-
PDF
- Split View
-
Views
-
Cite
Cite
Christiane Otto, Anna Särnefält, Anne Ljungars, Siegmund Wolf, Beate Rohde-Schulz, Iris Fuchs, Jenny Schkoldow, Mikael Mattsson, Richardus Vonk, Axel Harrenga, Christoph Freiberg, A Neutralizing Prolactin Receptor Antibody Whose In Vivo Application Mimics the Phenotype of Female Prolactin Receptor-Deficient Mice, Endocrinology, Volume 156, Issue 11, 1 November 2015, Pages 4365–4373, https://doi.org/10.1210/en.2015-1277
Close - Share Icon Share
The prolactin receptor (PRLR) has been implicated in a variety of physiological processes (lactation, reproduction) and diseases (breast cancer, autoimmune diseases). Prolactin synthesis in the pituitary and extrapituitary sites is regulated by different promoters. Dopamine receptor agonists such as bromocriptine can only interfere with pituitary prolactin synthesis and thus do not induce a complete blockade of PRLR signaling. Here we describe the identification of a human monoclonal antibody 005-C04 that blocks PRLR-mediated signaling at nanomolar concentrations in vitro. In contrast to a negative control antibody, the neutralizing PRLR antibody 005-C04 inhibits signal transducer and activator of transcription 5 phosphorylation in T47D cells and proliferation of BaF3 cells stably expressing murine or human PRLRs in a dose-dependent manner. In vivo application of this new function-blocking PRLR antibody reflects the phenotype of PRLR-deficient mice. After antibody administration female mice become infertile in a reversible manner. In lactating dams, the antibody induces mammary gland involution and negatively interferes with lactation capacity as evidenced by reduced milk protein expression in mammary glands and impaired litter weight gain. Antibody-mediated blockade of the PRLR in vivo stimulates hair regrowth in female mice. Compared with peptide-derived PRLR antagonists, the PRLR antibody 005-C04 exhibits several advantages such as higher potency, noncompetitive inhibition of PRLR signaling, and a longer half-life, which allows its use as a tool compound also in long-term in vivo studies. Therefore, we suggest that this antibody will help to further our understanding of the role of auto- and paracrine PRLR signaling in health and disease.
Prolactin (PRL) is a member of the GH, placental lactogen family of polypeptide hormones and is synthesized in lactotroph cells of the pituitary and in several extrapituitary tissues such as lymphocytes, mammary gland, and prostate epithelial cells. In humans, two distinctly regulated promoters control pituitary and extrapituitary PRL synthesis (1). The PRL receptor (PRLR) belongs to the class 1 cytokine receptor superfamily. Alternative splicing of the PRLR mRNA results in six different isoforms of the PRLR protein in humans: the long isoform, the intermediate isoform, the ΔS1 isoform (lacking the entire D1 domain of the receptor), the S1a and S1b isoforms (that seem to be inactive from a signaling perspective), and a soluble PRLR isoform consisting of the extracellular domain of the PRLR (see reference 2 for review). PRLRs are expressed predimerized at the cell membrane. PRL binding is required to induce signal transduction by these preformed receptor dimers leading predominantly to activation of the Janus kinase/signal transducer and activator of transcription 5 (STAT5) pathway (see reference 2 for review). After PRL binding, receptor-associated Janus kinases transphosphorylate each other and the PRLR. The phosphorylated PRLR binds to SH2-domain containing proteins such as STATs. Receptor bound STATs are subsequently phosphorylated, dissociate from the receptor, and translocate into the nucleus in which they stimulate transcription of PRLR target genes (see reference 2 for review).
As evidenced by the analysis of PRLR-deficient mice, PRLR-mediated signaling plays a role in a variety of processes such as lactation, reproduction, mammary gland tumor growth, hair regrowth, and autoimmune diseases (3–5). Locally produced PRL seems to be involved in several diseases such as lupus or breast and prostate cancer (6). Currently it is possible to interfere with pituitary-produced PRL only by use of dopamine 2 receptor agonists such as bromocriptine and cabergoline (6). These drugs, however, do not inhibit extrapituitary PRL synthesis in cancer cells and therefore failed in the treatment of patients suffering from breast cancer, although PRL has been implicated in this disease (7). To completely block PRLR-mediated signaling, PRLR antagonists are required. The first identified pure PRLR antagonist devoid of any agonistic activity was Δ1–9G129R-hPRL, a PRL variant lacking the first nine amino acids of PRL and containing an amino acid exchange in position 129 (8). This competitive PRLR inhibitor exhibited 10-fold lower affinity for the PRLR than PRL and served as valuable tool in in vitro assays and in short-term in vivo experiments (8). However, PRL and Δ1–9G129R-hPRL had relatively short half-lives (15–20 min) (8). In long-term in vivo studies, Δ1–9G129R-hPRL had to be administered by osmotic minipumps, but this strategy yielded insufficient plasma levels of the antagonist, which did not allow for a complete blockade of PRLR signaling (8).
