-
PDF
- Split View
-
Views
-
Cite
Cite
Amer Youssef, Victor K. M. Han, Low Oxygen Tension Modulates the Insulin-Like Growth Factor-1 or -2 Signaling via Both Insulin-Like Growth Factor-1 Receptor and Insulin Receptor to Maintain Stem Cell Identity in Placental Mesenchymal Stem Cells, Endocrinology, Volume 157, Issue 3, 1 March 2016, Pages 1163–1174, https://doi.org/10.1210/en.2015-1297
Close - Share Icon Share
Abstract
Placental mesenchymal stem cells (PMSCs) are readily available multipotent stem cells for potential use in regenerative therapies. For this purpose, PMSCs must be maintained in culture conditions that mimic the in vivo microenvironment. IGFs (IGF-1 and IGF-2) and oxygen tension are low in the placenta in early gestation and increase as pregnancy progresses. IGFs bind to two receptor tyrosine kinases, the IGF-1 receptor (IGF-1R) and the insulin receptor (IR), and their hybrid receptors. We hypothesized that IGF-1 and IGF-2 signal via distinct signaling pathways under low-oxygen tension to maintain PMSC multipotency. In preterm PMSCs, low-oxygen tension increased the expression of IGF-2 and reduced IGF-1. IGF-1 stimulated higher phosphorylation of IGF-1Rβ, ERK1/2, and AKT, which was maintained at steady lower levels by low oxygen tension. PMSC proliferation was increased by IGF-1 more than IGF-2,and was potentiated by low-oxygen tension. This IGF/low oxygen tension-mediated proliferation was receptor dependent because neutralization of the IGF-1R inhibited PMSC proliferation in the presence of IGF-1 and the IR in presence of IGF-2. These findings suggest that both IGF-1R and the IR can participate in mediating IGF signaling in maintaining PMSCs multipotency. We conclude that low-oxygen tension can modify the IGF-1 or IGF-2 signaling via the IGF-1R and IR in PMSCs.
Maintaining stem cells in vitro under appropriate culture conditions is critical to preserve stem cell identity and to determine fate toward self-renewal or differentiation (1). For tissue regeneration therapy, stem cells must maintain their characteristics in vitro, which can be achieved by mimicking the natural microenvironment (growth factors, extracellular matrices, oxygen tension, etc) before in vivo transplantation (2, 3). IGFs (IGF-1 and IGF-2) and oxygen tension are major stem cell niche factors in the human placenta (4–6). IGFs can determine stem cell fate to either maintain multipotency or induce differentiation, whereas low-oxygen tension inhibits stem cell differentiation and retains pluripotency (7–10).
IGF-1 and IGF-2 signal mainly through the IGF-1 receptor (IGF-1R), a receptor tyrosine kinase, which triggers ERK1/2 and AKT pathways to promote growth, differentiation, and inhibit apoptosis (4, 11, 12). IGF-1 has the highest binding affinity to IGF-1R with an affinity constant of 1 nM, whereas IGF-2 has a 10-fold lower affinity (13). IGF-1R shares structural homology with the insulin receptor (IR), which is expressed in two isoforms IR-A and IR-B, and can form hybrids (HR-A and HR-B) with the IGF-1R (14, 15). Specifically, IGF-2 similar to insulin, can bind with a 10-fold higher affinity than IGF-1 to the IR-A, the insulin receptor lacking the translated region of exon 11 (16). However, the role of receptor/ligand combination in stem cells is not yet clear in mediating pluripotency.
In cell culture, IGF-1 in defined conditions–Heregulin-1, activin A, insulin-like growth factor-1, fibroblast growth factor-2 media (17) or IGF-2 in knockout serum replacement media (9) was required to maintain undifferentiated embryonic stem cells (ESCs) in vitro without the need for a feeder layer. IGF-1R is highly enriched in ESCs and blocking it reduces self-renewal and induces differentiation (9, 10, 17). IR-A has been shown to promote proliferation in fetal tissues and embryogenesis as well as in aggressive tumors (15, 18, 19). IR-B increases upon differentiation of progenitors to mature osteoblasts (20) or mature intestinal epithelial cells (21), indicating that differentiation leads to a higher ratio of IR-B to IR-A.
In vivo, stem cells reside in a perivascular compartment under low oxygen tension (ranges from < 1% up to 8% O2) (22), and maintaining stem cells under 3%–5% O2 can prevent differentiation in vitro (7). In fact, the hypoxia inducible factor 2, induced by low-oxygen tension, can interact with the promoter regions of pluripotency genes OCT4, NANOG, and SOX2 and enhances their expression in human ESCs (23, 24). Also, low-oxygen tension enhances induced pluripotent cell reprogramming efficiency by increasing the number and colony size and the expression of pluripotency genes; it was possible to form colonies without one or two of the four transcription factors (octamer transcription factor [Oct]-3/4, Krüppel-like factor-4, +/− Sry-type high-mobility-group box transcription factor-2 [Sox2], +/− c-Myc) only if placed in low oxygen tension (25). Therefore, low-oxygen tension may modulate stem cell identity.
In a previous study, we demonstrated that IGF-1 and low-oxygen tension can maintain the multipotency and proliferation of placental mesenchymal stem cells (PMSCs) from different gestations via the IGF-1R signaling (6). However, compared with IGF-1, IGF-2 is more abundantly expressed at an early stage of development (26) and can reach a 3-fold higher concentration (300 vs 100 ng/mL in umbilical cord venous blood) (27). For this purpose, we used preterm PMSCs as a model to study the differences between IGF-1 and IGF-2 signaling at low-oxygen tension. We hypothesized that, under physiological low-oxygen tension, IGF-1 and IGF-2 regulate PMSC self-renewal by utilizing different signaling pathways transduced via IGF-1R or other receptors. We showed the following: 1) oxygen tension can regulate IGF-1 and IGF-2 expression, 2) IGF signaling via its receptors mediates PMSC signaling through ERK1/2 and AKT pathways to promote PMSC proliferation, and 3) low-oxygen tension up-regulates the expression of OCT4, NANOG, and SOX2. Most importantly, we showed that the IR, in addition to the IGF-1R, can mediate IGF signaling in PMSCs under low-oxygen tension to maintain proliferation and multipotency.
