-
PDF
- Split View
-
Views
-
Cite
Cite
Theresa A Stangl, Chantal M Wiepjes, Annemieke C Heijboer, Martin den Heijer, The influence of gender-affirming hormone therapy on serum concentrations of hormone-binding proteins, European Journal of Endocrinology, Volume 192, Issue 5, May 2025, Pages 614–620, https://doi.org/10.1093/ejendo/lvaf038
Close - Share Icon Share
Abstract
Background
Corticosteroid-binding globulin (CBG), thyroid-binding globulin (TBG), sex hormone–binding globulin (SHBG), and insulin-like growth factor–binding protein 3 (IGF-BP3) regulate the bioavailability and transport of hormones, affecting hormone concentration measurements and therapy plans. This study investigates to what extent gender-affirming hormone therapy (GAHT) impacts serum concentrations of these binding proteins.
Methods
This prospective study included 41 transfeminine persons starting oral or transdermal 17β-estradiol in combination with cyproterone acetate (CPA) or gonadotropin-releasing hormone analogs (GnRHa) and 38 transmasculine persons starting testosterone gel or injections. Serum concentrations of CBG (mg/L), TBG (nmol/L), SHBG (nmol/L), and IGF-BP3 (mg/L) were measured at baseline and after 3 and 12 months. Changes were analyzed using mixed models and reported as percentage change.
Results
In oral estradiol and CPA users, CBG increased by 29% (95% CI, 16, 44%), TBG by 24% (95% CI, 16, 32%), SHBG by 81% (95% CI, 61, 105%) and in oral estradiol and GnRHa users by 47% (95% CI, 7, 101%), 48% (95% CI, 9, 101%), and 242% (95% CI, 104, 474%), respectively. The IGF-BP3 remained unchanged. In transdermal estradiol users, only SHBG changed (+63% [95% CI, 3, 157%]), when combined with GnRHa. In transmasculine participants, CBG, TBG, SHBG, and IGF-BP3 decreased by 13% (95% CI, −21, −4%), 11% (95% CI, −15, −6%), 43% (95% CI, −48, −36%), and 10% (95% CI, −18, −2%) respectively, with no difference in gel vs injections.
Conclusion
The GAHT led to an increase of CBG, TBG, and SHBG in estradiol users, more specifically oral estradiol, and to a decrease of SHBG, CBG, TBG, and IGF-BP3 in testosterone users. Recognizing these alterations is crucial for ensuring accurate hormone measurements and optimal patient care.
Hormone-binding proteins play a crucial role in regulating hormone bioavailability and transport of hormones, yet their alterations after the start of gender-affirming hormone therapy (GAHT) are not well understood. This study describes the extent to which feminizing hormone treatment increases and masculinizing hormone treatment decreases serum concentrations of corticosteroid-binding globulin, thyroid-binding globulin, sex hormone–binding globulin, and insulin-like growth factor–binding protein 3. The findings of this study highlight the importance of healthcare professionals being aware of variations in hormone-binding proteins during GAHT. Such alterations can affect hormone measurements of persons using GAHT, leading to possible misdiagnosis, and may also have broader implications for patient care, including the management of conditions like hypothyroidism and the interpretation of cortisol-related diagnostic tests.
