-
PDF
- Split View
-
Views
-
Cite
Cite
Bahaaldin Alsoufi, Raina Sinha, Courtney McCracken, Janet Figueroa, Firat Altin, Kirk Kanter, Outcomes and risk factors associated with tricuspid valve repair in children with hypoplastic left heart syndrome, European Journal of Cardio-Thoracic Surgery, Volume 54, Issue 6, December 2018, Pages 993–1000, https://doi.org/10.1093/ejcts/ezy198
Close - Share Icon Share
Abstract

Tricuspid valve (TV) competence is important for successful palliation of hypoplastic left heart syndrome (HLHS). We report our experience with TV repair in HLHS patients with a focus on TV and right ventricular (RV) function and associated clinical outcomes.
From 2002 to 2012, 219 neonates with HLHS underwent the Norwood operation. Thirty patients who underwent TV repair at various stages comprised our current series cohort. Echocardiographic and clinical data were reviewed to determine the effectiveness of TV repair and outcomes of the patients.
Thirty patients received TV repair during Norwood (n = 4), Glenn (n = 17) and Fontan (n = 9) operations. Median age at TV repair was 188 days (range 3–1498). Preoperatively, all patients had ≥moderate TV regurgitation and 4 (13%) patients had ≥moderate RV dysfunction. After repair, TV regurgitation was none or trivial (n = 12, 40%), mild (n = 8, 27%), ≥moderate (n = 10, 33%), whereas 10 (33%) had ≥moderate regurgitation at last follow-up; ≥moderate RV dysfunction was present in 5 (17%) patients following TV repair and 10 (33%) patients at last follow-up. Competing risk analysis showed that 10 years following TV repair, 21% of patients had TV reoperation, 18% died or underwent transplantation and 61% were alive without subsequent reoperation. Overall, 10-year survival, transplant-free survival and freedom from second TV reoperation were 89%, 71% and 78%, respectively; ≥moderate RV dysfunction following TV repair was associated with diminished transplant-free survival (P = 0.0277).
Although TV repair is successful in reducing regurgitation in the majority of HLHS patients, outcomes are restricted by limited repair durability with recurrent significant regurgitation in one-third of the patients. RV dysfunction in these patients is progressive and a major determinant of transplant-free survival.
INTRODUCTION
Accomplishment of multistage palliation of hypoplastic left heart syndrome (HLHS) depends on a set of anatomic and haemodynamic criteria [1]. The presence of significant tricuspid valve (TV) regurgitation results in unfavourable conditions that can affect the success of this palliation strategy. Consequently, TV competence in these patients has important implications on both patient survival and functional status [2–7].
The mechanism of TV regurgitation is commonly multifactorial and related to both functional and anatomic elements. TV regurgitation can be exacerbated by shunt-related volume overload leading to annular dilatation and poor leaflet coaptation [2, 4, 8–13]. Additionally, TV competence is intimately related to right ventricular (RV) function and consequently RV dysfunction due to ischaemia, or other factors can be commonly coupled with TV regurgitation [2–4, 12]. Finally, structural abnormalities of the leaflets and subvalvular components of the TV are fairly common in children with HLHS and can lead to TV regurgitation that is not largely affected by volume loading conditions or RV function [8, 14].
Following the Norwood operation, almost 10% of HLHS patients will develop new-onset significant TV regurgitation [2, 5]. Additionally, significant TV regurgitation has been reported to occur in up to 37% of HLHS patients during various points of their multistage palliation [2, 4, 5]. Several groups have advocated TV repair in patients with HLHS and significant TV regurgitation with the hope of improving haemodynamic conditions and subsequently the functional status and late survival [3–5, 10, 15, 16]. A report from Toronto suggested that up to 25% of HLHS survivors would receive TV repair during their lifetime [5].
In this study, we aim to report the characteristics of HLHS patients who required TV repair at our institution during various points of their multistage palliation strategy. We focus on the progression of TV and RV function after TV repair and at subsequent follow-up. We examine variables associated with early and late failures that were defined as second TV reoperation, heart transplantation or death.