Materials and Methods
PMSC Isolation, cell culture, and incubation in low-oxygen tension
As described previously (6), we isolated preterm PMSCs from early-gestation human placentae of 10–13 weeks. After informed consent, placentae were collected from patients who underwent therapeutic pregnancy termination. PMSCs were isolated from the fetal side chorionic villi and processed as previously described (6). For each experiment, at least three primary cell cultures were used with three technical replicates. Cells were cultured and maintained using DMEM/F12 media supplemented with 15% fetal bovine serum (FBS) and fibroblast growth factor-2 (100 ng/mL; Gibco). Before treatment, cells were cultured in DMEM/F12 supplemented with 10% FBS only. Upon treatment, PMSCs were switched to serum-free media containing only IGF-1 or IGF-2 (0–100 ng/mL). For IGF-1R or IR neutralization, cells were grown in serum-free media for 24 hours and then switched for 2 hours in serum-free media with 1.33× final concentration of IGF-1Rα-Ab or IRα-Ab (in 75% final volume of cell culture media). Second, serum-free media containing 4× final concentration of IGF-1 or IGF-2 (in 25% final volume of cell culture media) was added and incubated for a further 24 hours. Proliferation was measured using the WST1 reagent assay (Roche) following the manufacturer's protocol or cell counting. For proliferation, PMSCs were seeded at 5000 cells per 100 μL in 96-well plates. For cell counting, PMSCs were seeded at 40 000 cells/well of 24-well plate. After 24 hours, serum-free media containing various concentrations of IGFs were added; cells were counted using the Countess automated cell counter (Invitrogen). Cell cultures were placed in either room air with 5% CO2 incubator or a hypoxia chamber as described previously (6).
IGF-1R and IR knockdown by small interfering RNA (siRNA)
PMSCs were plated at 200 000 cells/well in 6-well plates for 48 hours in 10% FBS. Upon siRNA transfection, cells were washed with Opti-MEM media (number 31985; Gibco) and transfected according to siRNA transfection protocol. Ten microliters of 10 μM control siRNA-A (sc-37007), IGF-1Rα/β siRNA (sc-29358), or IR siRNA (sc-29370) (Santa Cruz Biotechnology) and 5 μL of Lipofectamine 2000 (number 11668; Invitrogen) were diluted in Opti-MEM media and combined to make 500 μL volume per well. After 5 hours, 2 mL of fresh DMEM/F12 was added to each well containing 100 ng/mL of IGF-1 or IGF-2 per triplicate treatment and incubated for 48 hours in room air or low-oxygen tension.
IGF-1 and IGF-2 ELISA
PMSCs were cultured for 72 hours in 3 mL of serum- and IGF-free DMEM/F12 in six-well plates in room air or low-oxygen tension. Using IGF-1 and IGF-2 ELISA kits (BEK1082, BEK1085; Insight Genomics), 10 μL of tissue culture supernatant from each treatment was assayed following the manufacturer's protocol.
Real-time PCR
Total RNA was extracted from PMSCs using the PureLink RNA minikit (Ambion) as per the manufacturer's protocol. Cells were lysed in lysis buffer with 1% β-mercaptoethanol, scraped, collected, and homogenized using a 21-gauge syringe needle (10 times). Lysates were then applied to the column for binding RNA, washed and eluted using ribonuclease-free water. One to 3 μg total RNA was reverse transcribed using a Superscript III first-strand synthesis system for RT-PCR (Invitrogen) and oligo(deoxythymidine)20 primers. Quantitative PCR was performed on 100 ng of cDNA using the Platinum SYBR-Green qPCR SuperMix-UDG with ROX (Invitrogen) following the manufacturer's protocol. PCR products were less than 200 bp and each primer concentration used was 0.4 μM. All reactions were run in 10 μL using the Applied Biosystems ViiA7 system. Human RPL13a mRNA was used as the reference endogenous control for normalization of the target mRNAs. Amplification conditions were run at 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 15 seconds. The primer sequence for each gene is shown in Supplemental Figure 1).
Immunoblotting
Ten to 20 μg each of cell lysate samples were resolved by SDS-PAGE and then transferred onto polyvinyl difluoride membranes (Millipore). The membranes were blocked with 5% BSA or in 5% nonfat-dry milk in 1× Tris-buffered saline (TBS) for 1 hour at room temperature. Blots were then washed in 1× TBS in 0.1% Tween 20 (3 times for 5 min) followed by incubation at 4°C overnight with primary antibodies as per the manufacturer's protocols. Blots then were washed using TBS in 0.1% Tween 20 (3 times for 10 min) and were incubated with the corresponding secondary horseradish peroxidase-labeled antibody for 1 hour at room temperature. Immunocomplexes were detected by enhanced chemiluminescence and documented using a VersaDOC imaging system (Bio-Rad Laboratories).
Antibodies
For confirmation of hypoxia, hypoxia inducible factor-1 (HIF-1)-α antibody (H206, sc-10790; Santa Cruz Biotechnology, Inc) was used. To investigate the IGF-1 vs IGF-2 activated signaling proteins in PMSCs, we used the following antibodies: phospho-p44/42 MAPK (number 4377), p44/42 MAPK (number 9102), phospho-AKT (Ser473, number 4051), AKT (number 9272), phosphorylated (p)-IGF-IRβ (number 3918; Cell Signaling Technologies), IGF-1Rα (N-20, sc-712), IGF-1Rβ (C-20, sc-713; Santa Cruz Biotechnologies), p-IR (number 04–299; Millipore). For receptor neutralization, we used IGF-1Rα (1H7, sc461-L; Santa Cruz Biotechnologies) and IRα (MA-20, NB400–142; Novus Canada) or (MAB1137Z; Millipore). For multipotency markers, we used OCT3/4 antibody (N-19, sc-8628; Santa Cruz Biotechnologies), SOX-2 (2683–1), and NANOG (3369–1; Epitomics). For loading control, we used pan-Actin Ab-5 (number MS-1295; Thermo Fisher Scientific). The secondary antibodies used for immunoblotting were goat antirabbit (number 170–6515), antimouse (number 170–6516) horseradish peroxidase-conjugated antibodies (Bio-Rad laboratories), or donkey antigoat antibody (sc-2020; Santa Cruz Biotechnologies).
Statistical analysis
All experiments were run in triplicates and the results were analyzed from three independent experiments each; whenever possible, three or more PMSC primary lines were used for confirmation. All graphs and analyses were generated using GraphPad Prism Software version 5.0 (GraphPad Software). A two-way ANOVA with Bonferroni post hoc test was used for the PMSC WST1 and cell counting proliferation assay and densitometry quantifications. Data are expressed as mean ± SEM; the values were considered significant when P < .05.