Introduction
Most hormones circulate in the bloodstream bound to hormone-binding proteins that function as carrier proteins and are mainly produced in the liver. According to the free hormone hypothesis, only the free (unbound) fraction of those hormones is biologically active.1,2 Previous studies demonstrated that serum concentrations of corticosteroid-binding globulin (CBG), thyroid-binding globulin (TBG), sex hormone–binding globulin (SHBG), and insulin-like growth factor 1–binding protein 3 (IGF-BP3) increase under the influence of estradiol, for example, in pregnant women,3 and in cisgender women using contraceptive pills containing ethinylestradiol.4-12 This increase is especially seen in users of oral estradiol, which can be accounted to the first pass effect in the liver.13 While estradiol seems to increase concentrations of binding hormones, testosterone leads to a decrease of for example SHBG, possibly due to a decrease of adiponectin which is thought to affect SHBG synthesis in the liver.14,15
In clinical practice, the concentration of binding proteins can impact the interpretation of laboratory parameters in diagnostics, can influence therapy plans, and can play a significant role in the etiology of diseases. For example, the measurement of total testosterone depends on the amount of SHBG. Low SHBG concentration leads to a lower total testosterone, while high SHBG results in higher total testosterone concentrations, while free testosterone concentrations remain stable.16 Similarly, in diagnostics involving assessment of the hypothalamic pituitary adrenal axis status, high CBG serum concentrations lead to the measurement of high total cortisol levels. This potentially causes misinterpretation and severe patient-related outcomes.17,18 Additionally, the amount of binding proteins can also have direct implications for therapy plans: in hypothyroid patients, an increase in TBG can lead to less available T4, necessitating a higher dosage of exogenous T4 supplementation.8,19 Regarding disease etiology, binding proteins can also play an important role. A low SHBG concentration, for instance, is related with an increased risk to develop type 2 diabetes and fatty liver.20-23
While it is established that sex hormones influence serum concentrations of binding proteins, most studies have focused on cis women using medical agents that are not used in current protocols for feminizing hormone therapy or in cis men with hypogonadism receiving testosterone supplementation. However, changes in binding protein concentrations may have clinical implications for another, growing population: people with gender incongruence who choose gender affirming hormone therapy (GAHT). In the Netherlands, according to the current therapy protocols, feminizing hormone therapy consists of oral or transdermal 17β estradiol, often combined with testosterone-suppressing medication like gonadotropin-releasing hormone analogs (GnRHa) or cyproterone acetate (CPA). Masculinizing hormone therapy involves transdermal or intramuscular testosterone agents.24
This study aims to investigate to what extent GAHT affects serum CBG, TBG, SHBG, and IGF-BP3 concentrations in transgender individuals during the first year of hormone use. We hypothesize that oral estradiol increases serum concentrations of hormone-binding hormones to a greater extent compared to transdermal agents, that users of CPA show less increase than users of GnRHa, and lastly that users of testosterone agents show a decrease of hormone-binding hormones.
Methods
Population
This study is part of the European Network for Investigation of Gender Incongruence (ENIGI) study, which is an ongoing multicenter prospective observational study. Five gender identity clinics are participating in the ENIGI collaboration: Ghent University Hospital, Belgium; Amsterdam UMC, the Netherlands; Rikshospitalet in Oslo, Norway; Tel Aviv Sourasky Medical Center, Israel; and University Hospital in Florence, Italy. People could be included in the ENIGI study since 2010 if they were diagnosed with gender dysphoria, if they were about to start with GAHT, if they had never used gender-affirming hormones before, and if they spoke the native language. Transfeminine persons started using oral or transdermal 17β-estradiol in combination with antiandrogen therapy (CPA or GnRHa) and transmasculine persons started transdermal or intramuscular testosterone. For the current analysis, only participants from Amsterdam were included, if they were between 18 and 50 years old at the time of start hormones and had blood serum samples stored in the freezer at baseline and after 3 and/or 12 months. The ENIGI study was reviewed by the local Medical Ethics Committee of the VU University Medical Center, Amsterdam. Informed consent has been given according to institutional guidelines. This study was conducted in accordance with the principles of the Declaration of Helsinki.
