AgRP neurons. Estrogenic inhibition of NPY is well recognized and thought to play a crucial metabolic role (17, 48), and central administration of estradiol is also unable to reduce food intake in mice lacking AgRP (16). The lack of colocalization of ERα and Npy-GFP in experiment 3 suggests that this inhibition is indirect rather than being mediated via direct crosstalk with leptin signaling. Similar findings for NPY neurons have been reported previously (16).
Xu et al (34) in 2011 demonstrated the importance of estradiol signaling in the brain for control of appetite and metabolism. Brain-specific ERα KO caused a reduction in energy expenditure, increased food intake, and overall increased body weight and adiposity. This was extended by showing that deleting ERα selectively in steroidogenic factor-1-positive neurons, which are mainly localized in VMH, impairs estrogenic regulation of energy output. This is consistent with an earlier report showing that virus-induced silencing of ERα selectively in the VMH results in obesity (41). It should be noted that most ERα in the VMH is selectively expressed in the ventral-lateral portion, in which LepRb is not expressed. Future studies are needed to unravel the exact role these ERα-expressing neurons play. Estradiol, administered peripherally in this experiment by sc implants, could also influence metabolic function indirectly, for example, by regulation of adipocyte function (50) or ghrelin expression in the stomach (51).
Our Leprb-STAT3 KO results differ from those of a previous report showing that estradiol is unable to mediate its satiety effects in the absence of STAT3 in the brain (4). Importantly, however, the STAT3 deletion was not limited to LepRb cells as was the case in the present experiment. Therefore, estradiol may indeed require action on STAT3 in brain cells, but its anorexigenic effects appear to be independent of direct LepRb signaling cross talk. Comparison of body weights in brain and Leprb-specific STAT3 KO mice indicate that the loss of STAT3 from the entire brain causes a much more severe metabolic effect than Leprb-specific STAT3 KO (21, 52). Taken together, the possibility remains that STAT3 in non-Leprb neurons plays a critical role for estradiol's anorexigenic effects. It should also be noted, however, that another group (31) reported a more severe Leprb-STAT3 KO obesity phenotype (∼60% body weight increase relative to floxed littermate controls) than that seen in the current study. Whereas both experiments used hemizygous Leprb-Cre mice to drive STAT3 deletion, Piper et al used a different Leprb-Cre line (53) from us (29). Thus, the possibility remains that differing levels of Cre recombinase expression could contribute to the severity of the phenotype in these mice.
Interestingly, most Leprb-ERα KO females were unable to deliver or rear pups despite apparently normal puberty onset and estrous cyclicity. It is possible that this reduced fecundity was caused by impaired sexual and/or maternal behaviors due to deficits in preoptic LepRb and ERα-coexpressing cells because this region (in which we observed extensive Leprb-ERα coexpression) is responsible for estrogenic control of such behaviors (39, 49). Further investigation of a range of reproductive parameters in these mice is warranted to clarify this.
In summary, we report here that LepRb and ERα coexpression is very low in the mediobasal hypothalamus and that conditional deletion of either STAT3 and ERα from LepRb cells did not compromise the ability of estradiol to suppress body weight. These data do not support the dogma that estradiol exerts direct interactions with LepRb cells to produce its anorexigenic effects; rather, we show that these can occur entirely independently of LepRb-STAT3 signaling. In contrast, some aspects of female fecundity may require LepRb-ERα interactions.
Acknowledgments
This work was supported by The Royal Society of New Zealand Marsden Fund and the Health Research Council.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- AgRP
agouti-related peptide
- Arc
arcuate nucleus
- DMH
dorsomedial hypothalamus
- EB
estradiol benzoate
- ERα
estrogen receptor-α
- GFP
green fluorescent protein
- KO
knockout
- LepRb
leptin receptor
- MnPO
median preoptic nucleus
- NPY
neuropeptide-Y
- OGTT
oral glucose tolerance test
- OVX
ovariectomy
- POA
preoptic area
- pSTAT3
phosphorylated STAT3
- RER
respiratory exchange ratio
- rPOA
rostral POA
- STAT
signal transducer and activator of transcription
- VMH
ventromedial hypothalamus.



