-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah Nordmeyer, Peter Murin, Antonia Schulz, Friederike Danne, Johannes Nordmeyer, Johanna Kretzschmar, Daria Sumbadze, Katharina Rose Luise Schmitt, Oliver Miera, Mi-Young Cho, Nicodeme Sinzobahamvya, Felix Berger, Stanislav Ovroutski, Joachim Photiadis, Results of aortic valve repair using decellularized bovine pericardium in congenital surgery, European Journal of Cardio-Thoracic Surgery, Volume 54, Issue 6, December 2018, Pages 986–992, https://doi.org/10.1093/ejcts/ezy181
Close - Share Icon Share
Abstract
The search for an optimal patch material for aortic valve reconstruction (AVR) is an ongoing challenge. In this study, we report our experience of AVR using decellularized bovine pericardial patch material in congenital heart surgery.
Data of 40 consecutive patients who underwent AVR using the CardioCel® patch (Admedus Regen Pty Ltd, Perth, WA, Australia) between February 2014 and August 2016 were retrospectively reviewed. The median age of the patients at operation was 9 (2–34) years, and 18 patients were younger than 7 years. Twenty-six patients initially presented with aortic valve insufficiency (AI) and 14 with stenosis. Clinical and echocardiographic data were available until August 2017 for a median postoperative follow-up (FU) of 22 (6–42) months.
Nine of 40 (23%) patients experienced an event during FU (death: n = 1, 2.5%; reoperation: n = 8, 20%). Overall, the probability of freedom from reoperation or death was 97 ± 3%, 76 ± 9% and 57 ± 12% at 12, 24 and 36 months of FU, respectively. Reason for reoperation was stenosis in 3 (37.5%) patients, insufficiency in 4 (50%) patients and 1 (12.5%) patient was diagnosed with aortic valve endocarditis. Of the remaining 31 patients, 2 patients are scheduled for reoperation (aortic valve stenosis: n = 1 and AI: n = 1) and 9 patients exhibit worsening of aortic valve function with moderate AI. Freedom from developing combined end point [death/reoperation/moderate degree of aortic valve dysfunction (aortic valve stenosis, AI)] after AVR was 92 ± 5%, 55 ± 9% and 28 ± 9% at 12, 24 and 36 months, respectively.
AVR using decellularized bovine pericardial patch material in patients with congenital aortic valve disease show unsatisfactory results within the first 3 years of FU.
INTRODUCTION
Surgical management of aortic valve disease in childhood is hampered by technical, material and growth limitations. Valve replacement remains a temporary solution with the need for rereplacement during follow-up (FU) [1]. Known limitations of valve replacement are the lack of growth potential and early degeneration of biological prostheses and the unavailability of small size prostheses and the need for rigid anticoagulation of mechanical prostheses. Concerning the Ross procedure, good results have been reported in terms of autograft function. However, reoperations are still necessary for either dilation of the neoaortic root or dysfunction of the graft implanted in the pulmonary position [2, 3]. Aortic valvuloplasty including cusp reconstruction, especially in paediatric patients, has become more and more popular during the last decades. Nowadays, novel surgical techniques and growing experience allow the application of challenging surgical procedures on small-sized aortic roots and leaflets, with the aim of postponing aortic valve replacement as long as possible. However, the lack of optimal tissue for cusp repair still limits the durability of the reconstruction outcomes [1, 4].
We opt for aortic valve repair as often as possible; thus, we are regularly confronted with the choice of adequate tissue for cusp reconstruction. Recently, decellularized bovine pericardial patch material was introduced, showing promising early results [5–7]; this study reports our initial experience with this novel patch material for aortic valve repair in congenital heart disease.
MATERIALS AND METHODS
Study design
This retrospective study covers the period from February 2014 to August 2017. Data of all consecutive patients who underwent aortic valve reconstruction (AVR) using decellularized bovine pericardial patch material were reviewed. Emphasis was placed on the combined end points of reoperation or death and echocardiographic parameters early after operation and at FU for the assessment of aortic valve appearance and performance and left ventricular function. Informed consent to review the outcome data was waived by the institutional review board and was not required for this retrospective study. Patients and/or guardians of the patients consented to the operation according to a standardized protocol and, additionally, for the use of the novel bovine pericardial patch material for reconstruction purposes of the aortic valve. As this was not a prospective trial, there were no specific patient selection criteria. The surgical team decided on the material that would be used for AVR on an individual basis. Factors that were taken into account for this decision included the underlying aortic valve morphology and the planned reconstruction technique (e.g. cusp extension versus cusp replacement). Additionally, we retrospectively report the end point of reoperation or death in patients who received AVR with patch material other than decellularized bovine pericardial patch material within this time period at our institution.
Patient characteristics and management
Forty patients [median age 9 (1.7–34) years, median weight 26 (9–75) kg] underwent AVR using decellularized bovine pericardial patch material at our institution between February 2014 and August 2016. Eighteen (45%) children were younger than 7 years, and 10 (25%) patients were older than 18 years.