Results
PMSC expression of IGF-1 and IGF-2 is controlled by oxygen tension and IGF stimulation
PMSCs isolated from different gestations maintained under low-oxygen tension expressed lower levels of IGF-1 and higher IGF-2 (data not shown). We noted that under low-oxygen tension, IGF-2 was expressed higher in preterm PMSCs, which had higher levels of OCT4. Under low-oxygen tension, PMSCs stabilized and accumulated the HIF-1α, confirming the hypoxic condition (Supplemental Figure 2). In cell culture media, IGF-1 and IGF-2 peptides were present as measured by an IGF-ELISA. IGF-1 was decreased (30%) in low-oxygen tension, whereas IGF-2 was increased (70%) (Figure 1, A and B). At the mRNA level, increasing exogenous IGF-1 increased IGF-1 expression in room air at 100 ng/mL (suggesting a positive feedback) that was abolished by low-oxygen tension (Figure 1C). Similarly, exogenous IGF-2 stimulation (10 and 50 ng/mL) increased IGF-1 expression, which was also repressed by low-oxygen tension (Figure 1D). Therefore, low-oxygen tension prevented the up-regulation of IGF-1 expression; however, it increased endogenous IGF-2 expression (≥2-fold) (Figure 1, E and F). This was further increased by exogenous IGF-1 (50 and 100 ng/mL) stimulation (Figure 1E) and exogenous IGF-2 (100 ng/mL) only in low-oxygen tension (Figure 1F). Therefore, low-oxygen tension is a positive regulator of IGF-2 expression and secretion in PMSCs.
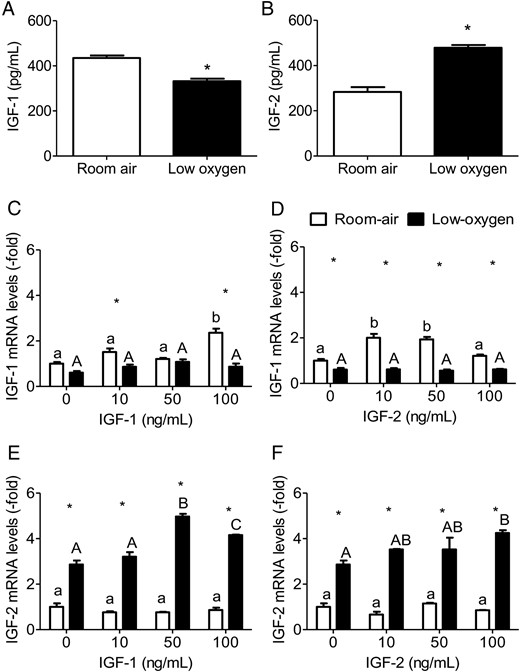
Expression of IGF-1 and IGF-2 is dependent on oxygen tension. By ELISA, IGF-1 (A) and IGF-2 (B) levels were measured in cell culture media from PMSCs cultured for 72 hours in serum-free media in either room air or low oxygen (one way ANOVA, P < .05, n = 6). *, Comparison between room air and low oxygen. By real-time PCR, expression level of IGF-1 (C and D) and IGF-2 (E and F) mRNA were measured relative to RPL13a in PMSCs treated for 48 hours in either room air or low-oxygen tension with varying (0, 10, 50, 100 ng/mL) IGF-1 or IGF-2 concentration. Values are presented as relative expression compared with 0 ng/mL IGF-1 or IGF-2 in room air, which was set to 1.0 (two way ANOVA, P < .05, n = 3). a, Comparison between IGF concentration in same oxygen tension; *, comparison between room air and low oxygen. Low-oxygen tension decreased IGF-1 but increased IGF-2 expression in PMSCs.
IGF-1R and IR expression is dependent on oxygen tension and IGF concentration
PMSCs expressed IGF-1R, IGF-2R, and IR, whereas the mitogenic and metabolic actions are mediated through either the IGF-1R or IR. IGF-1R and IR levels were up-regulated in the absence of IGFs and increased further by low-oxygen tension (Figure 2, A and B). In room air, increasing concentrations of IGF-1 decreased both receptors (IGF-1R and IR) in a dose-dependent manner (Figure 2, C and E). Low-oxygen tension maintained higher receptor levels in the absence of IGF-1, but increasing IGF-1 concentration decreased both IGF-1R and IR levels. IGF-2 had less effect on reducing the levels of both receptors in room air, whereas low-oxygen tension maintained higher levels (Figure 2, D and F). These findings suggest that IR is similar to IGF-1R in its response to increasing concentrations of IGFs, indicating similar mechanisms of regulating the expression of the two receptors. IR, which is expressed in two isoforms (IR-A and IR-B), was increased by low-oxygen tension (20). In PMSCs, the expression of IR-B, which was dominant in room air, switched to IR-A in low-oxygen tension (Figure 3A). In the presence of either IGF-1 or IGF-2, IR-B expression was opposite to IR-A with an increased IR-A to IR-B ratio that increased in a dose-dependent manner in low-oxygen tension (Figure 3, B and C). This observation suggests that IR-A may play a role in mediating IGF-signaling in PMSCs.
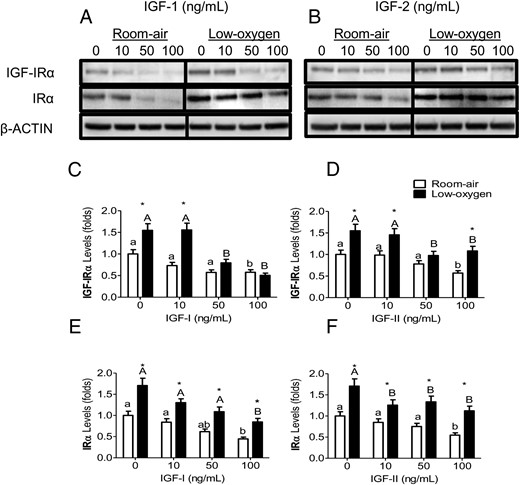
IGF-1R and IR levels in IGF-1 and IGF-2 and low-oxygen tension. PMSCs were cultured for 48 hours in either room air or low-oxygen tension with IGF-1 or IGF-2. By Immunoblotting, protein expression levels of IGF-1Rα or IRα were detected with increasing concentrations (0, 10, 50, 100 ng/mL) of IGF-1 (A) or IGF-2 (B). β-Actin is a protein loading control. C–F, Quantification of protein band intensity level (a two way ANOVA, n = 3, P < .05). a, Comparison between IGF concentration in the same oxygen tension; *, comparison between room air and low oxygen. Low-oxygen tension maintained higher levels of both receptors that was decreased with increasing concentrations of IGFs.