Here we describe the identification of a novel function-blocking monoclonal human PRLR antibody 005-C04, which inhibits PRLR-mediated signaling in cell lines expressing the human or mouse PRLR in vitro and in mice in vivo. This antibody is suitable for long-term in vivo studies, and its application mimics prominent hallmarks of the phenotype of female PRLR-deficient mice.
Materials and Methods
Please refer to the Supplemental Materials and Methods section.
Results
Screening of a human antibody phage display library yielded several single-chain fragment variable and fragment antigen-binding (Fab) antibody fragments that were able to bind to cellularly expressed human PRLR and to inhibit PRL signaling. The most potent Fab being cross-reactive on human and mouse PRLR was transformed into full-length human IgG1 and mouse IgG2a format for subsequent analysis. The amino acid sequences of the variable light and heavy chains of this PRLR antibody named 005-C04 are depicted in Supplemental Figure 1. Binding affinities of antibody 005-C04 to the purified extracellular domains (ECDs) of mouse and human PRLR and to mouse and human PRLRs expressed by stably transfected HEK293 cells are shown in Supplemental Table 1. The PRLR antibody 005-C04 exhibited high affinity within the single- to double-digit nanomolar range for the human and mouse PRLR (dissociation constant [KD] values of 12.2 and 3.2 nM, respectively) (Supplemental Table 1). Whereas the antibody did not bind the S1 subdomain, it showed strong interaction with the S2 subdomain (KD value of 0.4 nM on the recombinantly expressed S2 subdomain). Surface plasmon resonance analyses revealed that human PRL interacted with the ECD of human PRLR when the ECD is captured by the PRLR antibody 005-C04 (Supplemental Table 2). For comparison, we also included in this analysis the PRLR antibody 006-H08, which binds to the human PRLR in a competitive manner with regard to PRL (Supplemental Table 2). When the ECD of the human PRLR is captured by 06-H08, PRL is not able to bind to the ECD of the human PRLR, indicating that antibody 006-H08 and prolactin bind to the same or overlapping epitope of the human PRLR ECD (Supplemental Table 2). Vice versa, when the ECD of the human PRLR is captured via PRL, antibody 005-C04 but not antibody 006-H08 can still bind to the ECD of the human PRLR (Supplemental Table 2). These results indicate that antibody 005-C04 binds to the human PRLR in a noncompetitive manner with regard to prolactin.
Antibody 005-C04 showed approximately the same potency with regard to binding (using cells expressing the human or mouse PRLR, EC50 values) as well as to inhibition of cell proliferation (IC50 values, Supplemental Table 1). The obtained IC50 values for inhibition of cell proliferation were in the range of the KD values for receptor binding (Supplemental Table 1). In contrast to antibody 005-C04 (closed symbols in Supplemental Figure 2), a negative control antibody (open symbols in Supplemental Figure 2) had no effect on proliferation of BaF3 cells stably expressing the human PRLR (Supplemental Figure 2). When proliferation assays were performed using increasing concentrations of PRL up to 500 ng/mL (resembling levels seen in lactating mice), the IC50 value for antibody 005-C04 (binding to the human PRLR in a noncompetitive manner with regard to PRL) remained constant, whereas the IC50 value of antibody 006-H08 (binding to the human PRLR in a competitive manner with regard to PRL) increased with increasing PRL concentrations (Supplemental Table 3). The first generation PRLR antagonist G129R-hPRL inhibited cell proliferation of BaF3 cells expressing the human PRLR with an IC50 value of approximately 260 nM (Figure 1A), whereas antibody 005-C04 showed an IC50 value of 3.8 nM (Figure 1B), demonstrating that the PRLR antibody was almost 70-fold more potent in this readout paradigm.