We excluded participants with estradiol serum concentration < 180 or >700 pmol/L in users of feminizing hormone therapy or total testosterone serum concentration < 8 nmol/L in users of masculinizing hormone therapy at the 3- and 12-month measurement, because this thresholds align with the reference intervals pursued at our gender clinic at the time of the study. Participants with risk of liver damage were excluded as well, such as users of potentially hepatotoxic comedication (amiodarone, antiviral medication, chlorpromazine, isoniazid, methotrexate, and antibiotics) and >7 alcoholic beverages/week; participants with a medical history of liver cirrhosis, hemochromatosis or hepatitis, intravenous use of illegal substances, and elevation of liver enzymes (>3× upper limit of normal). Participants who had a diagnosis of hypothyroidism, hyperthyroidism, polycystic ovary syndrome, type 1 or 2 diabetes, and insulin resistance as well as participants who used oral or potent transdermal corticosteroids (with moderate [category 4] to high potency), progestogens, or contraceptive pill at baseline and 3 or 12 months of measurement were excluded. Individuals with a BMI > 30 kg/m2 were excluded due to the higher risk of fatty liver or fibrosis, which could affect hormone-binding protein concentrations.
Data collection and measurements
Clinical data and blood samples were collected at baseline and after 3 and 12 months after the start of GAHT. Besides measurements as part of regular care, extra blood was drawn and stored at −80 °C in the Amsterdam UMC Biobank until analysis. Regular care measurements that were used in this study involved total testosterone (nmol/L), 17β-estradiol (pmol/L), and SHBG (nmol/L). In some participants, also IGF-BP3 was measured within the scope of regular care, following local protocols before 2017. In 2023, the frozen samples were collected from the Biobank and analyzed at the Endocrinology Laboratory of the Amsterdam UMC location AMC. In this study, we did not account for the timing of the last medication intake or patch application before blood sampling, assuming stable estradiol serum concentrations and consistent testosterone suppression by antiandrogens. With hormone-binding proteins having half-lives of 5-7 days (SHBG, TBG, and CBG), we expected daily hormone fluctuations to have minimal impact on the results.
Laboratory measurements
Part of the serum samples was analyzed within the scope of patient care (between 2014 and 2023), and part of the serum samples was stored in freezers and analyzed in 2023. Serum CBG was measured using a radioimmunoassay (Diasource, Louvain-la-Neuve, Belgium) with an intraassay variation (CV) of 4.2% at a level of 47 mg/L and an interassay CV of 6.2% at a level of 17 mg/L and a lower limit of quantitation (LLOQ) of 10 mg/L. For the analysis of TBG, a radioimmunoassay (BRAHMS, Thermo Scientific, Massachusetts, USA) with an intraassay CV of 7.3% at a level of 378 nmol/L, an interassay CV of 8.3% at a level of 598 nmol/L, and a LLOQ of 75 nmol/L was used. For the measurement of SHBG, a chemiluminescence immunoassay (Architect and Alinity, Abbott Diagnostics, Illinois, USA) was used. The intraassay CV was 3.2% at a level of 49 nmol/L, the interassay CV was 4.8% at a level of 51 nmol/L, and the LLOQ was 4.5 nmol/L. The IGF-BP3 was measured using an ELISA (DRG, Marburg, Germany) with an intraassay CV of 1.8% at a level of 2.1 mg/L, an interassay CV of 8.6% at a level of 2.3 mg/L, and an LLOQ of 0.4 mg/L. For the measurement of the serum estradiol concentrations, an in-house developed liquid chromatography–tandem mass spectrometry method (25) was used with an intraassay CV of 5% at a level of 220 pmol/L, an interassay CV of 7% at a level of 54-283 pmol/L, and an LLOQ of 20 pmol/L. Testosterone was measured with a second-generation competitive immunoassay in the samples analyzed until 2016 (Architect, Abbott, Abbott Park, Illinois, USA). For this assay, the intraassay CV was 4% at a level of 2-20 nmol/L and 8% at a level of 0.2 nmol/L, the interassay CV was 6 at a level of 2-20 nmol/L and 10% at a level of 0.2 nmol/L, and the LLOQ was 0.1 nmol/L. From 2016 on, the in-house LC-MS/MS method was used to determine serum testosterone concentrations (26). The intraassay CV is 3.2% at a level of 4 nmol/L and 2% at a level of 25 nmol/L, the interassay CV is 4% at a level of 24 nmol/L and 6% at a level of 0.6 nmol/L, and the LLOQ is 0.1 nmol/L. The results of these testosterone methods were comparable as found by an extensive method comparison.