Patient characteristics are listed in Table 1. Fifteen patients had a previous aortic valve surgery, and another 14 patients underwent previous transcatheter balloon aortic valvuloplasty previously. At the time of operation, 14 patients experienced predominant aortic valve stenosis (AS), whereas predominant aortic valve insufficiency (AI) was observed in 26 patients. Lesions consisted of native stenosis (n = 7), post-surgical or postinterventional stenosis (n = 7), native regurgitation (n = 10), post-surgical or postinterventional regurgitation (n = 16). Aortic valve diameters are summarized according to AS and AI in Table 2. Native aortic valves were unicuspid in 15 (38%) cases, bicuspid in 14 (35%) cases and tricuspid in 11 (28%) cases.
. | ||
|---|---|---|
| Preoperative data . | AS (n = 14) . | AI (n = 26) . |
| Female gender, n (%) | 9 (64) | 5 (19) |
| Age (years), median (range) | 11 (2–34) | 6 (2–27) |
| Weight (kg), median (range) | 33 (15–75) | 21 (9–74) |
| Length (cm), median (range) | 147 (98–174) | 123 (80–181) |
| Body surface area (m2), median (range) | 1 (0.6–1.9) | 0.9 (0.4–1.9) |
| LVEF (%), median (range) | 75 (64–83) | 64 (47–76) |
| LVEDD (mm), median (range) | 40 (33–51) | 46 (30–67) |
| Mean pressure across aortic valve (mmHg), median (range) | 47 (30–70) | 12 (2–39) |
| Percentage of moderate or severe AI (%), median (range) | 36 | 100 |
. | ||
|---|---|---|
| Preoperative data . | AS (n = 14) . | AI (n = 26) . |
| Female gender, n (%) | 9 (64) | 5 (19) |
| Age (years), median (range) | 11 (2–34) | 6 (2–27) |
| Weight (kg), median (range) | 33 (15–75) | 21 (9–74) |
| Length (cm), median (range) | 147 (98–174) | 123 (80–181) |
| Body surface area (m2), median (range) | 1 (0.6–1.9) | 0.9 (0.4–1.9) |
| LVEF (%), median (range) | 75 (64–83) | 64 (47–76) |
| LVEDD (mm), median (range) | 40 (33–51) | 46 (30–67) |
| Mean pressure across aortic valve (mmHg), median (range) | 47 (30–70) | 12 (2–39) |
| Percentage of moderate or severe AI (%), median (range) | 36 | 100 |
AI: aortic valve insufficiency; AS: aortic valve stenosis; IVSD: interventricular septal end-diastolic dimension; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction.
. | ||
|---|---|---|
| Preoperative data . | AS (n = 14) . | AI (n = 26) . |
| Female gender, n (%) | 9 (64) | 5 (19) |
| Age (years), median (range) | 11 (2–34) | 6 (2–27) |
| Weight (kg), median (range) | 33 (15–75) | 21 (9–74) |
| Length (cm), median (range) | 147 (98–174) | 123 (80–181) |
| Body surface area (m2), median (range) | 1 (0.6–1.9) | 0.9 (0.4–1.9) |
| LVEF (%), median (range) | 75 (64–83) | 64 (47–76) |
| LVEDD (mm), median (range) | 40 (33–51) | 46 (30–67) |
| Mean pressure across aortic valve (mmHg), median (range) | 47 (30–70) | 12 (2–39) |
| Percentage of moderate or severe AI (%), median (range) | 36 | 100 |
. | ||
|---|---|---|
| Preoperative data . | AS (n = 14) . | AI (n = 26) . |
| Female gender, n (%) | 9 (64) | 5 (19) |
| Age (years), median (range) | 11 (2–34) | 6 (2–27) |
| Weight (kg), median (range) | 33 (15–75) | 21 (9–74) |
| Length (cm), median (range) | 147 (98–174) | 123 (80–181) |
| Body surface area (m2), median (range) | 1 (0.6–1.9) | 0.9 (0.4–1.9) |
| LVEF (%), median (range) | 75 (64–83) | 64 (47–76) |
| LVEDD (mm), median (range) | 40 (33–51) | 46 (30–67) |
| Mean pressure across aortic valve (mmHg), median (range) | 47 (30–70) | 12 (2–39) |
| Percentage of moderate or severe AI (%), median (range) | 36 | 100 |
AI: aortic valve insufficiency; AS: aortic valve stenosis; IVSD: interventricular septal end-diastolic dimension; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction.