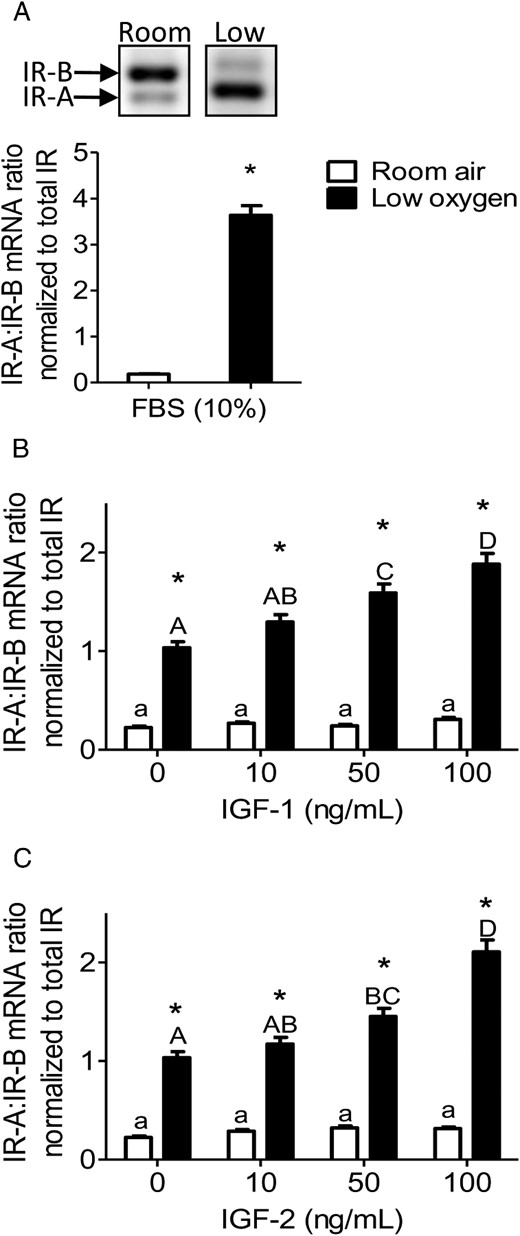
IR isoforms (A and B) are affected by low-oxygen tension and IGF concentration. PMSCs were cultured for 48 hours in either room air or low-oxygen tension with 10% FBS (A) or in the presence or absence of IGF-1 (B) or IGF-2 (C) (0, 10, 50, 100 ng/mL). By end-point PCR, mRNA levels of IR-A vs IR-B were measured and a ratio was calculated and normalized to total IR (two way ANOVA, n = 3, P < .05). a, Comparison between IGF concentration in same oxygen tension; *, comparison between room air and low oxygen. Low-oxygen tension increased the ratio of IR-A to IR-B, which is enhanced by increasing IGF-1 or IGF-2 concentration.
Activation of IGF-1R and IR signaling pathways in PMSCs is regulated by oxygen tension
Both IGF-1 and IGF-2 bind and activate the IGF-1R, but due to differences in binding affinity, IGF-1R phosphorylation and activation by IGF-1 or IGF-2 can vary, especially in short-term stimulation (15 min). In room air, IGF-1Rβ phosphorylation was more sensitive to IGF-1 than IGF-2 with a gradual increase (Figure 4, A and B). Low-oxygen tension reduced p-IGF-1Rβ to lower levels, hence reducing sensitivity to IGFs, although total receptor levels were unchanged. The levels of p-IRβ were also detected after IGF-1 and IGF-2 short-term stimulation (Figure 4, C and D). In room air, IGF-1 (100 ng/mL) increased p-IRβ levels, in comparison with IGF-2, which induced no effect (Figure 4, C and D). Also, in low-oxygen tension, p-IRβ levels were reduced below room air levels with IGF-1 and with no effect of IGF-2 (Figure 4, C and D). Therefore, IGF-1 is more potent than IGF-2 in the short-term activation of the IGF-1R and IR, which is lowered by low-oxygen tension in PMSCs.
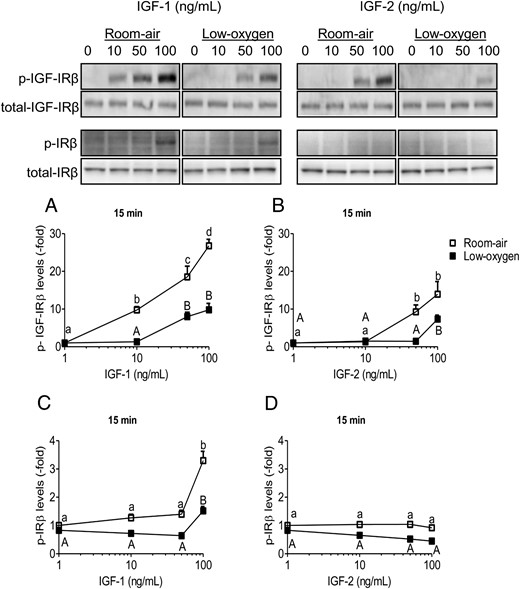
IGF-1Rβ and IR phosphorylation is dependent on IGF ligand, concentration, and oxygen tension. PMSCs were serum deprived for 24 hours in room air or low-oxygen tension and then stimulated for 15 minutes in the presence of increasing concentrations (0, 10, 50, 100 ng/mL) of IGF-1 or IGF-2. A and B, Levels of p-IGF-1Rβ with IGF-1 and IGF-2, respectively. C and D, Levels of IRβ with IGF-1 and IGF-2, respectively. Quantifications show levels of p-receptor normalized to total receptor and β-actin (two way ANOVA, n = 3, P < .05). a, Comparison between IGF concentration in the same oxygen tension. Low-oxygen tension deceased levels of IGF-1Rβ activation/phosphorylation, making it less sensitive to IGF-1 or IGF-2. Also, IRβ phosphorylation was less sensitive to IGF-2 than IGF-1.
The IGF short-term stimulation was determined by ERK1/2 and AKT phosphorylation. In room air, stimulation of ERK1/2 phosphorylation was similar between IGF-1 and IGF-2 (Figure 5, A and B). However, low-oxygen tension increased p-ERK1/2 levels in response to IGF-1 greater than that induced in room air (Figure 5A). The effect of IGF-2 on p-ERK1/2 was not seen in low-oxygen tension (Figure 5B). Insulin stimulation, similar to IGF-2, did not elevate p-ERK1/2 levels (Supplemental Figure 3, A and B). AKT phosphorylation was more sensitive to IGF-1 than IGF-2 and was similar to IGF-1Rβ phosphorylation response. In room air, AKT phosphorylation plateaued at 50 ng/mL of IGF-1, whereas IGF-2 induced the highest activation at 100 ng/mL (Figure 5, E and F). In contrast, low-oxygen tension lowered p-AKT in a similar pattern to p-IGF-1Rβ (Figure 5, E and F). Similar to IGF-2, insulin stimulation alone did not activate p-AKT levels in low-oxygen tension but was able to induce an increase in room air at supraphysiological levels (1 μg/mL) (Supplemental Figure 3, A and C), which triggers IGF-1R signaling (11). Overall, low oxygen tension reduced phosphorylation of AKT in the presence of IGF-1 or IGF-2, whereas ERK1/2 phosphorylation in response to IGF-1 was increased.