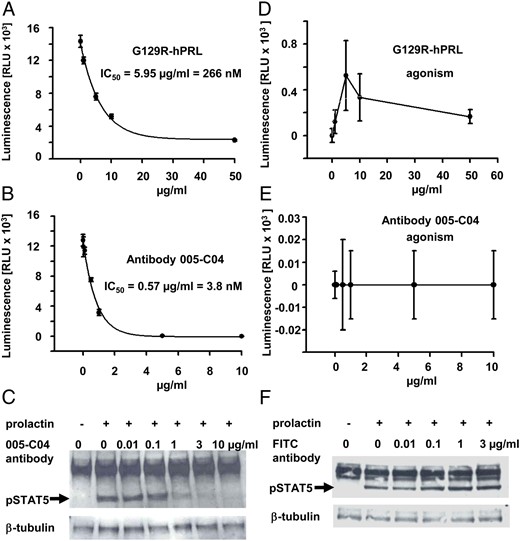
Inhibition of cell proliferation and STAT5 phosphorylation by PRLR antibody 005-C04. BaF3 cells stably expressing the human PRLR were subjected to proliferation assays in response to 20 ng/mL PRL in the presence of increasing concentrations of the PRLR antagonist G129R-hPRL (A) or antibody 005-C04 (B). In addition, agonistic activity of G129R-hPRL (D) or antibody 005-C04 (E) was examined in the absence of PRL. Antibody 005-C04 inhibited cellular proliferation with approximately 70-fold higher potency if compared with the PRLR antagonist G129R-hPRL (A and B). Whereas antibody 005-C04 was devoid of any significant agonistic activity (E), the PRLR antagonist G129R-hPRL showed some agonistic activity on cell proliferation in cells stably transfected with the human PRLR (D). Serum-starved T47D cells were incubated for 30 minutes with vehicle (−) or 20 ng/mL human PRL (+) in the presence of different concentrations of the neutralizing PRLR antibody 005-C04 (C) or in the presence of different concentrations of the negative control antibody (F). Cellular lysates were analyzed by Western blot for phospho-STAT5 and β-tubulin expression (loading control). The neutralizing PRLR antibody 005-C04 inhibited STAT5 phosphorylation in a dose-dependent manner (arrow). FITC, fluorescein isothiocyanate.
It was described previously that cellular proliferation assays had the highest sensitivity to detect agonistic properties of peptide-derived PRLR antagonists (9). Therefore, we analyzed the PRLR antibody 005-C04 in comparison with G129R-hPRL also in proliferation assays without the addition of PRL. When we used BaF3 cells stably transfected with the human PRLR, we could not detect any agonistic activity of antibody 005-C04 (Figure 1E), whereas there was some agonistic activity seen with G129R-hPRL (Figure 1D). Because the extent of agonistic activity may depend on the cellular context, we also used rat Nb2 cells endogenously expressing the PRLR (Supplemental Figure 3). Antibody 005-C04 inhibited the proliferation of Nb2 cells with an IC50 value of 4.6 nM (Supplemental Figure 3A), whereas G129R-hPRL showed an IC50 value of 152 nM (Supplemental Figure 3C). Maximal agonistic activity of G129R-hPRL reached almost 25% (Supplemental Figure 3D) of maximal cell proliferation seen in the absence of G129R-hPRL (Supplemental Figure 3C). In contrast, antibody 005-C04 was devoid of any significant agonistic activity with regard to the stimulation of Nb2 cell proliferation (Supplemental Figure 3B). As a second readout for functional blockade of PRLR-mediated signaling, we examined STAT5 phosphorylation in T47D cells. Compared with vehicle treatment, PRL induced a clear increase in STAT5 phosphorylation in serum-starved T47D cells (Figure 1C, F). Increasing doses of the neutralizing PRLR antibody 005-C04 inhibited STAT5 phosphorylation (Figure 1C), whereas the negative control antibody was without any effect (Figure 1F). Almost complete blockade of STAT5 phosphorylation was achieved with 1 μg/mL antibody 005-C04 (Figure 1C), resembling a concentration of 6.6 nM, which was in line with the IC50 values obtained for inhibition of BaF3 cell proliferation stably expressing the human PRLR (Supplemental Table 1).