Statistical analysis
For statistical analysis, Stata version 17 (StataCorp LP) was used. Descriptive statistics were used to present baseline characteristics separately for users of feminizing and masculinizing hormone therapy. Gaussian continuous variables are reported as means with standard deviation and non-Gaussian continuous variables as medians with interquartile ranges (IQRs). Data analyses with a linear mixed model with measurements clustered within participants were performed to analyze the serum concentrations of CBG, TBG, SHBG, and IGF-BP3 over the course of time (at baseline and after 3 and 12 months). To calculate the percentage change of binding proteins between baseline and after 12 months, the binding protein serum concentrations were log transformed prior to regression analysis and back transformed to ratios which are expressed as a percentage of change for presentation. All analyses were stratified for type of hormone administration.
Sensitivity analyses were performed to assess if associations differed in groups. We checked for effect modification by including BMI categories (BMI ≤ 20 kg/m2, 21-25 kg/m2, and 25-30 kg/m2) and tertiles of age as interaction terms to the models.
Pearson correlation coefficients between the percentages of change of the hormone-binding proteins were calculated to assess the association between the magnitudes of change between these hormone-binding proteins.
Results
The flowchart of inclusions is shown in Figure 1. In total, 41 users of feminizing hormone therapy and 38 users of masculinizing hormone therapy were included and baseline characteristics are presented in Table 1. Transdermal estradiol + CPA was used by 6 participants, 4 persons used transdermal estradiol + GnRHa, 27 persons used oral estradiol + CPA, and 4 persons used oral estradiol + GnRHa. Fourteen persons used testosterone gel and 24 persons testosterone injections. Among the participants, five transmasculine persons and six transfeminine persons had a missing value at either the 3-month or 12-month measurement.
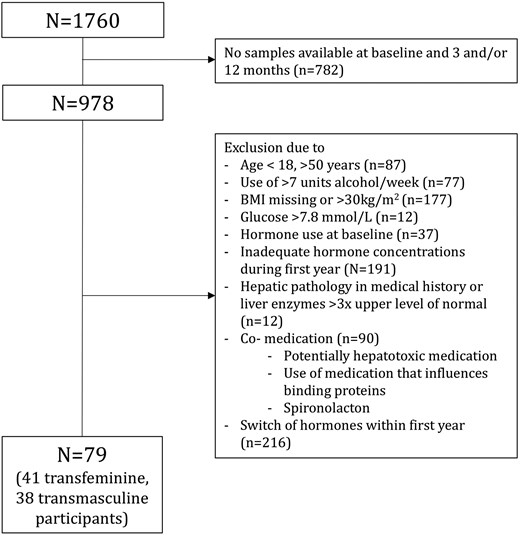
Flowchart of participant selection of participants in the ACOG study from Amsterdam.