Preoperative echocardiographic aortic valve parameters according to AS or AI
. | ||
|---|---|---|
| Preoperative data (mm) . | AS (n = 14) . | AI (n = 26) . |
| Aortic annulus | 17 (13–25) | 19 (11–37) |
| Sinus of Valsalva | 25 (18–30) | 24 (13–38) |
| Sinotubular junction | 19 (12–29) | 21 (12–32) |
| Aorta ascendens | 22 (13–37) | 22 (15–34) |
| z-score | ||
| Aortic annulus | 0.9 (−0.9 to 3.7) | 2.6 (−1.34 to 5.9) |
| Sinus of Valsalva | −0.4 (−2.9 to 1.6) | 1.3 (−2.8 to 4.9) |
| Sinotubular junction | −1.3 (−4.3 to 2.5) | 1.3 (−1.8 to 4.2) |
| Aorta ascendens | 0.5 (−2.2 to 6.4) | 1.6 (−0.4 to 5.8) |
. | ||
|---|---|---|
| Preoperative data (mm) . | AS (n = 14) . | AI (n = 26) . |
| Aortic annulus | 17 (13–25) | 19 (11–37) |
| Sinus of Valsalva | 25 (18–30) | 24 (13–38) |
| Sinotubular junction | 19 (12–29) | 21 (12–32) |
| Aorta ascendens | 22 (13–37) | 22 (15–34) |
| z-score | ||
| Aortic annulus | 0.9 (−0.9 to 3.7) | 2.6 (−1.34 to 5.9) |
| Sinus of Valsalva | −0.4 (−2.9 to 1.6) | 1.3 (−2.8 to 4.9) |
| Sinotubular junction | −1.3 (−4.3 to 2.5) | 1.3 (−1.8 to 4.2) |
| Aorta ascendens | 0.5 (−2.2 to 6.4) | 1.6 (−0.4 to 5.8) |
Data are presented as median (range).
AI: aortic valve insufficiency; AS: aortic valve stenosis.
Preoperative echocardiographic aortic valve parameters according to AS or AI
. | ||
|---|---|---|
| Preoperative data (mm) . | AS (n = 14) . | AI (n = 26) . |
| Aortic annulus | 17 (13–25) | 19 (11–37) |
| Sinus of Valsalva | 25 (18–30) | 24 (13–38) |
| Sinotubular junction | 19 (12–29) | 21 (12–32) |
| Aorta ascendens | 22 (13–37) | 22 (15–34) |
| z-score | ||
| Aortic annulus | 0.9 (−0.9 to 3.7) | 2.6 (−1.34 to 5.9) |
| Sinus of Valsalva | −0.4 (−2.9 to 1.6) | 1.3 (−2.8 to 4.9) |
| Sinotubular junction | −1.3 (−4.3 to 2.5) | 1.3 (−1.8 to 4.2) |
| Aorta ascendens | 0.5 (−2.2 to 6.4) | 1.6 (−0.4 to 5.8) |
. | ||
|---|---|---|
| Preoperative data (mm) . | AS (n = 14) . | AI (n = 26) . |
| Aortic annulus | 17 (13–25) | 19 (11–37) |
| Sinus of Valsalva | 25 (18–30) | 24 (13–38) |
| Sinotubular junction | 19 (12–29) | 21 (12–32) |
| Aorta ascendens | 22 (13–37) | 22 (15–34) |
| z-score | ||
| Aortic annulus | 0.9 (−0.9 to 3.7) | 2.6 (−1.34 to 5.9) |
| Sinus of Valsalva | −0.4 (−2.9 to 1.6) | 1.3 (−2.8 to 4.9) |
| Sinotubular junction | −1.3 (−4.3 to 2.5) | 1.3 (−1.8 to 4.2) |
| Aorta ascendens | 0.5 (−2.2 to 6.4) | 1.6 (−0.4 to 5.8) |
Data are presented as median (range).
AI: aortic valve insufficiency; AS: aortic valve stenosis.
All operations were performed via median sternotomy. During cardiopulmonary bypass, median time of perfusion, reperfusion and aortic cross-clamp time were 139 (99–352) min, 28 (11–107) min and 93 (61–227) min, respectively. The immediate postoperative result was evaluated using intraoperative transoesophageal echocardiography. Overall, in 7 cases, intraoperative rereconstruction was necessary due to a suboptimal initial result. Three experienced surgeons performed all operations.
Table 3 presents details on the approaches of aortic valve cusp surgery. In this cohort, all patients received leaflet extension of at least 1 leaflet and 13 patients received complete replacement of 1 leaflet next to a leaflet extension. Thirteen leaflets were replaced completely with bovine pericardial patch material and 64 leaflets were augmented. The reduction in the ascending aorta was performed in 6 patients. At the end of the procedure, 31 valves were tricuspid, and the remaining 9 were bicuspid. Conversion of a bicuspid valve to a tricuspid valve was performed by the creation of a new leaflet of CardioCel® and leaflet extension of at least 1 leaflet or by leaflet extensions only.
Valve cusp surgery performed in patients using the CardioCel® patch material
. | |||
|---|---|---|---|
| Techniques . | Right coronary cusp . | Left coronary cusp . | Non-coronary cusp . |
| Replacement | 12 | 0 | 1 |
| Augmentation | 23 | 18 | 23 |
. | |||
|---|---|---|---|
| Techniques . | Right coronary cusp . | Left coronary cusp . | Non-coronary cusp . |
| Replacement | 12 | 0 | 1 |
| Augmentation | 23 | 18 | 23 |
Valve cusp surgery performed in patients using the CardioCel® patch material
. | |||
|---|---|---|---|
| Techniques . | Right coronary cusp . | Left coronary cusp . | Non-coronary cusp . |
| Replacement | 12 | 0 | 1 |
| Augmentation | 23 | 18 | 23 |
. | |||
|---|---|---|---|
| Techniques . | Right coronary cusp . | Left coronary cusp . | Non-coronary cusp . |
| Replacement | 12 | 0 | 1 |
| Augmentation | 23 | 18 | 23 |
Median stay in the intensive care unit and hospital stay was 21 (16–121) h and 7 (5–12) days, respectively. All patients received Aspirin medication (1 mg/kg/day) during the 1st year after AVR.
