-
PDF
- Split View
-
Views
-
Cite
Cite
Nicola Martucci, Maura Tracey, Antonello La Rocca, Carmine La Manna, Giuseppe De Luca, Gaetano Rocco, A pilot prospective randomized, controlled trial comparing LigaSure™ tissue fusion technology with the ForceTriad™ energy platform to the electrosurgical pencil on rates of atrial fibrillation after pulmonary lobectomy and mediastinal lymphadenectomy, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages e13–e18, https://doi.org/10.1093/ejcts/ezu391
Close - Share Icon Share
Abstract
The use of bipolar sealing devices during pulmonary resection is particularly useful in thoracoscopic surgery. Theoretically, a bipolar device, which contains the current in a smaller area and completes the current cycle only through the tissue between the electrodes, may reduce the proportion of patients experiencing atrial fibrillation compared with monopolar devices such as the electrosurgical pencil using which the current completes the cycle through the patient. We investigated the impact of the LigaSure™ (LS) tissue fusion technology with the ForceTriad™ energy platform device on the incidence of postoperative atrial fibrillation and on the reduction of postoperative chest tube output and hospital length of stay after open pulmonary lobectomy.
A pilot prospective randomized, controlled trial comparing LS tissue fusion technology with the ForceTriad™ energy platform to the conventional electrosurgical pencil. Overall, 146 patients with resectable lung cancer were recruited at the Division of Thoracic Surgery of the Istituto Nazionale Tumori, Fondazione Pascale, IRCCS, between January 2011 and July 2013. Of these, 119 candidates to open lobectomy for non-small-cell lung cancer were randomized to either LS tissue fusion technology with the ForceTriad™ energy platform (LS: 57 patients) or standard haemostatic procedure (standard treatment, ST: 62 patients) for hilar and mediastinal nodal dissection. The primary end-point was to compare the incidence of postoperative atrial fibrillation of LS compared with ST. The secondary end-point was to compare the efficacy of LS compared with ST in terms of total chest tube drainage, daily chest tube drainage and chest tube duration.
There was no statistically significant difference between LS and ST in terms of postoperative atrial fibrillation (P = 0.31). However, LS was associated to significant reduction of duration of both mediastinal nodal dissection (P = 0.017) and the cumulative chest tube drainage (P = 0.025).
The incidence of atrial fibrillation with LS tissue fusion technology with the ForceTriad™ energy platform is not reduced as compared with conventional electrosurgical pencil. However, the use of LS during mediastinal nodal dissection is associated to shorter duration of lymphadenectomy and duration of chest tube drainage.
INTRODUCTION
In the treatment of early and certain subsets of locally advanced non-small-cell lung cancer (NSCLC), resection of the tumour and lymph node removal for staging is the treatment of choice for eligible patients [1, 2]. Staging of the disease is crucial for determining the treatment course and for estimating prognosis. There is some debate in the surgical community as to whether lymph node sampling is adequate or systemic lymph node dissection is needed [3, 4]. A recent trial performed by the American College of Surgeons (Z0030) demonstrated no increase in morbidity when systemic dissection is performed as opposed to sampling [5].
Energy devices are used in thoracic surgical procedures to either effect haemostasis or seal lung surfaces [6–8].
Atrial arrhythmias are a frequent postoperative complication in thoracic surgery [9] with a reported incidence of 3.4–30% [9]. Atrial arrhythmias, fibrillation, flutter and supraventricular tachycardia are most common [10]; atrial fibrillation tends to occur within postoperative days 2 and 3 [9]. Many factors have been thought to cause atrial fibrillation after pulmonary surgery [9, 11], namely advanced age, extent of the operation due to increasing clinical lung cancer stage, preoperative high heart rate, history of heart failure or peripheral vascular disease, male gender and intraoperative transfusions [12]. The LigaSure (LS) precise energy delivery, electrode pressure, automatic sensitization of tissue resistance and adjustment of output voltage represent important advantages in an effort to reduce lateral thermal damage [6]. We proposed that at least some arrhythmias may be induced by the use of monopolar electrocautery pencils used to harvest lymph nodes and for hilar dissection. Our hypothesis was that a bipolar device, which contains the current in a smaller area and completes the current cycle only through the tissue between the electrodes, may reduce the proportion of patients experiencing atrial fibrillation compared with monopolar devices such as the electrosurgical pencil using which the current completes the cycle through the patient.