PATIENTS AND METHODS
Inclusion criteria
We reviewed the records of all neonates with HLHS (n = 219) who underwent the Norwood operation at Children’s Healthcare of Atlanta, Emory University from 2002 to 2012. Patients with single ventricle anomalies other than HLHS who had the Norwood operation as their initial palliative surgery were excluded. Among those HLHS patients, our current study cohort included the patients who received TV repair during various stages of their multistage palliative strategy (n = 30). They were identified using our institutional surgical database. Demographic, anatomic, clinical, imaging, operative and hospital details were abstracted from medical records for analysis. Approval of this study was obtained from our hospital’s Institutional Review Board, and requirement for individual consent was waived for this observational study.
Follow-up
Echocardiographic data were abstracted from the reports present in our electronic chart system. For the purpose of this study, the last follow-up echocardiogram was the most recent in patients who were alive without subsequent reoperation, the last one prior to death or the last one prior to heart transplantation. Time-related outcomes were determined from recent office visits documented in the Children’s Healthcare of Atlanta electronic chart system or from direct correspondence with other paediatric cardiologists outside the system. Follow-up was 93% complete. Mean follow-up duration was 7.8 ± 3.8 years from the date of TV repair.
Statistical analysis
Statistical analyses were performed using SAS v9.4 (Cary, NC, USA), and statistical significance was assessed at the 0.05 level. Descriptive statistics are presented as counts and percentages for categorical variables and median, interquartile range (25th–75th percentiles) for continuous data with skewed distributions. Time-dependent outcomes (TV reoperation, death, death or heart transplantation) were analysed using survival analysis methods. Kaplan–Meier plots were constructed for each event of interest and stratified by clinical subgroups. The Wilcoxon test was used to compare survival distributions among patient subgroups. When reporting overall estimates of TV reoperation, death and transplantation were considered competing events for TV reoperation with the remaining patients alive without reoperation or transplantation. As a result, we used a non-parametric method to estimate the cumulative incidence function from a specific cause (i.e. TV reoperation) over time using the %CIF macro in SAS. Cumulative incidence at specific points in follow-up is presented with associated 95% confidence intervals (CIs).
RESULTS
Patient characteristics
Thirty patients with HLHS who underwent the Norwood operation between 2002 and 2012 received 37 TV reoperations. There were 16 (53%) males and 14 (47%) females. The anatomic subtype for these 30 patients on the pre-Norwood echocardiogram was mitral stenosis plus aortic atresia (n = 13, 43%), mitral atresia plus aortic atresia (n = 11, 37%), mitral stenosis plus aortic stenosis (n = 4, 13%) and mitral atresia plus aortic stenosis (n = 2, 7%). An atrial septal defect was considered restricted when the defect diameter was <3 mm, when the mean pressure gradient through the defect exceeded 5 mm Hg on colour Doppler, or both and this was the case in 11 (37%) patients. The mean diameter of the ascending aorta was 3.3 mm, and 12 (40%) patients had ascending aortic diameter ≤3 mm. Six of those (20%) patients had ≥moderate TV regurgitation on the pre-Norwood echocardiogram, and only 1 (3%) patient had moderate ventricular dysfunction. At the time of the Norwood operation, 24 (80%) patients received a right ventricle to pulmonary artery conduit (Sano) whereas the remaining 6 (20%) patients received a modified Blalock–Taussig shunt as the source of pulmonary blood flow.
The median age at the time of TV repair was 188 days (range 3–1498). The first TV repair was performed at the time of the Norwood operation (n = 4, 13%), at the time of the Glenn operation (n = 17, 57%), at the time of the Fontan operation (n = 8, 27%), or after the Fontan operation (n = 1, 3%). TV repair was accomplished with a variety of surgical techniques, including DeVega-type annuloplasty (n = 25 of 30), commissuroplasty (n = 12 of 30), closure of leaflet fenestrations (n = 2 of 30) and other (n = 3 of 30), with a number of patients receiving more than 1 repair technique (Table 1).
Operative findings and repair techniques in patients with HLHS who received TV repair at various palliation stages
| . | All patients (n = 30) . | TV repair at time of Norwood (n = 4) . | TV repair at time of Glenn (n = 17) . | TV repair at time or after Fontan (n = 9) . |
|---|---|---|---|---|
| Valve pathology | ||||
| Annular dilatation | 16 | 1 | 10 | 5 |
| Structural anomalies | 14 | 3 | 7 | 4 |
| Repair techniquea | ||||
| De Vega | 25 | 3 | 15 | 7 |
| Commissuroplasty | 12 | 2 | 7 | 3 |
| Fenestration closure | 2 | 1 | 1 | 0 |
| Other | 3 | 0 | 2 | 1 |
| . | All patients (n = 30) . | TV repair at time of Norwood (n = 4) . | TV repair at time of Glenn (n = 17) . | TV repair at time or after Fontan (n = 9) . |
|---|---|---|---|---|
| Valve pathology | ||||
| Annular dilatation | 16 | 1 | 10 | 5 |
| Structural anomalies | 14 | 3 | 7 | 4 |
| Repair techniquea | ||||
| De Vega | 25 | 3 | 15 | 7 |
| Commissuroplasty | 12 | 2 | 7 | 3 |
| Fenestration closure | 2 | 1 | 1 | 0 |
| Other | 3 | 0 | 2 | 1 |
Some patients required more than 1 surgical repair technique.