Within the same time period, 15 patients received AVR with patch material other than decellularized bovine pericardial patch material [Matrix patch (cell-free equine pericardial patch) = 2, autologous pericardium = 12, Cormatrix (porcine small intestinal submucosa) = 1]. The median age and body weight of the patients at operation were 9 (0–34) years and 25 (7–118) kg, respectively.
Decellularized bovine pericardium
The decellularized bovine pericardial patch [CardioCel patch (Admedus Regen Pty Ltd, Perth, WA, Australia)] is manufactured from bovine spongiform encephalopathy-free pericardium. Several tissue-engineering processes that include steps to remove lipids, cells and cell remnants, nucleic acids (DNA and RNA) and Gal epitopes are involved in the manufacturing. In addition, cross-linking is achieved using an ultra-low engineered glutaraldehyde concentration to minimize glutaraldehyde cytotoxicity levels (Allied Healthcare Group Ltd. proprietary). Additionally, the ADAPT® anticalcification process and a non-glutaraldehyde sterilization are performed, and the storage solution possesses anticalcification properties [5].
Echocardiography
During routine measurements, evaluation of AS was performed using continuous-wave Doppler measurements for the acquisition of mean pressure gradients across the aortic valve. AI was graded as severe (Grade 3), moderate (Grade 2), mild (Grade 1) or none (0) according to colour Doppler assessment. Significant stenosis is defined as mean aortic transvalvular gradient of 40 mmHg, significant regurgitation as Grade 3 (severe) or Grade 2 (moderate) valve insufficiency. Unfavourable related events such as infective endocarditis, valve thrombosis or calcification were specifically looked for.
Statistical analysis
Statistical analysis was performed using GraphPad PRISM Version 6.0f. Probability of freedom from event occurrence was estimated according to Kaplan-Meier method. A P-value <0.05 was considered statistically significant.
RESULTS
Freedom from reoperation or death
Nine of 40 (23%) patients experienced an event during FU (death: n = 1, 2.5%; reoperation: n = 8, 20%). Overall, the probability of freedom from reoperation or death was 97 ± 3%, 76 ± 9% and 57 ± 12% at 12, 24 and 36 months of FU, respectively (Fig. 1). Reason for reoperation was stenosis in 3 (37.5%) patients, insufficiency in 4 (50%) patients and 1 (12.5%) patient was diagnosed with endocarditis. Original diagnoses, surgical treatment and time and reason for reoperation or death are shown in Table 4. The median postoperative FU for the entire cohort was 22 (6–42) months [patients aged <18 years: 21 months (6–42), patients aged >18 years: 24 months (11–35)].
Baseline characteristics and follow-up details in patients, who experienced an event
. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age at operation (years) . | Initial DX . | Preoperative AV anatomy . | Postoperative AV anatomy . | Mean gradient (mmHg) at discharge . | Grade of AI at discharge . | DX at event . | FU until event (months) . | Thickened valves on echo . |
| 1 | 9 | AS | Unicuspid | Bicuspid | 30 | 0 | AS | 27 | Yes |
| 2 | 2 | AI | Tricuspid | Tricuspid | 5 | 1 | AI | 29 | Yes |
| 3 | 6 | AI | Bicuspid | Tricuspid | 7 | 0 | Death | 28 | Yes |
| 4 | 6 | AI | Tricuspid | Tricuspid | 3 | 1 | AI | 23 | Yes |
| 5 | 5 | AI | Unicuspid | Tricuspid | 2 | 1 | Endocarditis | 20 | Yes |
| 6 | 3 | AI | Bicuspid | Tricuspid | 4 | 1 | AS | 25 | Yes |
| 7 | 2 | AI | Bicuspid | Tricuspid | 13 | 1 | AS | 23 | Yes |
| 8 | 15 | AS | Unicuspid | Tricuspid | 6 | 1 | AI | 15 | Yes |
| 9 | 27 | AS | Unicuspid | Tricuspid | 14 | 1 | AI | 11 | Yes |
. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age at operation (years) . | Initial DX . | Preoperative AV anatomy . | Postoperative AV anatomy . | Mean gradient (mmHg) at discharge . | Grade of AI at discharge . | DX at event . | FU until event (months) . | Thickened valves on echo . |
| 1 | 9 | AS | Unicuspid | Bicuspid | 30 | 0 | AS | 27 | Yes |
| 2 | 2 | AI | Tricuspid | Tricuspid | 5 | 1 | AI | 29 | Yes |
| 3 | 6 | AI | Bicuspid | Tricuspid | 7 | 0 | Death | 28 | Yes |
| 4 | 6 | AI | Tricuspid | Tricuspid | 3 | 1 | AI | 23 | Yes |
| 5 | 5 | AI | Unicuspid | Tricuspid | 2 | 1 | Endocarditis | 20 | Yes |
| 6 | 3 | AI | Bicuspid | Tricuspid | 4 | 1 | AS | 25 | Yes |
| 7 | 2 | AI | Bicuspid | Tricuspid | 13 | 1 | AS | 23 | Yes |
| 8 | 15 | AS | Unicuspid | Tricuspid | 6 | 1 | AI | 15 | Yes |
| 9 | 27 | AS | Unicuspid | Tricuspid | 14 | 1 | AI | 11 | Yes |
AI: aortic valve insufficiency; AS: aortic valve stenosis; DX: diagnosis; FU: follow-up.