| . | Users of feminizing hormone therapy (n = 41) . | Users of masculinizing hormone therapy (n = 38) . |
|---|---|---|
| Age (y) | 27 (20, 33) | 24 (20, 26) |
| BMI (kg/m2) | 22 (3) | 23 (3) |
| Tobacco smoking (% current) | 11 | 0 |
| Alcohol intake (units/week) | 0 (0, 1) | 0 (0, 1) |
| CBG (mg/L) | 48 (7) | 54 (18) |
| TBG (nmol/L) | 359 (31) | 400 (75) |
| SHBG (nmol/L) | 33 (12) | 56 (25) |
| IGF-BP3 (mg/L)a | 2.94 (0.43) | 3.27 (0.55) |
| Testosterone (nmol/L) | 18.4 (5.9) | 1.1 (0.3) |
| Estradiol (pmol/L) | 78 (63, 100) | 161 (120, 254) |
| . | Users of feminizing hormone therapy (n = 41) . | Users of masculinizing hormone therapy (n = 38) . |
|---|---|---|
| Age (y) | 27 (20, 33) | 24 (20, 26) |
| BMI (kg/m2) | 22 (3) | 23 (3) |
| Tobacco smoking (% current) | 11 | 0 |
| Alcohol intake (units/week) | 0 (0, 1) | 0 (0, 1) |
| CBG (mg/L) | 48 (7) | 54 (18) |
| TBG (nmol/L) | 359 (31) | 400 (75) |
| SHBG (nmol/L) | 33 (12) | 56 (25) |
| IGF-BP3 (mg/L)a | 2.94 (0.43) | 3.27 (0.55) |
| Testosterone (nmol/L) | 18.4 (5.9) | 1.1 (0.3) |
| Estradiol (pmol/L) | 78 (63, 100) | 161 (120, 254) |
Baseline characteristics, displayed stratified for users of feminizing and masculinizing hormone therapy. Data are presented as means (SD), medians (IQR), or total numbers and percentages.
Abbreviations: BMI, body mass index, CBG, corticosteroid binding globulin; CPA, cyproterone acetate; GnRHa, gonadotropin-releasing hormone analog; IGF-BP3, insulin-like growth factor 1–binding protein 3; IQR, interquartile range; SD, standard deviation; SHBG, sex hormone–binding globulin; TBG, thyroid-binding globulin.
aKnown in 22 transfeminine and 25 transmasculine persons.
| . | Users of feminizing hormone therapy (n = 41) . | Users of masculinizing hormone therapy (n = 38) . |
|---|---|---|
| Age (y) | 27 (20, 33) | 24 (20, 26) |
| BMI (kg/m2) | 22 (3) | 23 (3) |
| Tobacco smoking (% current) | 11 | 0 |
| Alcohol intake (units/week) | 0 (0, 1) | 0 (0, 1) |
| CBG (mg/L) | 48 (7) | 54 (18) |
| TBG (nmol/L) | 359 (31) | 400 (75) |
| SHBG (nmol/L) | 33 (12) | 56 (25) |
| IGF-BP3 (mg/L)a | 2.94 (0.43) | 3.27 (0.55) |
| Testosterone (nmol/L) | 18.4 (5.9) | 1.1 (0.3) |
| Estradiol (pmol/L) | 78 (63, 100) | 161 (120, 254) |
| . | Users of feminizing hormone therapy (n = 41) . | Users of masculinizing hormone therapy (n = 38) . |
|---|---|---|
| Age (y) | 27 (20, 33) | 24 (20, 26) |
| BMI (kg/m2) | 22 (3) | 23 (3) |
| Tobacco smoking (% current) | 11 | 0 |
| Alcohol intake (units/week) | 0 (0, 1) | 0 (0, 1) |
| CBG (mg/L) | 48 (7) | 54 (18) |
| TBG (nmol/L) | 359 (31) | 400 (75) |
| SHBG (nmol/L) | 33 (12) | 56 (25) |
| IGF-BP3 (mg/L)a | 2.94 (0.43) | 3.27 (0.55) |
| Testosterone (nmol/L) | 18.4 (5.9) | 1.1 (0.3) |
| Estradiol (pmol/L) | 78 (63, 100) | 161 (120, 254) |
Baseline characteristics, displayed stratified for users of feminizing and masculinizing hormone therapy. Data are presented as means (SD), medians (IQR), or total numbers and percentages.
Abbreviations: BMI, body mass index, CBG, corticosteroid binding globulin; CPA, cyproterone acetate; GnRHa, gonadotropin-releasing hormone analog; IGF-BP3, insulin-like growth factor 1–binding protein 3; IQR, interquartile range; SD, standard deviation; SHBG, sex hormone–binding globulin; TBG, thyroid-binding globulin.
aKnown in 22 transfeminine and 25 transmasculine persons.