HLHS: hypoplastic left heart syndrome; TV: tricuspid valve.
Operative findings and repair techniques in patients with HLHS who received TV repair at various palliation stages
| . | All patients (n = 30) . | TV repair at time of Norwood (n = 4) . | TV repair at time of Glenn (n = 17) . | TV repair at time or after Fontan (n = 9) . |
|---|---|---|---|---|
| Valve pathology | ||||
| Annular dilatation | 16 | 1 | 10 | 5 |
| Structural anomalies | 14 | 3 | 7 | 4 |
| Repair techniquea | ||||
| De Vega | 25 | 3 | 15 | 7 |
| Commissuroplasty | 12 | 2 | 7 | 3 |
| Fenestration closure | 2 | 1 | 1 | 0 |
| Other | 3 | 0 | 2 | 1 |
| . | All patients (n = 30) . | TV repair at time of Norwood (n = 4) . | TV repair at time of Glenn (n = 17) . | TV repair at time or after Fontan (n = 9) . |
|---|---|---|---|---|
| Valve pathology | ||||
| Annular dilatation | 16 | 1 | 10 | 5 |
| Structural anomalies | 14 | 3 | 7 | 4 |
| Repair techniquea | ||||
| De Vega | 25 | 3 | 15 | 7 |
| Commissuroplasty | 12 | 2 | 7 | 3 |
| Fenestration closure | 2 | 1 | 1 | 0 |
| Other | 3 | 0 | 2 | 1 |
Some patients required more than 1 surgical repair technique.
HLHS: hypoplastic left heart syndrome; TV: tricuspid valve.
Prior to TV repair, TV regurgitation was moderate (n = 24, 80%) or severe (n = 6, 20%). The right ventricle function was normal (n = 23, 77%), mildly diminished (n = 3, 10%), moderately diminished (n = 3, 10%) or severely diminished (n = 1, 3%).
Operative technique
All operations were performed through median sternotomy, with the majority being redo-sternotomy. Standard cardiopulmonary bypass with aortic and bicaval cannulation was established, and mild hypothermia to 32–34°C was utilized except when repair of the associated arch obstruction required cooling to 18°C. The TV was exposed through a right atriotomy and tested for incompetence by injecting saline into the right ventricle. The majority of patients underwent De Vega annuloplasty. A pledgetted annuloplasty suture of 3-0 or 4-0 polypropylene sutures was started at the anteroseptal commissure and sewn intermittently to the junction of the annulus and the right ventricle along the anterior and posterior leaflets until just past the posteroseptal commissure. Another pledget was placed, and the suturing was reversed along the posterior and anterior leaflets 1–2 mm from the first row alternating the suture technique (taking bites on the second row where the suture was out on the first row and vice versa) until the original pledget at the anteroseptal commissure was reached. The suture was tied down snugly over a Hegar dilator calibrated to 2–3 mm larger than the predicted appropriate pulmonary annulus size derived from published nomograms [16]. Based on anatomic findings, alternative or additional techniques such as commissuroplasty using plegeted polypropylene sutures or direct closure of fenestrations using 5-0 or 6-0 sutures were performed. Following closure of the atrium and deairing manoeuvres, the cross-clamp was released and the simultaneous cardiac operations were completed on a beating heart. The detailed surgical techniques performed during various palliation stages are listed in Table 1.
Outcomes
The echocardiographic results of TV repair are demonstrated in Fig. 1. On the echocardiogram that was performed prior to hospital discharge following TV repair, TV regurgitation was found to be none or trivial (n = 12, 40%), mild (n = 8, 27%) and moderate (n = 10, 33%). The durability of the TV repair is also presented in Fig. 1, and 7 (23%) patients progressed to severe TV regurgitation.