Female sterility was one characteristic phenotype of PRLR-deficient mice (10). To assess whether the neutralizing PRLR antibody behaved also in vivo as a PRLR antagonist, we performed controlled mating studies in mice. Administration of PBS, a negative control antibody, or PRLR-specific antibody 005-C04 at a 10 mg/kg dose had no significant effect on pregnancy rates (Supplemental Figure 4A) and litter size (Supplemental Figure 4B). In sharp contrast, mice receiving 30 mg/kg of the neutralizing PRLR antibody 005-C04 were completely infertile (Supplemental Figure 4, A and B). When these females were mated again with male mice 3 weeks after the end of the first mating round, all became pregnant and delivered litters of normal size (data not shown), indicating that the contraceptive effect of the PRLR antibody was reversible. Further experiments are required to unravel the exact mechanism leading to infertility in female mice treated with antibody 005-C04. In a first attempt, we performed a small pilot experiment in which female mice remained either untreated or were treated with 30 mg/kg antibody 005-C04 using the same application scheme as in the fertility assay but were not mated with male mice. Two days after the last antibody administration, the mice were killed, the estrogen and progesterone hormone levels were determined, and the reproductive tissues were analyzed histologically. In this pilot experiment, we could not find any evidence that antibody application interfered with estrous cycles in mice (Otto C., unpublished observations). Therefore, future experiments should focus on the effects of the antibody treatment on blastocyst development and implantation to address the mechanism of action.
Because homozygous PRLR mutant mice were unable to lactate (10), we examined postpartal lactation capacity as a second readout for the in vivo efficacy of the neutralizing PRLR antibody. The litter size was adjusted to eight pups on postpartal day 1 and the dams remained untreated or were treated with negative control antibody or two doses of the 005-C04 antibody on postpartal days 1, 3, 6, 9, and 10. To monitor the lactation capacity of the dams, litter weight was determined daily and is depicted in Figure 2A. Because in the 30 mg/kg 005-C04 antibody group several litters had to be killed for ethical reasons (severe growth retardation) on postpartal day 10, statistical analysis of the raw data focused on the litter weight gain between postpartal days 9 and 1. Between postpartal day 1 and 9, litters showed a weight increase of 39.1 g, 35.1 g, 31.0 g, and 25.2 g from untreated dams (closed circles in Figure 2A), from dams treated with unspecific control antibody (open circles in Figure 2A), from dams treated with 10 mg/kg antibody 005-C04 (closed triangles in Figure 2A) and from dams treated with 30 mg/kg antibody 005-C04 (open triangles in Figure 2A), respectively.
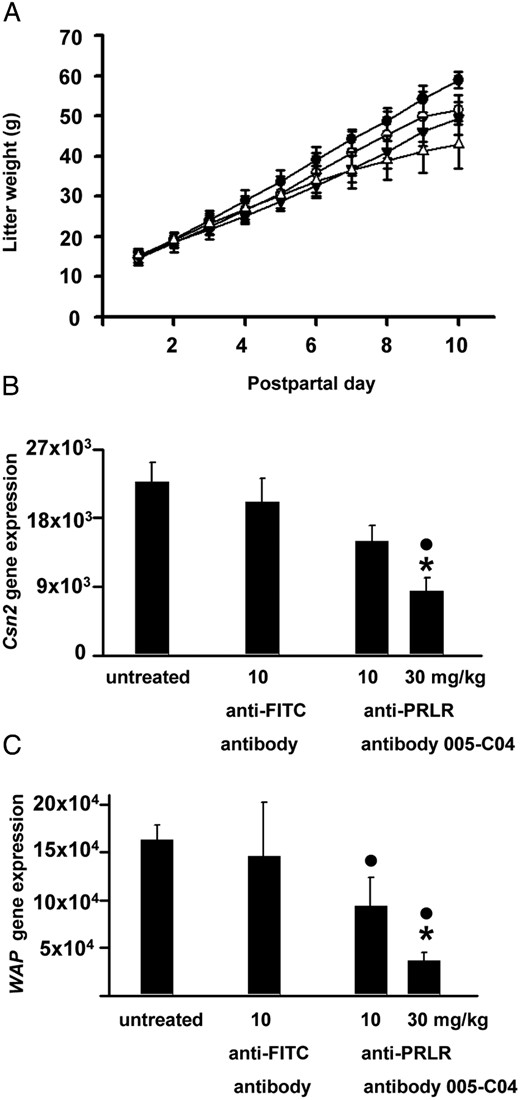
Reduced lactation in mice treated with the neutralizing PRLR antibody. Lactating dams (litter size = 8 pups) remained untreated (closed circles) or were treated with either 10 mg/kg anti-FITC antibody (open circles) or 10 mg/kg (closed triangles) and 30 mg/kg neutralizing PRLR receptor antibody 005-C04 in the IgG2a format (open triangles). Litter weight is depicted from postpartal day 1 to postpartal day 10 (A). Antibody 005-C04 at a dose of 30 mg/kg significantly inhibited lactation capacity as evidenced by the reduced litter weight gain between postpartal day 1 and 9 if compared with litters from untreated dams or dams treated with unspecific antibody. Milk protein expression was assessed on postpartal day 11 in mammary glands of lactating dams (B and C). Treatment with the neutralizing PRLR antibody induced a dose-dependent decrease in β-casein (Csn2) (B) and whey acidic protein (Wap) (C) gene expression. Data are presented as mean ± SD. *, P < .05 vs dams treated with negative control antibody; ●, P < .05 vs untreated dams. FITC, fluorescein isothiocyanate.