Baseline characteristics and follow-up details in patients, who experienced an event
. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age at operation (years) . | Initial DX . | Preoperative AV anatomy . | Postoperative AV anatomy . | Mean gradient (mmHg) at discharge . | Grade of AI at discharge . | DX at event . | FU until event (months) . | Thickened valves on echo . |
| 1 | 9 | AS | Unicuspid | Bicuspid | 30 | 0 | AS | 27 | Yes |
| 2 | 2 | AI | Tricuspid | Tricuspid | 5 | 1 | AI | 29 | Yes |
| 3 | 6 | AI | Bicuspid | Tricuspid | 7 | 0 | Death | 28 | Yes |
| 4 | 6 | AI | Tricuspid | Tricuspid | 3 | 1 | AI | 23 | Yes |
| 5 | 5 | AI | Unicuspid | Tricuspid | 2 | 1 | Endocarditis | 20 | Yes |
| 6 | 3 | AI | Bicuspid | Tricuspid | 4 | 1 | AS | 25 | Yes |
| 7 | 2 | AI | Bicuspid | Tricuspid | 13 | 1 | AS | 23 | Yes |
| 8 | 15 | AS | Unicuspid | Tricuspid | 6 | 1 | AI | 15 | Yes |
| 9 | 27 | AS | Unicuspid | Tricuspid | 14 | 1 | AI | 11 | Yes |
. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age at operation (years) . | Initial DX . | Preoperative AV anatomy . | Postoperative AV anatomy . | Mean gradient (mmHg) at discharge . | Grade of AI at discharge . | DX at event . | FU until event (months) . | Thickened valves on echo . |
| 1 | 9 | AS | Unicuspid | Bicuspid | 30 | 0 | AS | 27 | Yes |
| 2 | 2 | AI | Tricuspid | Tricuspid | 5 | 1 | AI | 29 | Yes |
| 3 | 6 | AI | Bicuspid | Tricuspid | 7 | 0 | Death | 28 | Yes |
| 4 | 6 | AI | Tricuspid | Tricuspid | 3 | 1 | AI | 23 | Yes |
| 5 | 5 | AI | Unicuspid | Tricuspid | 2 | 1 | Endocarditis | 20 | Yes |
| 6 | 3 | AI | Bicuspid | Tricuspid | 4 | 1 | AS | 25 | Yes |
| 7 | 2 | AI | Bicuspid | Tricuspid | 13 | 1 | AS | 23 | Yes |
| 8 | 15 | AS | Unicuspid | Tricuspid | 6 | 1 | AI | 15 | Yes |
| 9 | 27 | AS | Unicuspid | Tricuspid | 14 | 1 | AI | 11 | Yes |
AI: aortic valve insufficiency; AS: aortic valve stenosis; DX: diagnosis; FU: follow-up.
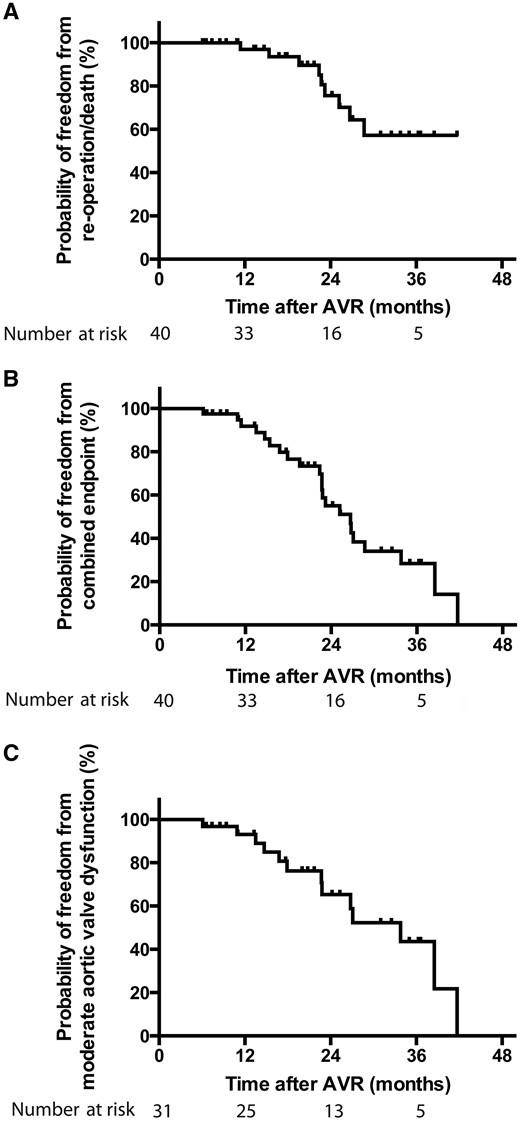
The Kaplan–Meier method. (A) Probability of freedom from death or reoperation (40 patients initially), (B) probability of freedom from combined end point (death or reoperation and/or moderate aortic valve dysfunction) (40 patients initially) and (C) probability of freedom from moderate aortic valve dysfunction (31 patients without reoperation or death). AVR: aortic valve reconstruction.