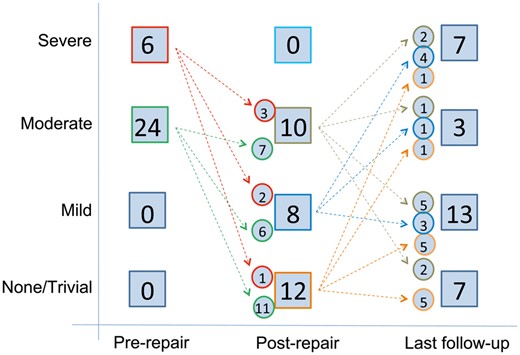
Diagram showing the degree of tricuspid valve (TV) regurgitation prior to TV repair, at the time of hospital discharge following TV repair and at last follow-up. The number of patients in each group and the number of patients crossing over between the groups are indicated.
The progression of right ventricle function is demonstrated in Fig. 2. Although right ventricle function was preserved in the majority of patients prior to TV repair (n = 26, 87%), some patients experienced persistent or worsening dysfunction (n = 5, 17%) as observed on the predischarge echocardiogram with a number of those patients (n = 10, 33%) significantly worsening on subsequent follow-up.
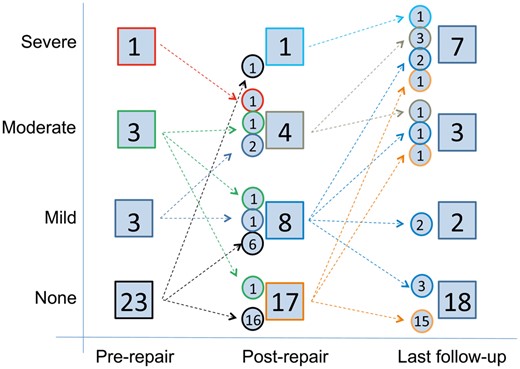
Diagram showing the degree of right ventricular dysfunction prior to tricuspid valve repair, at the time of hospital discharge following tricuspid valve repair and at last follow-up. The number of patients in each group and the number of patients crossing over between the groups are indicated.
The clinical progression of patients following TV repair is demonstrated in the flow chart in Fig. 3. Overall, TV reoperation was required in 6 (20%) patients and eventually, 3 patients underwent TV replacement. Heart transplantation was required in 6 (20%) patients, including 2 who received heart transplantation after TV reoperation. At the last follow-up, 20 (67%) patients were alive and free from heart transplantation.

A flowchart showing the clinical progress following TV repair in 30 patients with hypoplastic left heart syndrome. TV: tricuspid valve.
The hazard of death or heart transplantation was characterized as a high early phase within the first 6 months after TV repair, a lower constant phase that persisted for 1–3 years after surgery and a low late phase then after. The hazard of TV reoperation was characterized by a moderately high early phase that persisted until 2–3 years after initial TV repair. On competing risk analysis, at 1 year following TV repair, 14% of patients had required TV reoperation, 3% had died or undergone transplantation, and 83% were alive without subsequent reoperation. At 10 years following TV repair, 21% of patients had required TV reoperation, 18% had died or undergone transplantation, and 61% were alive without subsequent reoperation (Fig. 4).
![Competing risk analysis of outcomes [second tricuspid valve (TV) reoperation, death and transplantation] after TV repair in 30 children with hypoplastic left heart syndrome. Point zero is the time of TV repair. AVV: atrioventricular (TV) valve, TX: transplantation.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/54/6/10.1093_ejcts_ezy198/2/m_ezy198f4.jpeg?Expires=1750421643&Signature=ZIeymgJAWpNBomNuHzCoNdd4GwYbqnSUcS7SxmbaeI1FAProBCIxLMBkTOkbIppYemGDaPVaSBhazma1XNlp5p0T~pDG6sd1menHDB68iVE3eksX4pn8RA-r19h3h2CK0J1mbE7Xj5~rSJCeOfX3p8HO4k76~BTBmKFhuXUb9iC7X~yaePFfpTyjKrYUacT7Xtoe6rt~569NVFy0itAc804QNMh0NkNiES-amyz54HWnukgRvQcVoRyYv3Ks8rvmTvX2SyCvJmfFHXmyik-T0qttNv3FiccFtQFQetfCRa~gSkATLGPatYLv0LY2FobpILAJHT7pvaWgARDFdhGWXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Competing risk analysis of outcomes [second tricuspid valve (TV) reoperation, death and transplantation] after TV repair in 30 children with hypoplastic left heart syndrome. Point zero is the time of TV repair. AVV: atrioventricular (TV) valve, TX: transplantation.