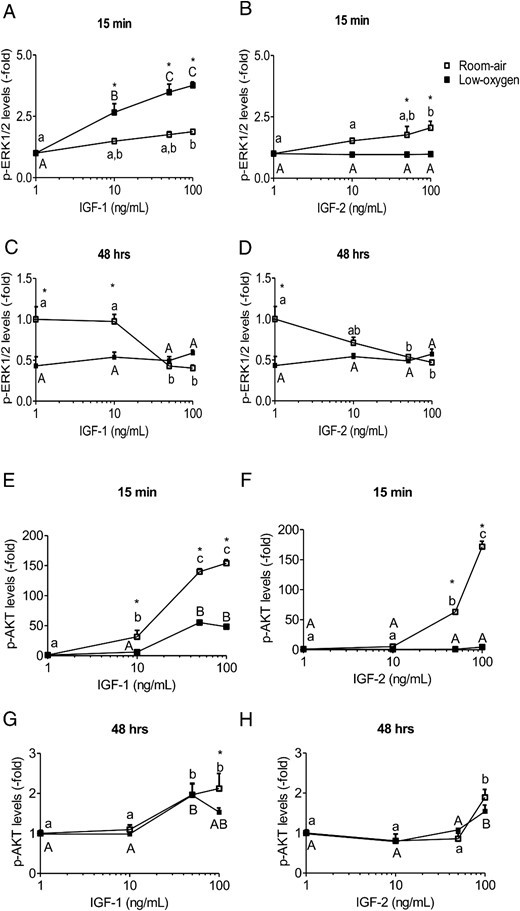
ERK1/2 and AKT phosphorylation is IGF concentration and oxygen tension dependent. PMSCs were stimulated for 15 minutes or 48 hours in room air or low-oxygen tension with increasing concentrations (0, 10, 50, 100 ng/mL) of IGF-1 or IGF-2. From immunoblotting, levels of p-ERK1/2 (A–D) and p-AKT (E–H) were normalized to total kinase and β-actin. Values are presented as relative expression compared with 0 ng/mL IGF-1 or IGF-2 in room air, which was set to 1.0 (two way ANOVA, n = 3, P < .05). a, Comparison between IGF concentration in same oxygen tension; *, comparison between room air and low oxygen. In the short term, low-oxygen tension potentiates p-ERK1/2 signal and represses p-AKT. In the long term, low-oxygen tension, maintains a tight lowered level of p-ERK1/2 and elevated p-AKT levels.
In long-term activation (48 h), p-ERK1/2 and p-AKT can indicate a stem cell state of proliferation or differentiation. Continuous stimulation of PMSCs by IGF-1 and IGF-2, in room air, caused a dose-dependent decrease in p-ERK1/2 levels that reached similar levels maintained by lo- oxygen tension (Figure 5, C and D). Interestingly, levels of p-ERK1/2 in low-oxygen tension were almost identical in response of IGF-1 and IGF-2 stimulation. Phospho-AKT had an opposite activation pattern compared with p-EKR1/2 that maintained a dose-dependent increase with IGF-1 or IGF-2 in both oxygen tensions (IGF-1 being more potent than IGF-2) (Figure 5, G and H). Therefore, low-oxygen maintained a narrow range of p-ERK1/2 levels but did not interfere with AKT phosphorylation dependence on IGF-1 or IGF-2 stimulation.
PMSC multipotency is regulated by low-oxygen tension and IGFs
Stem cell fate is determined by its microenvironment, and the expression of the pluripotency genes, OCT4, NANOG, and SOX2, determines the character of stemness. OCT4, NANOG, and SOX2 constitute a triad of transcription factors required in maintaining pluripotency in stem cells, and generating induced pluripotent cells (28). In PMSCs, OCT4 protein levels did not change with increasing concentrations of IGF-1 or IGF-2 in either room air or low-oxygen tension (Supplemental Figure 4, A and B), whereas, NANOG and SOX2 levels were increased by low-oxygen tension but decreased by IGF-1 or IGF-2 (Supplemental Figure 4, C–F). Therefore, unlike NANOG and SOX2, no change in OCT4 protein level suggests that OCT4 may not be the main regulator of PMSC multipotency and differentiation under these condition. At the transcription level, a continuous exposure to low-oxygen tension increased the expression level of all three OCT4, NANOG, and SOX2 expression, which was elevated further by IGFs (IGF-2 > IGF-1). However, increasing the concentrations of IGFs had different effects between IGF-1 and IGF-2 (Figure 6). IGF-1 decreased the expression of the three pluripotency genes in room air in a dose-dependent manner; however, they remained elevated by low-oxygen tension (Figure 6A). In contrast, IGF-2 did not alter the expression of pluripotency genes in room air, but they were increased in low-oxygen tension (Figure 6B). This shows that IGF-1 can reduce pluripotency gene expression in PMSCs even in low-oxygen tension, unlike IGF-2.
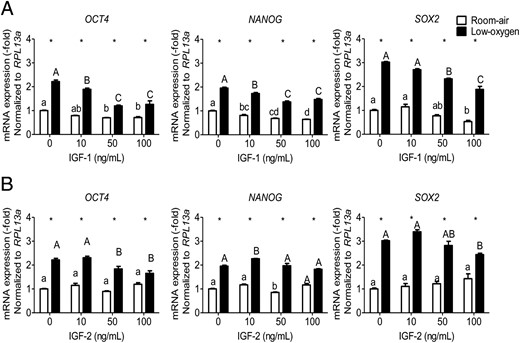
OCT4, NANOG, and SOX2 expression is affected by IGF-1 or IGF-2 concentration and oxygen tension. PMSCs were cultured for 48 hours in either room air or low-oxygen tension in the presence or absence of IGF-1 (A) or IGF-2 (B) (0, 10, 50, 100 ng/mL). By real-time PCR, the expression levels of OCT4 or NANOG or SOX2 mRNA were measured relative to RPL13a as an endogenous control in the presence of IGF-1 or IGF-2. Values are presented as relative expression compared with 0 ng/mL IGF-1 or IGF-2 in room air, which was set to 1.0 (two way ANOVA, n = 3, P < .05). a, Comparison between IGF concentration in same oxygen tension; *, comparison between room air and low oxygen. Low-oxygen tension increased multipotency gene expression in which IGF treatment reduces them (more with IGF-1 than IGF-2).