Probability of freedom from reoperation or death is presented for different groups separately (Fig. 2) (no significant differences were observed between groups):
Patients <18 years of age: 100%, 70 ± 12% and 46 ± 14% at 12, 24 and 36 months of FU, respectively.
Patients >18 years of age: 90 ± 10% at 12, 24 and 36 months of FU, respectively.
Patients with initial AI: 100%, 71 ± 12% and 52 ± 15% at 12, 24 and 36 months of FU, respectively.
Patients with initial AS: 100%, 83 ± 11% and 69 ± 16% at 12, 24 and 36 months of FU, respectively.
Patients with a unicuspid aortic valve: 100%, 74 ± 16% and 56 ± 20% at 12, 24 and 36 months of FU, respectively.
Patients with a bicuspid aortic valve: 100%, 79 ± 13% and 68 ± 16% at 12, 24 and 36 months of FU, respectively.
Patients with a tricuspid aortic valve: 90 ± 10%, 72 ± 18% and 36 ± 27% at 12, 24 and 36 months of FU, respectively.
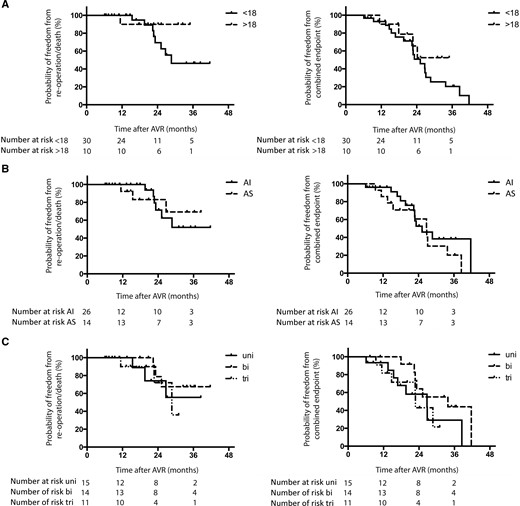
The Kaplan–Meier method. Probability of freedom from death or reoperation and probability of freedom from combined end point (death or reoperation and/or moderate aortic valve dysfunction) in (A) patients >18 years of age and <18 years of age at operation, (B) patients with initial AS and AI and (C) patients with initial uni-, bi- or triaortic valve morphology. AI: aortic valve insufficiency; AS: aortic valve stenosis; AVR: aortic valve reconstruction; Bi: bicuspid; Tri: tricuspid; Uni: unicuspid.
The patient who died experienced sudden left heart failure 28 months after AVR. Cardiac autopsy showed calcified degeneration of the aortic valve with concomitant non-infectious endocarditis, especially of the left aortic cusp and right aortic cusp. At AVR, all 3 cusps had been augmented. FU at Month 22 had shown reasonable results with mild AS and AI.
In patients who underwent AVR with patch material other than decellularized bovine pericardial patch material, the median postoperative FU was 50 (1–61) months and freedom from reoperation or death was 93 ± 6% at 12, 24 and 36 months.
Aortic valve cusp appearance at reoperation
All patients with stenosis and indication for reoperation showed immobile, thickened valves on echocardiography, and the same was described with regard to their intraoperative appearance (Fig. 3). One patient with AI 11 months after the initial repair showed only mildly thickened valves on echocardiography; however, 1 cusp was detached from the aortic wall, and thus, relevant AI was present. The remaining patients with AI and indication for reoperation showed valve cusp thickening on echocardiography, and the patient with endocarditis showed a mobile vegetation at 1 aortic valve cusp without global significant AS or AI.
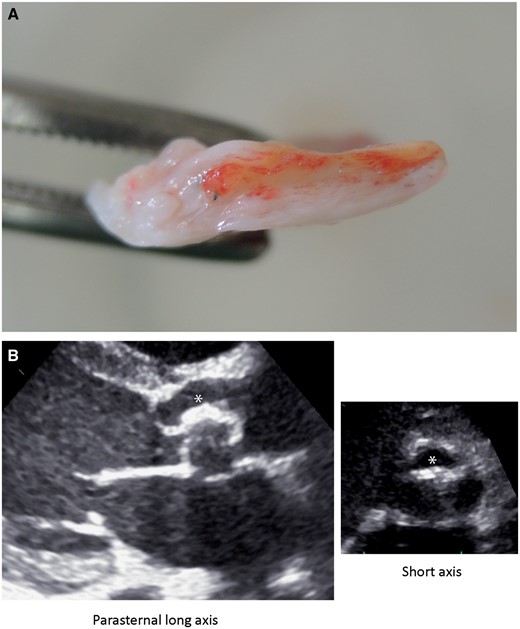
(A) An image of an explanted bovine pericardial patch with marked proliferation (white) surrounding the initial patch (yellow). (B) An echocardiographic image of a thickened valve leaflet (asterisks).