Overall, 10-year survival was 89% (95% CI 70–96%) whereas transplant-free survival was 71% (95% CI 51–75%) (Figs 5 and 6).
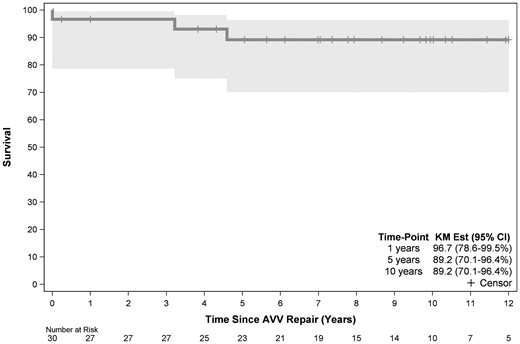
Time-related survival following tricuspid valve repair in 30 children with hypoplastic left heart syndrome. Point zero is the time of tricuspid valve repair. AVV: atrioventricular (TV) valve; CI: confidence interval; KM: Kaplan-Meier.
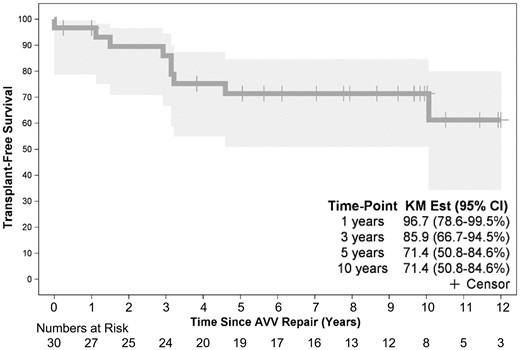
Time-related transplant-free survival following tricuspid valve repair in 30 children with hypoplastic left heart syndrome. Point zero is the time of tricuspid valve repair. AVV: atrioventricular (TV) valve; CI: confidence interval; KM: Kaplan-Meier.
The effect of multiple demographic, anatomic, operative and clinical variables on transplant-free survival was examined. The only factor that was found to be associated with diminished transplant-free survival was ≥moderate right ventricle dysfunction on echocardiograms performed following TV repair (P = 0.0277) (Fig 7). Although transplant-free survival was noted to be higher in patients who received their initial TV repair at or after the Fontan operation, this was not statistically significant in our study (P = 0.1773).
Freedom from TV reoperation was 78% (95% CI 58–90%) at 10 years following initial TV repair. No factors were identified in our analysis to be associated with TV reoperation.
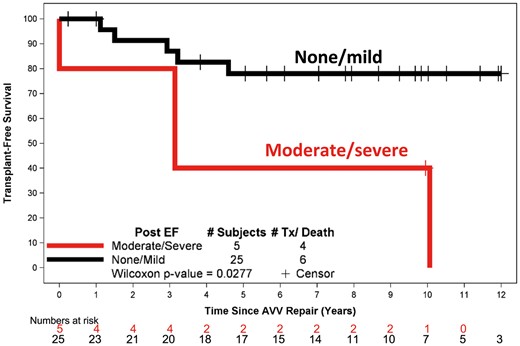
Transplant-free survival stratified post-repair right ventricular function. Point zero is the time of tricuspid valve repair. AVV: atrioventricular (TV) valve; EF: ejection fraction; TX: transplantation..
DISCUSSION
About 13% of all infants with HLHS who underwent the Norwood operation at our institution during the study period underwent TV repair. This number is likely an underestimate, given that there were a number of patients with significant TV regurgitation who had hospital or interstage mortality prior to undergoing TV repair. Our institutional policy, however, is to perform TV repair in Norwood hospital survivors with ≥moderate TV regurgitation.