PMSC proliferation is enhanced by IGFs in low-oxygen tension
Self-renewal is an important characteristic of stem cells. We assessed PMSC proliferation, as an indicator of self-renewal, in low-oxygen tension and by IGFs using the WST-1 reagent assay (Figure 7), and cell counting (Supplemental Figure 5). In room air, IGF-1 increased proliferation in a dose-dependent manner that plateaued at (50 ng/mL) but was increased further at (100 ng/mL) in low-oxygen tension (Figure 7A and Supplemental Figure 5A). Interestingly, proliferation decreased when PMSCs were exposed to IGF-1 concentrations greater than 100 ng/mL in room air but not in low-oxygen tension (data not shown). In comparison, IGF-2 had less effect on PMSC proliferation in room air but was enhanced by low-oxygen tension (Figure 7B and Supplemental Figure 5B). Therefore, IGF-1 was more potent in stimulating PMSC proliferation in room air, whereas, low-oxygen tension potentiated both IGF-1 and IGF-2 effects. The effects on proliferation mirror ERK1/2 phosphorylation.
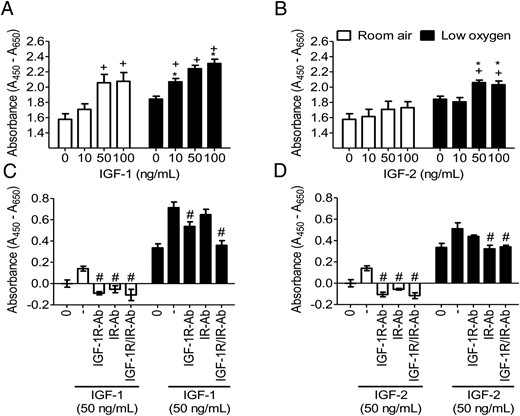
IGF-mediated proliferation is regulated by oxygen tension and specific-receptor stimulation. By WST-1, proliferation was measured with increasing concentrations (0, 10, 50, 100 ng/mL) of IGF-1 (A) or IGF-2 (B) for 48 hours or with 5 μg/mL neutralizing antibodies IGF-1R-Ab or IR-Ab with (50 ng/mL) IGF-1 (C) or IGF-2 D) for 24 hours in room air or low-oxygen tension (two-way ANOVA, n = 8, P < .05). +, Comparison between IGF concentration in same oxygen tension; *, comparison between room air and low oxygen; #, effect of antibody neutralization as compared with the absence of antibody. Low-oxygen tension enhanced PMSC proliferation and potentiated the IGF effect in increasing PMSC proliferation. IGF-1R with IGF-1 and IR with IGF-2 are responsible for IGF-mediated signaling, especially under low-oxygen tension conditions. Cell counting results are shown in Supplemental Figure 5.
Low-oxygen tension mediates a differential IGF signaling through the IGF-1R and IR to stimulate proliferation
Low-oxygen tension up-regulated both IGF-1R and IR levels (Figure 2) in PMSCs. To determine the respective roles of different receptors in mediating IGF-1 or IGF-2 actions, neutralization antibody against IGF-1Rα (IGF-1R-Ab) was used in culture. In room air, neutralizing the IGF-1R abolished the effects of IGF-1 or IGF-2, whereas in low-oxygen tension, the IGF-mediated effects were unchanged (Figure 7, C and D, and Supplemental Figure 5, C and D). Excess of IGF-1R-Ab of 10-fold did not inhibit further proliferation in low-oxygen tension (data not shown). This finding suggested that the effect of IGF-1 or IGF-2 on PMSC proliferation can be mediated by an additional receptor, possibly IR. In room air, IGF-1- or IGF-2-mediated proliferation was reduced by the IGF-1R neutralizing antibody and less by IR neutralizing antibody (Figure 7, C and D, and Supplemental Figure 5, C and D). In low-oxygen tension, the IGF-1-enhanced PMSC proliferation was lowered further by neutralizing the IGF-1R than the IR (Figure 7C and Supplemental Figure 5C). A combination of both receptor neutralizing antibodies completely abolished the IGF-1 effects. With IGF-2, neutralizing the IR had a greater effect than neutralizing IGF-1R (Figure 7D and Supplemental Figure 5D). This was confirmed by inhibiting both receptors. This PMSC IR-mediated proliferation was dependent on IGF stimulation because insulin alone did not increase proliferation and also decreased the pluripotency-associated protein levels of OCT4 and SOX2 in low-oxygen tension conditions (data not shown). Therefore, IGFs used both the IGF-1R and IR to promote PMSC proliferation, especially in low-oxygen tension.
As mediators of IGF-1R/IR signaling, we determined the phosphorylation of ERK1/2 and AKT during receptor neutralization. The presence of either IGF-1R or IR neutralizing antibodies negated the effect of IGF-1 by decreasing p-ERK1/2 levels (Supplemental Figure 6A), without affecting IGF-2 action (Supplemental Figure 6B). AKT was more responsive to IGF stimulation and was inhibited by IGF-1R and IR neutralizing antibodies (Supplemental Figure 6, C and D). IGF-1R neutralization in room air decreased only the IGF-1 induced p-AKT, whereas the IGF-2 induced p-AKT was decreased in both oxygen tensions. IR neutralization had a more significant effect in low-oxygen tension in the presence of both IGF-1 and IGF-2 (Supplemental Figure 6, C and D). Thus, AKT phosphorylation is more dependent on IGF signaling than ERK1/2 phosphorylation in PMSCs, and low-oxygen tension can differentially mediate the IGF-1 and the IGF-2 signaling via the IGF-1R or the IR.
Inhibition of IGF-1R or IR also affects PMSC multipotency
We showed that low-oxygen tension increased the expression level of OCT4, NANOG, and SOX2 genes and remained elevated in presence of IGFs compared with room air (Figure 6). In the presence of IGF-1R-Ab, IGF-2 increased the expression of all three genes in room air (Supplemental Figure 7, B, D, and F). In the presence of IR-Ab, IGF-1 decreased all three pluripotency genes in room air, whereas low-oxygen tension maintained higher levels (Supplemental Figure 7, A, C, and E). This demonstrates that a balance between IGF-1R and IR is required to maintain the expression of the pluripotency-associated genes.