The weight increase in litters from dams treated with 10 mg/kg 005-C04 is significantly less than in litters from untreated dams (P = .0477). The weight increase in these litters also tended to be less than those injected with unspecific control antibody, but this difference is not conclusive (P = .4876). 005-C04 at a dose of 10 mg/kg has therefore no specific effect on lactation capacity; the difference that is seen compared with weight gain in litters from untreated dams most likely reflects the injection stress (11) that is absent in untreated dams. Treatment with the PRLR-specific antibody 005-C04 at 30 mg/kg (open triangles in Figure 2A) led to significant decrease in litter weight gain between days 1 and 9 if compared with litters from the untreated dams (P < .001) and if compared with control antibody treatment (P = .0101).
We next examined gene expression of two major milk proteins, β-casein (Csn2) and whey acidic protein (Wap), in the mammary glands of the lactating dams (Figure 2, B and C). Whereas treatment with the control antibody had no effect, the PRLR antibody severely reduced the expression of Csn2 and Wap in a dose-dependent manner (Figure 2, B and C). Figure 3 shows the histological hematoxylin and eosin-stained sections of mammary glands from lactating dams of the different experimental groups. Mammary glands from untreated dams (Figure 3A) or from dams treated with the control antibody (Figure 3B) were completely filled with milk-producing alveoli. In contrast, there were signs of mammary gland involution in mothers treated with the neutralizing PRLR antibody at doses of 10 (Figure 3C) and 30 mg/kg (Figure 3D). Mammary gland involution in these latter two experimental groups was characterized by increased accumulation of fat tissue (asterisks in Figure 3, C and D) and lobuloalveolar regression. The changes in milk protein expression (Figure 2, B and C) and the tissue remodeling in response to treatment with the neutralizing PRLR antibody (Figure 3, C and D) were remarkable because these changes occurred despite elevated maternal PRL levels due to the presence of suckling pups. In contrast to the PRLR-deficient female mice (12), antibody-treated dams showed completely undisturbed maternal behavior. Each pup that was placed after daily weighing into the opposite corner of the nest was immediately retrieved by its dam into the nest. All dams showed intensive crouching and nursing behavior.
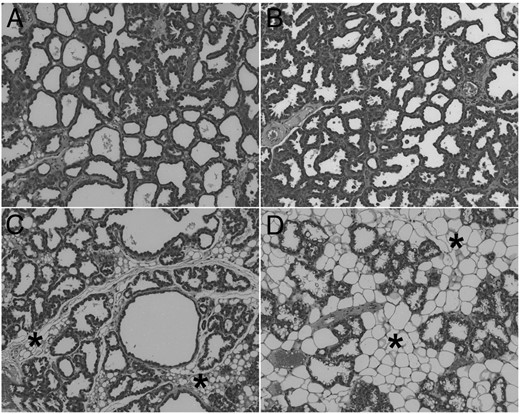
The neutralizing PRLR antibody 005-C04 induced mammary gland involution in lactating dams. Lactating dams remained untreated (A) or were treated with either 10 mg/kg anti-FITC antibody (B) or 10 mg/kg (C) and 30 mg/kg (D) neutralizing PRLR antibody 005-C04 in the IgG2a format as described in Materials and Methods. On postpartal day 11, dams were killed. Representative sections of mammary glands stained with HE are shown at the same magnification. Mammary glands of untreated dams (A) and dams treated with control antibody (B) showed complete alveolar development. Treatment with the neutralizing PRLR antibody 005-C04 (C and D) induced mammary gland involution characterized by alveolar regression and enhanced adipogenesis (asterisks) in a dose-dependent manner. H&E, hematoxylin and eosin
Studies using wild-type mice treated with PRL or grafted with PRLR-deficient skin (5, 13) have demonstrated that prolactin retards entry into the anagen phase of the hair cycle, leading to reduced hair regrowth after shaving, whereas grafts from the PRLR-deficient mice in wild-type mice showed more rapid hair cycling leading to increased hair regrowth within the graft region. In line with these findings, the PRLR-deficient mice show accelerated hair cycling in the second molting phase and have longer and coarser fur (12). To examine whether application of a neutralizing PRLR antibody mimics the phenotype of PRLR-deficient mice, we performed hair regrowth studies in 8-week-old female mice in which the anagen phase can be induced by shaving (14). Treatment with the specific PRLR antibody 005-C04 stimulated hair regrowth in normoprolactinemic mice (Figure 4, row C) if compared with untreated animals (Figure 4, row A) or mice treated with the control antibody (Figure 4, row B). Hair regrowth was delayed in the untreated hyperprolactinemic, pituitary-grafted animals (compare Figure 4, row D vs row A) and in hyperprolactinemic animals treated with the control antibody (compare Figure 4, row E vs row B) as demonstrated by the pinkish skin color in the hyperprolactinemic animals (Figure 4, rows D and E). Treatment with the specific antibody 005-C04 blocked PRLR signaling in hyperprolactinemic females and accelerated hair regrowth (Figure 4, row F vs rows D and E).