The course of the binding proteins is visualized in Figure 2. As there was no difference in the change of binding proteins in testosterone gel and injection users, we also present the pooled percentages of change of binding proteins in transmasculine persons in Figure 3. Furthermore, the concentrations of all binding proteins already shifted to nearly the same concentrations as the 12-month measurement (Figure 2). Therefore, we only describe the percentages of change after 12 months of hormone therapy in Figure 3.
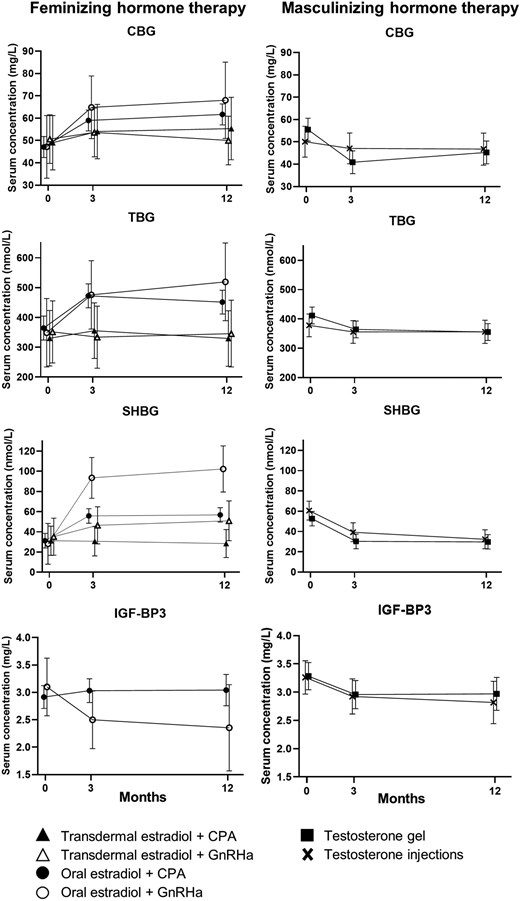
The effect of hormone therapy on mean (95% CI) serum concentrations of hormone-binding proteins after 3 and 12 months. CBG, corticosteroid-binding globulin; TBG, thyroid-binding globulin; SHBG, sex hormone–binding globulin; IGF-BP3, insulin-like growth factor 1–binding protein3; CPA, cyproterone acetate; GnRHa, gonadotropin-releasing hormone analog.
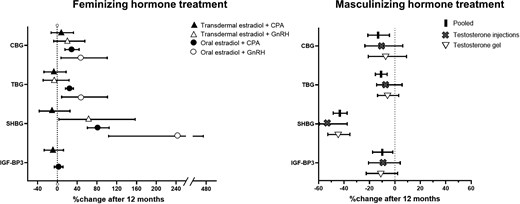
Percentages of change of hormone-binding proteins after 12 months of hormone therapy. CBG, corticosteroid-binding globulin; TBG, thyroid-binding globulin; SHBG, sex hormone–binding globulin; IGF-BP3, insulin-like growth factor 1–binding protein3; CPA, cyproterone acetate; GnRH, gonadotropin-releasing hormone analog.
Corticosteroid-binding globulin
At baseline, transfeminine persons had a mean (SD) CBG concentration of 48 (7) mg/L. In transdermal estradiol users (combined with either CPA or GnRHa), there was no difference in serum CBG concentrations between baseline and after 12 months. However, it increased by 29% (95% CI, 16, 44%) in oral estradiol + CPA users and by 47% (95% CI, 7, 101%) in oral estradiol + GnRHa users (Figure 3).
The mean (SD) CBG concentration of transmasculine persons at baseline was 54 (18) mg/L. After 12 months, CBG had decreased by 13% (95% CI, 4, 21%).