The mechanism of TV regurgitation in HLHS patients is multifactorial. Volume overload due to the presence of aortopulmonary or right ventricle to pulmonary artery conduit can lead to annular dilatation and subsequent lack of leaflet coaptation [2, 8–13]. Although some reports suggested that aortopulmonary shunts might be associated with higher volume loading of the RV and therefore higher incidence of significant TV regurgitation and the need for TV repair, other recent studies from the Single Ventricle Reconstruction Trial showed that TV regurgitation was comparable between the 2 shunt groups [2, 8–13]. In our current series, 80% of patients who received TV repair had a right ventricle to pulmonary artery conduit at the time of their Norwood operations; however, this is related to the fact that the right ventricle to pulmonary artery conduit was performed in 79% of HLHS patients who received the Norwood operation at our institution during that study period [17]. There is additionally an important relationship between TV performance and RV function. In HLHS patients, the RV is prone to ischaemic damage during the preoperative, intraoperative and postoperative periods. The subsequent remodelling process can lead to the development of RV dysfunction, dilatation and geometrical distortion of the RV and the associated TV annulus, leading to worsening TV regurgitation [2, 6, 7, 9, 11, 12]. This intimate association explains the challenging nature of TV regurgitation in HLHS, especially in those with persistent RV dysfunction. Finally, structural abnormalities of the TV leaflets and subvalvular apparatus are frequently encountered. A prior study from Toronto has demonstrated that up to 85% of patients with single ventricle and atrioventricular valve regurgitation have structural abnormalities and that pure functional regurgitation due to annular dilatation as a result of shunt-related systemic ventricle volume overload was present only in around 15% of those patients [8]. More specific to HLHS patients, a morphological study from London showed that more than one-third of patients with HLHS have significant TV dysplasia [14]. Our operative findings are compatible with those studies, and while annular dilatation was an important component of TV regurgitation in all our patients, a fair number of those patients (14 of 30) had associated structural anomalies such as leaflet prolapse, retraction, fenestration, cleft and other key structural anomalies. Given the multifactorial origin of TV regurgitation in HLHS patients, volume unloading at the time of second-stage bidirectional cavopulmonary connection is expected to improve TV regurgitation in a limited number of those patients with functional regurgitation whereas those with structural abnormalities are expected to continue to have significant regurgitation [9, 11–13, 18, 19]. Although a recent study from Edmonton has shown that volume unloading at the time of bidirectional cavopulmonary connection was associated with increased leaflet coaptation, decreased vena contracta width and decreased RV end-diastolic diameter, the degree of TV regurgitation remained unchanged [13]. Another study from the same group showed that TV repair was associated with additional decrease in TV regurgitation degree [10]. Those findings are parallel to recent findings from the Single Ventricle Reconstruction Trial [9, 11]. Therefore, the policy at our institution is to offer TV repair to HLHS patients with ≥moderate TV regurgitation, provided that they do not have significant prohibitive associated comorbidities. Interestingly, patients who required TV repair at the time of the Norwood operation were more likely to have structural anomalies, while those who required TV repair at the time of the Glenn operation were more likely to have annular dilatation only. The presence of structural anomalies did not influence early repair results, repair longevity or survival in our series, although we are limited by the small cohort size and the multitude of factors that affect those outcomes in our analysis.
Several techniques have been described for TV repair, including bicuspidization, edge-to-edge repair and DeVega partial annuloplasty [2, 3, 16, 20, 21]. DeVega annuloplasty was the most common technique applied at our institution, although one-third of patients required multiple techniques. Although the immediate post-repair TV regurgitation was ≤mild in two-thirds of patients, the durability of TV repair was not as high with the regurgitation progressing to severe in 23% of our patients at last follow-up. The interesting finding in our experience, however, was the lack of a predictive pattern for late TV function, including surgical techniques utilized at the time of TV repair. Interestingly, although a number of patients who had moderate TV regurgitation at the time of discharge showed improvement of the regurgitation degree on subsequent follow-up, a number of patients who had trivial or mild TV regurgitation on the post-repair echocardiogram had significant worsening of the regurgitation degree to moderate or severe. This finding might be explained by the complex multifactorial process that is involved in the development of TV regurgitation in patients with HLHS, especially the association with RV function and remodelling. Ultimately, 6 patients required 7 redo TV reoperations and 2 received TV replacement, with 1 dying and the other receiving a second TV replacement followed by heart transplantation.