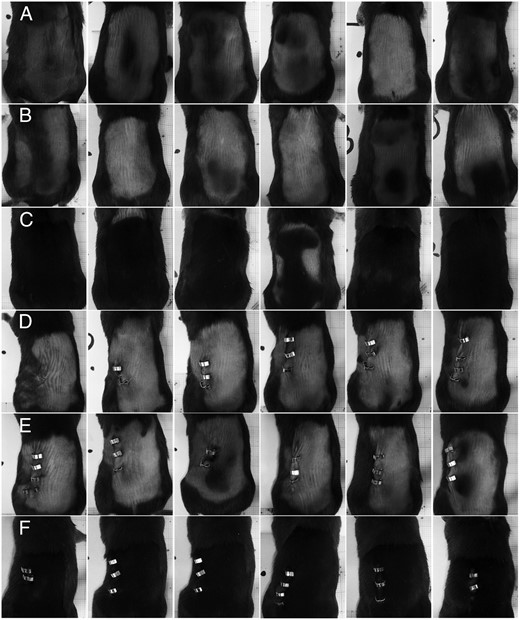
The neutralizing PRLR antibody 005-C04 stimulates hair regrowth in normal and hyperprolactinemic female mice. In this figure, all six animals per treatment group are depicted in a row-wise manner. Eight-week-old female C57BL/6 mice remained either unoperated (rows A–C) or received a pituitary isograft (rows D–F) under their kidney capsule to induce hyperprolactinemia and were shaved on their backs on day 0 (day of grafting). Animals remained untreated (rows A and D) or were treated with either 30 mg/kg control antibody (rows B and E) or specific neutralizing PRLR antibody 005-C04 (rows C and F) on days 0, 7, and 14. Animals were killed and photographed on day 15. Note the pinkish skin color in hyperprolactinemic animals (rows D and E) in comparison with the pink-gray skin color in normoprolactinemic animals (rows A and B), which indicates a delay in hair regrowth in response to hyperprolactinemia. Treatment with the specific neutralizing PRLR antibody 005-C04 accelerates hair regrowth in normal (row C) and hyperprolactinemic animals (row F) if compared with the respective normal (rows A and B) and hyperprolactinemic (rows D and E) controls.
Discussion
In this study we identified a human monoclonal antibody named 005-C05 that blocked signal transduction mediated by the mouse and human PRLR in a noncompetitive manner. In vitro, antibody 005-C04 inhibited PRL-induced STAT5 activation and cell proliferation with IC50 values in the single- to double-digit nanomolar range. The in vivo application of this neutralizing PRLR antibody rendered female mice sterile. The antibody inhibited STAT5-dependent milk protein expression (15) in vivo and induced mammary gland involution in lactating dams. Treatment of shaved female mice with the specific antibody stimulated hair regrowth, whereas hyperprolactinemia induced by pituitary grafting in untreated animals delayed hair regrowth. All these in vivo observations indicate that application of this neutralizing PRLR antibody mimicked several phenotypic changes observed after genetic inactivation of the PRLR in female mice (5, 10, 13, 16). There was only one exception: in contrast to PRLR-deficient mice, which showed severely disturbed maternal behavior (12) when confronted with foster pups, lactating dams treated with the specific PRLR antibody showed normal maternal behavior with regard to retrieval of pups to the nest and crouching behavior. This finding may indicate that the neutralizing PRLR antibody does not cross the blood-brain barrier because PRLRs in the medial preoptic brain area in rats have been implicated in pup retrieval (17). An alternative explanation for this discrepancy could be that neurogenesis took already place during early pregnancy (18), and thus, the mechanisms underlying undisturbed pub retrieval were already in place when postpartal treatment with antibody 005-C04 started.