Aortic valve function and freedom from developing significant aortic valve dysfunction
Probability of freedom from developing combined end point [death/reoperation/moderate degree of aortic valve dysfunction (AS, AI)] after AVR was 92 ± 5%, 55 ± 9% and 28 ± 9% at 12, 24 and 36 months, respectively (Fig. 1B). Echocardiographic findings concerning AS and AI at the time of hospital discharge and during FU for all 40 patients are summarized in Table 5.
Grade of aortic valve insufficiency and aortic mean transvalvular gradient in patients during follow-up according to the initial AI or AS
. | ||
|---|---|---|
| . | At the time of discharge from the hospital . | At the last follow-up . |
| Grade of AI | ||
| Patients with initial AI (n = 26) | ||
| 0 | 6 | 4 |
| 1 | 17 | 14 |
| 2 | 3 | 7 |
| 3 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| 0 | 4 | 1 |
| 1 | 9 | 7 |
| 2 | 1 | 4 |
| 3 | 0 | 2 |
| Aortic mean transvalvular gradient (mmHg) | ||
| Patients with initial AI (n = 26) | ||
| <10 | 16 | 11 |
| 10–30 | 10 | 13 |
| 31–49 | 0 | 1 |
| ≥50 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| <10 | 4 | 0 |
| 10–30 | 10 | 11 |
| 31–49 | 0 | 3 |
| ≥50 | 0 | 0 |
. | ||
|---|---|---|
| . | At the time of discharge from the hospital . | At the last follow-up . |
| Grade of AI | ||
| Patients with initial AI (n = 26) | ||
| 0 | 6 | 4 |
| 1 | 17 | 14 |
| 2 | 3 | 7 |
| 3 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| 0 | 4 | 1 |
| 1 | 9 | 7 |
| 2 | 1 | 4 |
| 3 | 0 | 2 |
| Aortic mean transvalvular gradient (mmHg) | ||
| Patients with initial AI (n = 26) | ||
| <10 | 16 | 11 |
| 10–30 | 10 | 13 |
| 31–49 | 0 | 1 |
| ≥50 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| <10 | 4 | 0 |
| 10–30 | 10 | 11 |
| 31–49 | 0 | 3 |
| ≥50 | 0 | 0 |
AI: aortic valve insufficiency; AS: aortic valve stenosis.
Grade of aortic valve insufficiency and aortic mean transvalvular gradient in patients during follow-up according to the initial AI or AS
. | ||
|---|---|---|
| . | At the time of discharge from the hospital . | At the last follow-up . |
| Grade of AI | ||
| Patients with initial AI (n = 26) | ||
| 0 | 6 | 4 |
| 1 | 17 | 14 |
| 2 | 3 | 7 |
| 3 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| 0 | 4 | 1 |
| 1 | 9 | 7 |
| 2 | 1 | 4 |
| 3 | 0 | 2 |
| Aortic mean transvalvular gradient (mmHg) | ||
| Patients with initial AI (n = 26) | ||
| <10 | 16 | 11 |
| 10–30 | 10 | 13 |
| 31–49 | 0 | 1 |
| ≥50 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| <10 | 4 | 0 |
| 10–30 | 10 | 11 |
| 31–49 | 0 | 3 |
| ≥50 | 0 | 0 |
. | ||
|---|---|---|
| . | At the time of discharge from the hospital . | At the last follow-up . |
| Grade of AI | ||
| Patients with initial AI (n = 26) | ||
| 0 | 6 | 4 |
| 1 | 17 | 14 |
| 2 | 3 | 7 |
| 3 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| 0 | 4 | 1 |
| 1 | 9 | 7 |
| 2 | 1 | 4 |
| 3 | 0 | 2 |
| Aortic mean transvalvular gradient (mmHg) | ||
| Patients with initial AI (n = 26) | ||
| <10 | 16 | 11 |
| 10–30 | 10 | 13 |
| 31–49 | 0 | 1 |
| ≥50 | 0 | 1 |
| Patients with initial AS (n = 14) | ||
| <10 | 4 | 0 |
| 10–30 | 10 | 11 |
| 31–49 | 0 | 3 |
| ≥50 | 0 | 0 |
AI: aortic valve insufficiency; AS: aortic valve stenosis.
Probability of freedom from developing combined end point is presented for different groups separately (Fig. 2) (no significant differences are observed between groups):
Patients <18 years of age: 93 ± 5%, 51 ± 11% and 20 ± 9% at 12, 24 and 36 months of FU, respectively.
Patients >18 years of age: 90 ± 10%, 66 ± 16% and 53 ± 18% at 12, 24 and 36 months of FU, respectively.
Patients with AI: 96 ± 4%, 53 ± 12% and 38 ± 12% at 12, 24 and 36 months of FU, respectively.
Patients with AS: 86 ± 9%, 61 ± 14% and 20 ± 13% at 12, 24 and 36 months of FU, respectively.
Patients with a unicuspid aortic valve: 93 ± 6%, 58 ± 15% and 29 ± 16% at 12, 24 and 36 months of FU, respectively.