Thyroid-binding globulin
Transfeminine persons had a mean (SD) TBG concentration of 359 (31) nmol/L at baseline. After 12 months, the TBG concentration of transdermal estradiol (with CPA or GnRHa) users had not been changed. Oral estradiol + CPA users showed an increase of 24% (95% CI, 16, 32%), and oral estradiol + GnRHa users showed an increase by 48% (95% CI, 9, 101%).
The mean (SD) TBG concentration of transmasculine persons at baseline was 400 (75) nmol/L. After 12 months, TBG had decreased by 11% (95% CI, 6, 15%).
Sex hormone–binding globulin
At baseline, participants who were about to start feminizing hormone therapy had a mean (SD) SHBG serum concentration of 33 (12) nmol/L. In transdermal estradiol + CPA users, the SHBG concentration was not changed after 12 months of hormone therapy. After 12 months, the SHBG concentration was increased by 63% (95% CI, 3, 157%) in transdermal estradiol + GnRHa users, by 81% (95% CI, 61, 105%) in oral estradiol + CPA users, and by 242% (95% CI, 104, 474%) in oral estradiol + GnRHa users.
The mean (SD) SHBG concentration in transmasculine persons was 56 (25) nmol/L at baseline, and the percentage of change in SHBG after 12 months of using masculinizing hormone therapy was −43% (95% CI, −48, −36%).
Insulin-like growth factor–binding protein 3
Serum concentrations of IGF-BP3 were measured in 22 transfeminine and 25 transmasculine persons.
Transfeminine persons had a mean (SD) baseline IGF-BP3 concentration of 2.94 (0.43) mg/L. Twelve months after starting to use oral estradiol + CPA or transdermal estradiol + CPA, the mean IGF-BP3 concentration was not changed. There were no measurements of IGF-BP3 available in GnRHa users.
The mean (SD) IGF-BP3 concentration in serum of transmasculine persons at baseline was 3.27 (0.55) mg/L. After 12 months, IGF-BP3 had decreased by 10% (95% CI, 2, 18).
Sensitivity analysis
The course of all binding proteins over time was not different in groups of BMI or tertiles of age (data not shown).
Correlation
The correlation coefficients (95% CI) between the percentages changes of CBG and TBG were 0.15 (−0.04, 0.34) for feminizing hormone therapy users and 0.17 (−0.13, 0.47) for masculinizing hormone therapy users. For SHBG and CBG, the coefficients were 0.35 (0.22, 0.49) and 0.13 (−0.5, 0.32), and for SHBG and TBG, they were 0.35 (0.24, 0.47) and −0.11 (−0.33, 0.12), respectively.
Discussion
In this study, we assessed the relationship between the use of GAHT and serum CBG, TBG, SHBG, and IGF-BP3 concentrations. The use of feminizing hormone therapy, especially of oral estradiol, led to an increase, while masculinizing hormone therapy led to a decrease of binding protein concentrations after 3 and 12 months of hormone therapy. In addition, the binding proteins did not change with the same magnitude and the magnitudes of change between hormone-binding proteins were weakly to very weakly correlated. Of all binding proteins assessed in this study, SHBG was the most sensitive to the sex hormones while IGF-BP3 concentrations were only influenced mildly. Furthermore, oral estradiol increased hormone-binding proteins to a greater extent than transdermal estradiol, and CPA users showed a smaller change of SHBG during the use of estradiol.