METHODS
Mechanism of action
The LS Vessel sealing system utilizes a combination of heat generated via bipolar radiofrequency energy, and precise jaw pressure to denature the collagen and elastin in a blood vessel or in tissue. These proteins, along with other constituents in the vessel or tissue, re-anneal to form an amalgam that effectively ligates the vessel or tissue. The LS Vessel sealing system (Covidien, Mansfield, MA, USA) comprises seven open configuration instruments and three instruments configured for laparoscopic application. These instruments are compatible with both the LS generator and the recently released Force Triad generator. The Force Triad generator has monopolar, bipolar and LS ‘u’ vessel sealing output, which has a novel, closed loop seal algorithm that further optimizes tissue effect. The generator detects the impedance of the tissue and defines the correct amount of energy required to achieve an optimal seal. Collectively, the instruments work as a system with either generator. The FDA has approved the LS ‘u’ system for sealing isolated and bundled systemic arteries, veins and lymphatics up to 7 mm in diameter.
Preoperative patients' characteristics
Between January 2011 and July 2013, 146 candidates for pulmonary lobectomy were entered in the study. Of these, 27 were excluded because a different extent of lung resection than lobectomy was needed for either metastases to the lung or benign disease. As a result, 119 lobectomy candidates with preoperative diagnosis of lung cancer were randomized to receive either LS or standard electrocautery during hilar and lymph node dissection. Continuous and categorical variables were selected according to currently reported predictors for atrial fibrillation—Table 2 [12]. Sixty-two patients were randomized to receive electrosurgical pencil [standard treatment (ST); Valleylab, Covidien, CO, USA] whereas LS was used in 57; a CONSORT flow diagram is included (Fig. 1). In this series, there were 87 male and 32 female patients; their median age was 66 years (range, 31–82 years). Forty-five patients (38%) were aged 70 years or older. The median BMI was 26.8 (range, 17.8–44.4); in particular, 69% of the patients had a BMI greater than 25. At the moment of the operation, 67 patients (56%) were on anticoagulants or antiplatelet medications (33 in the ST and 34 in the LS subgroup; P = 0.98) for their peripheral vascular disease (10 patients), previous myocardial infarction (5) hypertension (55) or arrhythmias (9). In addition, 10 patients (8%) had received induction chemotherapy before surgery (3 in the ST and 7 in the LS group, respectively; P = 0.31). While 24 patients were assigned an American Society of Anesthesiology (ASA) score of 1 (1 patient) to 2 (23 patients), an ASA score of 3 (92 patients) to 4 (3 patients) was attributed to the remaining 95 patients; in particular, the distribution of patients with ASA3 score was significantly different between the ST and LS groups (P = 0.039, Table 1).
Comparison of baseline characteristics between patients treated with LS versus ST
| Preoperative . | P . | LS . | ST . | Preoperative . | LS (SD) . | ST (SD) . | P . |
|---|---|---|---|---|---|---|---|
| Gender | 0.78 | M/F 41/16 | M/F 46/16 | Age Mean | 64.4 (8.8) | 65.9 (10.0) | 0.21 |
| Age70 | 0.33 | 19/57 | 26/62 | BMI Mean | 28.3 (5.8) | 27.1 (3.6) | 0.50 |
| BMI25 | 0.91 | 39/57 | 43/62 | ASA Mean | 3 (0.4) | 3 (0.5) | 0.061 |
| ASA3 | 0.039 | 50/57 | 45/62 | FEV1% Mean | 92.1 (18.7) | 97.8 (17.0) | 0.30 |
| Hx of arrhythmias | 0.19 | 7/57 | 2/62 | Smoker | 33/57 | 38/62 | 0.42 |