Knockdown of IGF-1R and IR effect on PMSC multipotency
To further analyze the role of IGF-1 and IGF-2 signaling in mediating PMSC multipotency, IGF-1R and IR expression were decreased by transcript-specific siRNAs. This knockdown can generate endogenous IGF-1R or IR signaling and eliminate the role of hybrid receptors. The siRNA knockdown of IGF-1R and IR significantly reduced the level of each receptor (78% and 60%, respectively) (Supplemental Figure 8), without affecting PMSC morphology and survival after 48 hours. OCT4, the master regulator switch of pluripotency, had a stable protein level with IGF-1 or IGF-2 treatments after 48 hours, regardless of oxygen tension (Supplemental Figure 4, A and B). Upon knockdown of IGF-1R, OCT4 increased in room air and low-oxygen tension with IGF-2 and did not change with IGF-1 (Supplemental Figure 9A), similar to antibody inhibition. SOX2 and NANOG show similar protein levels (data not shown). In contrast, IR knockdown decreased OCT4 levels with IGF-1 in room air and with IGF-2 in low-oxygen tension (Supplemental Figure 9A). This observation was also confirmed with the dual knockdown of IGF-1R/IR. Therefore, the balance between IGF-1R and IR is required to maintain PMSC multipotency.
IGF-1R and IR can induce basal autophosphorylation levels and mediate signal transduction in absence of ligand binding (29). For this purpose, p-ERK1/2 and p-AKT levels were measured after IGF-1R and IR knockdown. The knockdown of IGF-1R increased p-ERK1/2 levels in room air with IGF-1 and decreased in both oxygen conditions with IGF-2 (Supplemental Figure 9B), whereas p-AKT levels were decreased regardless of IGF or oxygen tension (Supplemental Figure 9C). This observation shows a dependence of p-AKT on the IGF-1R signaling similar to the antibody neutralization effect. On the other hand, when IR was knocked down, p-ERK1/2 and p-AKT levels were not decreased in low-oxygen tension with IGF-1 and IGF-2 in both oxygen tension (Supplemental Figure 9, B and C), suggesting that both IGF-1 and IGF-2 still use the IGF-1R to mediate signaling via ERK1/2 and AKT. Dual siRNA for both receptors decreased p-AKT levels similar to IGF-1R-only knockdown (Supplemental Figure 9, B and C). As noticed, p-AKT did not decrease with IR knockdown, which is required for OCT4 maintenance; therefore, the levels of IGF-1R were measured. As expected, knockdown of IR did not affect IGF-1R levels, which were increased in low-oxygen tension (Supplemental Figure 9D). Hence, the loss of OCT4 in IR knockdown can be independent of p-AKT levels, which were possibly mediated via the IGF-1R signaling. Therefore, the specific IR signaling pathways required for multipotency maintenance are still to be elucidated.
Discussion
In this study, we demonstrated that oxygen tension of the culture environment can modulate PMSC response to IGF-mediated signaling, proliferation, and multipotency, as would be expected in vivo. We showed that in contrast to room air, low-oxygen tension increased IGF-2 expression and decreased IGF-1, whereas it enhanced IGF-1R and IR expression. In addition, low-oxygen tension decreased the IGF-1-mediated phosphorylation of IGF-1Rβ, IRβ and AKT in the short term, whereas it potentiated ERK1/2 phosphorylation. After a prolonged stimulation with IGFs, low-oxygen tension maintained a lower level of p-ERK1/2 and caused a dose-dependent increase in p-AKT levels. Also, pluripotency-associated genes were reduced by IGF-1 or IGF-2 in room air, whereas IGF-2, more than IGF-1, increased their expression levels in low-oxygen tension. Low-oxygen tension stimulated PMSC proliferation and potentiated the effects of IGF-1 and IGF-2 via the cooperative actions between IGF-1R and IR.
It is known that the microenvironmental factors such as nutrition, oxygen tension, tissue pH, and local paracrine factors may play a significant role in growth, proliferation, and differentiation of all cells including, stem cells. During development, IGFs exist in tissues as autocrine/paracrine factors or endocrine factors for cellular growth and repair (30). In ESC cultures, IGF-2 is expressed by surrounding differentiated fibroblasts to support ESC pluripotency via the IGF-1R (9). In particular, PMSCs can express IGF-1 as a paracrine factor, which can provide an IGF-1R mediated protective and survival mechanism to surrounding cells (31). In this study, we demonstrated that PMSCs express and secrete both IGFs, and low oxygen tension increased IGF-2 and reduced IGF-1.
In the human placental chorionic mesodermal core, in which PMSCs reside in the perivascular space, IGF-2 is expressed abundantly as early as 6 weeks of gestation, whereas IGF-1 is expressed in low abundance later, after 12 weeks of gestation (32). The early expression of IGF-2 correlates with the lower oxygen tension in the placenta (17.9 mm Hg) preceding the IGF-1 expression, which rises as oxygen tension increases (60 mm Hg) (5, 33). This suggests that IGF-2 plays a role in early placental development (34, 35). Unlike IGF-1, IGF-2 promoter contains a hypoxia-responsive element that is bound directly by the HIF-1 to increase transcription in low-oxygen tension (36, 37). Hence, IGF-2 is normally increased in low-oxygen tension in many primary cell cultures (38, 39), and this increased expression of IGF-2 by low-oxygen tension in PMSCs is potentially required for stem cell identity. Thus, maintaining PMSCs in vitro in a microenvironment that mimics their natural conditions in vivo can program PMSCs to maintain their stemness.
Activation of ERK1/2 and AKT signaling pathways is important in the maintenance of stem cell proliferation and self-renewal and plays an important role in maintaining OCT4 levels in PMSCs (6). Low-oxygen tension decreased phosphorylation of ERK1/2 and AKT, although it increased the expression of IGF-1R and IR; the mechanism underlying these changes is unknown, whether it is due to a decrease in IGF binding sensitivity to IGF-1R or alteration in downstream phosphorylation cascade. However, low-oxygen tension transiently increased IGF-1-induced ERK1/2 phosphorylation, which was shown to be important for proliferation (40, 41). A prolonged stimulation (48-hrs) with IGF-1 and IGF-2 induced opposite effect on ERK1/2 and AKT phosphorylation, in which the latter increased in a dose-dependent manner. Phospho-ERK1/2 levels decreased at higher IGF concentrations in both room air and low-oxygen tension. IGF-1R activation induces a transient insulin receptor substrate-1 (IRS-1) phosphorylation via targeted degradation after a prolonged IGF-1 stimulation, which causes a decrease in p-ERK1/2 levels (42). In PMSCs, IRS-1 levels are decreased after 1 hour of IGF-1 stimulation in room air, whereas it is maintained lower in low-oxygen tension (6). Therefore, low-oxygen tension can decrease the prolonged p-ERK1/2 levels required for differentiation. In PMSCs, ERK1/2 was less dependent on IGF stimulation than AKT as shown by receptor activation. This was confirmed by the receptor neutralization and knockdown that IGF-1R signaling is more critical for AKT than ERK1/2 phosphorylation, suggesting that PMSC proliferation and multipotency via AKT phosphorylation is IGF dependent.