Patients with a bicuspid aortic valve: 100%, 64 ± 14% and 44 ± 16% at 12, 24 and 36 months of FU, respectively.
Patients with a tricuspid aortic valve: 82 ± 12%, 43 ± 18% and 22 ± 18% at 12, 24 and 36 months of FU, respectively.
DISCUSSION
Decellularized bovine pericardial patch material has been previously used for the closure of cardiac septal defect [6]. Mazzitelli et al. [7] recently reported its use for total replacement of aortic valve cusps in 3 selected patients with congenital aortic valve disease, with promising results; however, long-term results were not reported.
This study constitutes the first relatively large series of 40 patients undergoing AVR using decellularized bovine pericardial patch material.
Reported advantages of this novel tissue are reduced immunogenicity, less calcification and repopulation of the decellularized material with host-specific cells [6, 8, 9]. However, the present work shows worrying results with regard to longevity of aortic valve function after AVR in patients with congenital aortic valve disease when bovine pericardial patch material was used; 2.5% mortality, 2.5% aortic valve endocarditis, and freedom from reoperation or death at 36 months of FU was 57%.
Aortic valve repair with cusp extension in paediatric population with median age <18 years is not reported extensively in the literature. However, varying results were found on AVR using autologous pericardial patch material [10–12]. Very detailed information about the incidence of valve-related complications after aortic valve repair in 640 patients, in whom at least 30% had reported congenitally malformed aortic valves (unicuspid, bicuspid or quadricuspid), is described in a large study by Aicher et al. [13]. They reported a hospital mortality of 0.8% for isolated aortic valve repair, the incidence of thromboembolism of 0.2% per patient per year and the incidence of endocarditis of 0.16% per patient per year. Furthermore, they described a freedom from reoperation of approximately 98% at 12 months. Freedom from reoperation at 5 and 10 years was 88% and 81% in the bicuspid and 97% and 93% in the tricuspid aortic valves [13], respectively, which is notably better than the results presented in our study. In a large cohort of paediatric patients after AVR with autologous pericardial patch, d’Udekem et al. [1] described an overall freedom from reintervention of 97% at 12 months and 80% at 7-year interval. Early mortality in their cohort was 2% with 3 deaths occurring during the early postoperative period. In direct comparison, we could show similar short-term results with freedom from reoperation or death of 97% after 12 months. However, with freedom from reoperation or death of 57% at 36 months of FU for bovine pericardial patch material used in this study, we described a much worse mid-term outcome. In our patient cohort, cusp extension of at least 1 cusp was performed in all patients. Thus, results in patients with leaflet replacement or leaflet extension cannot be presented separately. Polimenakos et al. [11] described cusp extension valvuloplasty using autologous pericardial tissue for aortic valve repair as a safe option for treating aortic valve disease in patients <19 years of age, albeit with a freedom from reoperation of approximately 70% at 5 years of FU and 60% at 18 years of FU. Our cohort was small and heterogenous with patients with congenital abnormal aortic valves who received AVR with leaflet extensions. Failure of AVR can have multiple reasons; however, outcomes after AVR in this cohort are worse than the results reported in the literature, when compared with patients who were operated on within the same time period with patch material other than bovine pericardial tissue.
Intraoperatively, replaced and/or augmented aortic valve cusps in cases of reoperation in our study cohort appeared as thickened leaflets with reduced mobility. This suggested that the decellularized bovine pericardial patch material did not behave as expected in these patients.
An expected advantage of decellularized bovine pericardial patch material, which is treated with a low-glutaraldehyde solution and stored in a non-glutaraldehyde solution, is a reduced immunogenic response to toxicity, because glutaraldehyde preservation of patch material thought to be associated with degenerative processes and finally, device failure and the need for reintervention [14, 15]. However, in our study cohort, changes in material properties and subsequent aortic valve dysfunction seemed to be the most realistic reason for this dissatisfactory results within the first 3 years of FU. Furthermore, very recent studies have described failure of the CardioCel patch especially in the ascending aortic position in infants [16] and have shown calcification of the CardioCel patch material in Minipigs at 12 months of FU [17].
CONCLUSION
In the younger population, AVR is preferable to any other valve replacement technique as long as it achieves durability and good ventricular function. Cusp reconstruction is technically demanding and requires reliable material. Autologous glutaraldehyde-treated pericardium, when available, is predominantly used, but it has limitations, particularly in terms of calcification. Thus, the search for an optimal patch material is an ongoing challenge. The results using decellularized bovine pericardial patch material that are presented in this series, however, seem worse than published results that were achieved by the use of autologous pericardial patch material. Based on our experience, decellularized bovine pericardial patch material should be used with caution for reconstruction purposes of the aortic valve leaflets in patients with congenital aortic valve pathology.
ACKNOWLEDGEMENTS
The authors thank Vera Bäuml, Ruza Meyer and Anke Olsson for their involvement in collecting the follow-up data.
Conflict of interest: Mi-Young Cho, Joachim Photiadis and Stanislav Ovroutski disclose a financial relationship with Admedus Ltd. All other authors declared no conflict of interest.
REFERENCES
Author notes
The Stanislav Ovroutski and Joachim Photiadis authors contributed equally to this study.