Another important issue is the timing of TV repair in HLHS patients. The presence of significant TV regurgitation prior to the Norwood operation poses a medical challenge. TV repair in neonates is a difficult task due to tissue friability, associated risk factors (e.g. low weight) and higher morbidity and mortality associated with increased magnitude of surgery during the Norwood operation [22–24]. Although all 4 patients who received TV repair concomitant with the Norwood procedure survived in our current series, this is not the similar experience in other published reports and we are very cautious in offering TV repair during the Norwood operation. In neonates presenting with severe TV regurgitation and RV dysfunction, very limited options are available at the disposal of surgeons, especially with the long waiting period for infants listed for heart transplantation while they are waiting for a suitable donor heart [25]. On the other hand, neonates with moderate TV regurgitation and preserved RV function are usually offered the Norwood operation without TV repair. In neonates with severe TV regurgitation and preserved RV function, the role of hybrid first-stage palliation might be considered either to assess whether regurgitation degree improves with volume unloading or as a bridge to heart transplantation or comprehensive second-stage operation and concomitant TV repair in those patients with persistent severe regurgitation [1]. These approaches have not been utilized in our patient cohort. Most commonly, TV repair is performed at the time of bidirectional cavopulmonary connection. Despite increased operative complexity, hospital morbidity and mortality are not commonly increased in patients undergoing bidirectional cavopulmonary connection with concomitant procedures, such as atrioventricular valve repair, Damus–Kaye–Stansel anastomosis, arch or pulmonary artery augmentation [26, 27]. Finally, TV repair concomitant with the Fontan operation is controversial. Several surgeons have advocated a staged approach with earlier TV repair followed by a later assessment for Fontan candidacy. This is due to the unpredictable worsening of RV function following TV repair that is not well tolerated in patients with a Fontan physiology, in addition to the fact that early and late outcomes in patients who have undergone prolonged bypass and concomitant procedures at the time of the Fontan operation have been shown to be inferior in several series in the past [28]. On the other hand, several groups including ours have reported favourable outcomes in selected patients who underwent TV repair concomitant with the Fontan operation [29, 30]. Due to our institutional bias, we are not in a position to assess the superiority of a concomitant versus staged TV repair strategy, and in fact patients who underwent TV repair at the time of Fontan operation had the best transplant-free survival in our series. Nonetheless, this finding might be also limited by selection bias and those patients generally had good RV function and few associated risk factors. Thus, we believe that the decision to perform concomitant TV repair at the time of Fontan operation should be individualized taking into consideration RV function, the likelihood of achieving successful TV repair and other risk factors.
The most important factor associated with transplant-free survival in our series was post-repair RV function. Although TV repair was associated with significant improvement of regurgitation in the majority of patients, the coexisting RV function seemed to be unaffected and in fact, showed early or late deterioration in a fair number of those patients. Similar to TV regurgitation, there was no clear pattern of progression, and although these patients who had RV dysfunction following TV repair continued to do so on subsequent follow-up, a significant number of additional patients who had good or mildly depressed RV function following TV repair showed worsening of their RV function to moderate or severe dysfunction and subsequently received heart transplantation or died. Consequently, transplant-free survival in our patients was only 71%. This finding is in line with other studies that showed that RV dysfunction was not reversible despite successful TV repair in the majority of patients and that persistent poor RV function despite successful TV repair was associated with poor survival [2–4, 12]. Based on those findings, we believe that patients with significant RV dysfunction should be managed differently and be listed early for transplantation. Given the young age of those patients and donor shortage, it is expected that those patients will suffer from high transplant waiting-list mortality. Mechanical circulatory support in those patients is naturally challenging due to several anatomic and logistic considerations. Nonetheless, given the poor outcomes of those patients with conventional surgical approaches, ongoing research exploring mechanical circulatory support options in single ventricle patients is likely to offer a significant survival advantage if it is successful in the future.
CONCLUSIONS
Significant TV regurgitation in HLHS patients is not infrequent and necessitates surgical repair at various points of the multistage palliation strategy. TV repair is challenging; however, it can be associated with improvement of regurgitation in the majority of patients. Nonetheless, late recurrence of TV regurgitation and worsening of RV function limit the durability of this repair. Patients with RV dysfunction have poor survival. Different management strategies are warranted in these high-risk patients and might involve the mode of initial palliation, the timing of TV repair and the timing of listing for transplantation.
Conflict of interest: none declared.
Footnotes
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–11 October 2017.
REFERENCES
- heart transplantation
- hypoplastic left heart syndrome
- echocardiography
- repair of tricuspid valve
- repair of single ventricle with aortic outflow obstruction and aortic arch hypoplasia (hypoplastic left heart syndrome) (eg, norwood procedure)
- child
- follow-up
- repeat surgery
- vomiting
- palliative care
- transplantation