In general, PRLR antagonists are valuable tools for complete blockade of PRLR signaling activated by PRL secreted from pituitary and extrapituitary sites. Compared with PRLR antagonists that were structurally derived from PRL such as G129R-hPRL or Δ1–9G129R-hPRL, the neutralizing PRLR antibody offered several advantages. First, the antibody blocked PRLR signaling in a noncompetitive manner, ie, the antibody did not compete with the endogenous ligand PRL for receptor binding and the IC50 values for functional receptor blockade did not change with increasing PRL concentrations. In contrast, Δ1–9G129R-hPRL acted as a competitive PRLR antagonist and a 10- to 50-fold molar excess compared with PRL was required to block PRLR signal transduction in vitro (8). Second, compared with the natural ligand PRL, peptide-derived PRLR antagonists such as G129R-hPRL and Δ1–9G129R-hPRL exhibited a 10-fold lower affinity for the PRLR (8). When we compared the IC50 values for inhibition of cell proliferation in cells stably expressing the human PRLR or in Nb2 cells endogenously expressing the rat PRLR, we demonstrated that the PRLR antibody 005-C04 was approximately 40- to 70-fold more potent than the G129R-hPRL antagonist. Third, PRL and its peptide-derived antagonists had a relatively short half-life in the range of 15–20 minutes (8), whereas the half-lives of 14 days were reported for many approved human IgG1 antibodies (19). Due to its reduced potency and the competitive interaction with the PRLR, Δ1–9G129R-hPRL was unable to interfere with STAT5 activation in the mammary glands of pregnant mice in which PRL reached levels of up to hundreds of nanograms per milliliter (8).
Prereduction of endogenous PRL levels by bromocriptine injections was required to observe an inhibition of STAT5 phosphorylation by Δ1–9G129R-hPRL when coinjected with PRL at 100-fold molar excess (8). In sharp contrast, the neutralizing PRLR antibody described in this study blocked the PRLR in a noncompetitive manner and inhibited the expression of STAT5-dependent target genes in the mammary glands of dams in a dose-dependent manner, although the PRL levels were dramatically elevated due to the presence of suckling pups. For successful lactation inhibition with bromocriptine twice-daily applications or pellet implantation was required in mice due to the reduced half-life of this compound (20). The effect sizes we observe on litter weight gain with antibody 005-C04 at a dose of 30 mg/kg were comparable with those seen after daily or continuous bromocriptine administration during early lactation in mice (20). Whether 30 mg/kg antibody 005-C04 in the chosen application scheme already produces the maximum possible effect on early lactation in mice needs to be addressed in further studies. The aim of the present study was to analyze whether antibody 005-C04 has any effect on early lactation in mice at all. Neutralizing PRLR antibodies might present a therapeutic alternative to bromocriptine for lactation inhibition in women who suffer from stillbirth or cannot breast-feed their babies due to severe illness of the newborn. Side effects such as nausea, headache, dizziness, fatigue, vomiting, hypotension, and hallucinations, which can occur under bromocriptine therapy (21), are not expected under therapy with neutralizing PRLR antibodies.
The major drawback of peptidergic PRLR antagonists, a short half-life, did not allow the analysis of long-term in vivo effects after PRLR antagonist application (8). The use of osmotic minipumps was required but yielded plasma levels far below those required for complete PRLR blockade (8). As an alternative, transgenic mice ubiquitously expressing Δ1–9G129R-hPRL under the control of the methallothionein promoter were used to analyze the in vivo consequences of PRLR blockade in mice overexpressing PRL within the prostate (22). For the generation of double-transgenic mice, matings were arranged with female mice overexpressing Δ1–9G129R-hPRL (22). This experimental procedure clearly indicated that overexpression of Δ1–9G129R-hPRL did not significantly alter female fertility (22), a finding that was not in line with the infertility phenotype of PRLR-deficient mice (16). Although overexpression of the PRLR antagonist was able to inhibit many of the consequences of PRL overexpression in the prostate, female fertility in the presence of Δ1–9G129R-hPRL and only partial reduction of prostate weight in double-transgenic mice expressing the PRLR antagonist and PRL might indicate that only incomplete PRLR blockade was achieved in this experimental approach (22).