PMSC proliferation was dependent on both the IGF-1R and IR, and it was regulated by oxygen tension. Both IGF-1R and IR participated in mediating IGF actions in low-oxygen tension. Traditionally, IGF-1R induces more mitogenic and differentiation signals than the IR (43), although both receptors share a structural homology (84%) (44). However, IGF-1R and IR have a greater diversity (44%) in the C terminus of the β-subunit (44). Two autophosphorylated tyrosine residues at the C-terminus have an inhibitory effect on insulin-induced mitogenesis without affecting metabolic signaling (45, 46). In PMSCs, low-oxygen tension increased the C-terminal IRβY1334 phosphorylation (data not shown). This residue is a direct alternative binding site, in addition to IRS-1, for the SH2 domain of p85 subunit of phosphatidylinositol 3-kinase (PI3K), in addition to SYP (a tyrosine-phosphatase) (47). Therefore, some signaling pathways between the two receptors (eg, MAPK-ERK1/2 and PI3K-AKT) are common; however, several others are up- or down-regulated by either IGF-1R or IR signaling (43). This can be attributed to the diversity of IGF-1R and IR sequences, which may participate in stem cell proliferation and pluripotency.
IR signaling can mediate pluripotent stem cell self-renewal and proliferation via PI3K signaling, inactivating glycogen synthase kinase-3 and the tumor suppressor P53, thereby inhibiting cell death and differentiation (48). In addition, IGF signaling can maintain pluripotency by phosphorylating p53, which acts as a negative regulator at OCT4 and NANOG promoters, and prevents senescence and pluripotency gene repression (49). Inhibition studies demonstrate that AKT activation is more important to maintain stem cell potency as measured by OCT4, NANOG, and SOX2 levels (50). Also, the induction of the PI3K/AKT signaling pathway, more than MAPK/ERK1/2, was shown to be central for pluripotency maintenance of human ESCs (49). We also reported that PMSC multipotency is dependent on the PI3K/AKT pathway to maintain OCT4 levels (6). However, the difference between IGF-1R vs IR-mediated signaling via PI3K/AKT in maintaining stem cell multipotency is unknown. Using the receptor knockdown studies in PMSCs, we observed that AKT phosphorylation was dependent on the IGF-1R and not the IR, although loss of OCT4 occurred in the absence of IR. This could be due to differences between the inhibition of PI3K with LY294002 and abrogation of AKT and other regulated proteins that affect PMSC multipotency and the IR knockdown effect, which can regulate other multipotency maintaining targets independent of PI3K/AKT signaling. Therefore, the loss of IR may have specific signaling pathways, yet to be discovered, to maintain PMSC multipotency.
Developmentally, the IR gene has acquired an additional exon, exon 11, which encodes for 12 amino acids present in the α-subunit of IR-B and absent from IR-A (19). In neural progenitor stem cells, proliferation is dependent on IGF-2, which is mediated by IGF-1R, whereas self-renewal is more dependent on IR-A (51). Because PMSCs express both higher IGF-2 and IR-A in low-oxygen tension, it is possible that IGF-2 signaling via IR-A is important in PMSC multipotency and proliferation. In mice, IGF-2 plays a major role in placental development because Igf2(−/−), but not the Igf1(−/−) or Igf1r(−/−), leads to smaller placentae. This reduced placental growth is not mediated via the Igf-1r because the double knockout of Igf2 and Igf1r results in a placental phenotype similar to Igf2(−/−) alone, suggesting that IR can be an additional receptor that mediates IGF-2 actions in the placenta (11, 52). Also, IR-A is the predominant IR isoform in fetal tissues that mainly mediates IGF-2 signaling, whereas IR-B is expressed in insulin-target postnatal tissues (18). Our data demonstrating the up-regulation of IR and the switch to IR-A by low-oxygen tension suggest that IGF-2 signaling occurs in PMSCs via IR-A. Therefore, IGF-2 may use IR-A in addition to IGF-1R to maintain PMSC multipotency. The dual expression of IGF-1R and IR in PMSCs can favor the formation of hybrid receptors IGF-1R/IR that preferentially binds to IGFs than insulin. In the human placenta, a high percentage of IGF-1R (55%) is assembled as hybrid receptors to specifically bind IGF-1 than insulin (53). IR-A and IGF-1R/IR-A are up-regulated in various cancers to mediate more aggressive malignant cancer forms (19). Similarly, IGF-1R/IR-A hybrid receptors may be used by PMSCs to maintain their multipotency. In fact, the knockdown or neutralization of IGF-1R or IR caused the decrease in pluripotency-associated gene expression, which can be mediated by either the IGF-1R, IR or the hybrid receptors. The functional role of hybrid receptors in PMSCs and placental physiology need further investigation.
In this study, we showed that culturing PMSCs under low-oxygen tension modifies IGF signaling through the IGF-1R or IR via ERK1/2 and AKT and that those two kinases participate in the regulation of stem cell fate. We also showed that under low-oxygen tension conditions, PMSCs can maintain multipotency by using either IGF-1R and/or IR to mediate IGF actions. Therefore, culturing stem cells under low-oxygen tension and in defined conditions with IGF-1 and/or IGF-2 or insulin will maintain pluripotency prior to preparation for regenerative therapies.
Acknowledgments
This work was supported by Canadian Institutes for Health Research Grant 111024.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- HIF-1
hypoxia-inducible factor-1
- IGF-1R
IGF-1 receptor
- IR
insulin receptor
- IRS-1
insulin receptor substrate-1
- Oct
octamer transcription factor
- p
phosphorylated
- PI3K
phosphatidylinositol 3-kinase
- PMSC
placental mesenchymal stem cell
- siRNA
small interfering RNA
- Sox2
Sry-type high-mobility-group box transcription factor-2
- TBS
Tris-buffered saline.