The findings of the current study on CBG, TBG, and SHBG are consistent with the previous studies in the transgender population.25-28 However, some studies that included transfeminine persons using CPA as antiandrogen therapy in combination with oral or transdermal estradiol found no changes in CBG.27,28 Likewise, no change in vitamin D–binding protein was found in transfeminine persons using oral estradiol + CPA after 3 months. Vitamin D–binding protein is mainly produced in the liver as well. The authors argue that the use of CPA might have influenced their results.29 Consistently, in the current study, oral estradiol + CPA users had a smaller increase of SHBG compared to oral estradiol + GnRHa users. The sample size of oral estradiol + GnRH users was however small, which might have influenced our results. Still, CPA has strong progestogenic and weak glucocorticoid30 properties which might have suppressed concentrations of SHBG.16,31,32
The considerable increase in binding proteins with oral estradiol usage may be ascribed to two underlying mechanisms. The main production site of CBG, TBG, SHBG, and IGF-BP3 is the liver. With the intake of oral estradiol, the serum estradiol concentration in the hepatic circulation rises to a greater extent than in the systemic circulation, which is referred to as the first pass effect.33,34 On one side, the high estradiol concentration in the liver seems to stimulate hepatic binding protein production.35 On the other side, estradiol affects the amount of sialic acid residues the binding proteins carry. A higher degree of glycosylation decreases the clearance from the circulation by the liver and consequently leads to higher serum binding protein concentrations.8,9,36,37 Estradiol administered transdermally does not pass through the gut and liver metabolism, which might explain why the increase of binding proteins of users of transdermal estradiol in this study was smaller. Earlier studies on changes in hormone-binding proteins primarily focused on individuals using ethinylestradiol, a significantly more potent form of estrogen often used in oral contraceptive pills, compared to the 17β-estradiol used in our study. Despite this, we observed significant changes in hormone-binding proteins, which closely resemble the percentages of change observed in users of oral hormonal contraception.7
In testosterone users, the decrease of binding proteins might be attributed to a decrease in adiponectin15 which leads to an inhibition of expression of SHBG.14,38,39 However, also estradiol concentrations in users of masculinizing hormone therapy might be decreased through the use of testosterone and a possible suppression of the menstrual cycle in some persons.40 The decrease in hormone-binding proteins therefore might be influenced not only by higher testosterone but also by lower estradiol serum concentrations, a possibility that future studies should explore. The relatively small sample size among users of GnRHa represents a limitation of this study; therefore, these results should be interpreted with caution. Also, IGF-BP3 was only measured in CPA and no GnRHa users. Nevertheless, the insights on changes of hormone-binding proteins across different therapy options are valuable, particularly because we were able to control for numerous factors that could influence binding protein concentrations.
In this study, a rise in CBG, TBG, and SHBG concentrations in individuals using feminizing hormone therapy and a decrease in these concentrations in users of masculinizing hormone therapy was observed. All changes in binding protein concentrations had occurred by the time of the 3-month measurement, with no further changes at the time of the 12-month measurement. The findings of this study underscore the importance of healthcare professionals being aware of variations in hormone-binding proteins during GAHT. Such alterations can affect hormone measurements of persons using GAHT, leading to possible misdiagnosis, and may also have broader implications for patient care, including the management of conditions like hypothyroidism and the interpretation of cortisol-related diagnostic tests. Measuring free hormone concentrations would be beneficial in certain contexts, for example, measuring free cortisol instead of total cortisol will provide a more accurate reflection of biologically active cortisol. However, due to the very low concentration, measuring free hormones is challenging and may therefore not necessarily lead to an improvement in diagnostic accuracy.41 While prior studies have proposed reference values for CBG and cortisol in women using ethinylestradiol,42 further research is needed to determine whether distinct reference intervals for hormone-binding proteins should be established for individuals receiving GAHT. This may help improve diagnostic accuracy and individualized care in this population.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Authors’ contributions
Theresa A. Stangl (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Project administration [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Chantal M. Wiepjes (Methodology [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal]), Annemieke C. Heijboer (Conceptualization [equal], Data curation [equal], Investigation [equal], Methodology [equal], Supervision [equal], Writing—review & editing [equal]), and Martin den Heijer (Conceptualization [equal], Data curation [equal], Methodology [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal])
Data availability
Data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Author notes
Conflict of interest: None declared.