These data and our findings may suggest that minimal PRLR signaling in female mice seems to be sufficient to maintain fertility. The dose of 30 mg/kg of the antibody was required to induce complete infertility. At first sight it might be surprising that a dose of 10 mg/kg 005-C04 was without any effect in fertility studies, whereas a dose of 30 mg/kg 005-C04 led to complete infertility. In contrast to many small molecules, PRLR antibodies display nonlinear pharmacokinetics, ie, area under the curve, and a half-life increase with the dose. This is due to the fact that target-mediated drug disposition plays a more significant role on exposure at low compared with high antibody drug doses. Therefore, a 3-fold difference in doses (10 vs 30 mg/kg) does not translate into a 3-fold difference but into a much higher difference in exposures (Otto, C., unpublished observations). We did not determine the pharmacokinetic-pharmacodynamic driver in fertility experiments. Nevertheless, for other in vivo readouts, it turned out that the level of 005-C04 needed to be above a threshold concentration over time (Otto, C., unpublished observation). Therefore, the higher influence of target-mediated drug disposition at the 10 mg/kg dose might prevent sufficient antibody exposures over time, leading to undisturbed fertility. To our knowledge, neutralizing PRLR antibody 005-C04 seems to be the first PRLR antagonist that mimics the infertility phenotype of PRLR-deficient mice in vivo. The recently described PRLR antibody LFA102 (23) showed no effects on cell proliferation in cells stably expressing the mouse PRLR and had no effect in fertility studies (Otto, C., unpublished observations).
It remains to be shown whether PRLR antibodies could be suitable for nonhormonal contraception in females. Experiments using exogenous PRL application or bromocriptine failed to demonstrate a role for PRL in regulating luteal function in primates (24), whereas in mice PRL seemed to play a pivotal role in preventing corpus luteum apoptosis (16). However, there were several publications supporting the hypothesis that prolactin prevented also in humans the apoptosis of luteinized granulosa cells (25) or that minimal endometrial PRL expression was required for maintenance of early pregnancy in women (26). A recent publication described the phenotypic consequences of a transdominant negative human PRLR mutation (27). Possibly due to interindividual different balances of normal PRLR dimers and mutant homo- and heterodimers, there was some phenotypic variance. One of three sisters showed the same phenotype as PRLR-deficient mice (16), eg, infertility in the presence of normal estrous cycles (27). Nevertheless, fertility studies in monkeys and women are necessary to address the question whether neutralizing PRLR antibodies bear a contraceptive potential also in humans.
Taken together, the results from this study underline that the newly identified PRLR antibody 005-C04 is a potent noncompetitive antagonist of the PRLR in vitro that acts also in vivo as a PRLR antagonist because its application to female mice mimics to a large extent the phenotype of female PRLR-deficient mice. We hope that this antibody will be a valuable tool for investigating PRL’s role in healthy and diseased conditions.
Acknowledgments
We thank Adelheid Engelhaupt (Bayer Pharma AG, Berlin, Germany) and Oliver Gernetzki (Bayer Pharma AG) for help with the cellular proliferation assays; Silke Aigner, Sabine Raschat, and Michael Haas for help with the cell binding and surface plasmon resonance analyses; Sandra Hartke for the cloning of expression constructs; and Mark Trautwein, Anke Mayer Bartschmid, Lars Linden, Sandra Bruder, Simone Greven, and colleagues (Global Biologics Research, Wuppertal, Germany) for the antibody and antigen expression, purification, and analytics.
Present address for C.O.: Experimental Medicine Cardiology and Hematology, Bayer Pharma AG, Aprather Weg 18a, 42113 Wuppertal, Germany.
Present address for A.S.: Department of Process Development, Bavarian Nordic A/S, Hejreskovvej 10A, DK-3490 Kvistgaard, Denmark.
Present address for A.H.: Bayer Intellectual Property GmbH, Alfred-Nobel-Straße 10, 40789 Monheim, Germany.
Present address for C.F.: GD Biologics Business Unit, Genedata AG, Margarethenstrasse 38, 4053 Basel, Switzerland.
Disclosure Summary: A.H., S.W., B.R.-S., I.F., J.S., R.V., and C.O. are employees of Bayer Pharma AG. M.M. and A.L. are employees of BioInvent International AB. A.S. was previously employed by BioInvent International AB. C.F. was previously employed by Bayer Pharma AG.
Abbreviations
- ECD
extracellular domain
- GH
growth hormone
- KD
dissociation constant
- PRL
prolactin
- PRLR
PRL receptor
- STAT5
signal transducer and activator of transcription 5.