| MI | 0.062 | 4/57 | 1/62 | Steroid | 11/57 | 6/62 | 0.23 |
| Hx of peripheral vascular disease | 0.423 | 6/57 | 4/62 | Chronic renal insufficiency | 6/57 | 16/62 | 0.19 |
| Hypertension | 0.55 | 28/57 | 27/62 | Left atrial enlargement | Mild = 2 Mod = 9 Norm = 43 | Mild = 2 Mod = 5 Norm = 56 | 0.33 |
| Induction chemotherapy | 0.42 | 6/57 | 4/62 | Extent of mitral valve dysfunction | Mild = 11 Mod = 0 Sev = 1 Norm = 42 | Mild = 4 Mod = 2 Sev = 0 Norm = 57 | 0.081 |
| Side | 0.23 | R/L 33/24 | R/L 32/30 |
| Preoperative . | P . | LS . | ST . | Preoperative . | LS (SD) . | ST (SD) . | P . |
|---|---|---|---|---|---|---|---|
| Gender | 0.78 | M/F 41/16 | M/F 46/16 | Age Mean | 64.4 (8.8) | 65.9 (10.0) | 0.21 |
| Age70 | 0.33 | 19/57 | 26/62 | BMI Mean | 28.3 (5.8) | 27.1 (3.6) | 0.50 |
| BMI25 | 0.91 | 39/57 | 43/62 | ASA Mean | 3 (0.4) | 3 (0.5) | 0.061 |
| ASA3 | 0.039 | 50/57 | 45/62 | FEV1% Mean | 92.1 (18.7) | 97.8 (17.0) | 0.30 |
| Hx of arrhythmias | 0.19 | 7/57 | 2/62 | Smoker | 33/57 | 38/62 | 0.42 |
| MI | 0.062 | 4/57 | 1/62 | Steroid | 11/57 | 6/62 | 0.23 |
| Hx of peripheral vascular disease | 0.423 | 6/57 | 4/62 | Chronic renal insufficiency | 6/57 | 16/62 | 0.19 |
| Hypertension | 0.55 | 28/57 | 27/62 | Left atrial enlargement | Mild = 2 Mod = 9 Norm = 43 | Mild = 2 Mod = 5 Norm = 56 | 0.33 |
| Induction chemotherapy | 0.42 | 6/57 | 4/62 | Extent of mitral valve dysfunction | Mild = 11 Mod = 0 Sev = 1 Norm = 42 | Mild = 4 Mod = 2 Sev = 0 Norm = 57 | 0.081 |
| Side | 0.23 | R/L 33/24 | R/L 32/30 |
Variables with P < 0.1 were entered in the multiple regression model. Italic values are statistically significant.
Age70: 70-year age cut-off; ASA3: American Society of Anesthesiology Classification; BMI 25: value of BMI cut-off; Hx: History; MI: myocardial infarction; FEV1%: percentage preoperative forced expiratory volume at 1 s; ST: standard treatment (see text); LS: LigaSure (see text); Mod: moderate; Sev: severe; Norm: normal; SD: standard deviation.
Comparison of baseline characteristics between patients treated with LS versus ST
| Preoperative . | P . | LS . | ST . | Preoperative . | LS (SD) . | ST (SD) . | P . |
|---|---|---|---|---|---|---|---|
| Gender | 0.78 | M/F 41/16 | M/F 46/16 | Age Mean | 64.4 (8.8) | 65.9 (10.0) | 0.21 |
| Age70 | 0.33 | 19/57 | 26/62 | BMI Mean | 28.3 (5.8) | 27.1 (3.6) | 0.50 |
| BMI25 | 0.91 | 39/57 | 43/62 | ASA Mean | 3 (0.4) | 3 (0.5) | 0.061 |
| ASA3 | 0.039 | 50/57 | 45/62 | FEV1% Mean | 92.1 (18.7) | 97.8 (17.0) | 0.30 |
| Hx of arrhythmias | 0.19 | 7/57 | 2/62 | Smoker | 33/57 | 38/62 | 0.42 |
| MI | 0.062 | 4/57 | 1/62 | Steroid | 11/57 | 6/62 | 0.23 |
| Hx of peripheral vascular disease | 0.423 | 6/57 | 4/62 | Chronic renal insufficiency | 6/57 | 16/62 | 0.19 |
| Hypertension | 0.55 | 28/57 | 27/62 | Left atrial enlargement | Mild = 2 Mod = 9 Norm = 43 | Mild = 2 Mod = 5 Norm = 56 | 0.33 |
| Induction chemotherapy | 0.42 | 6/57 | 4/62 | Extent of mitral valve dysfunction | Mild = 11 Mod = 0 Sev = 1 Norm = 42 | Mild = 4 Mod = 2 Sev = 0 Norm = 57 | 0.081 |
| Side | 0.23 | R/L 33/24 | R/L 32/30 |
| Preoperative . | P . | LS . | ST . | Preoperative . | LS (SD) . | ST (SD) . | P . |
|---|---|---|---|---|---|---|---|
| Gender | 0.78 | M/F 41/16 | M/F 46/16 | Age Mean | 64.4 (8.8) | 65.9 (10.0) | 0.21 |
| Age70 | 0.33 | 19/57 | 26/62 | BMI Mean | 28.3 (5.8) | 27.1 (3.6) | 0.50 |
| BMI25 | 0.91 | 39/57 | 43/62 | ASA Mean | 3 (0.4) | 3 (0.5) | 0.061 |
| ASA3 | 0.039 | 50/57 | 45/62 | FEV1% Mean | 92.1 (18.7) | 97.8 (17.0) | 0.30 |
| Hx of arrhythmias | 0.19 | 7/57 | 2/62 | Smoker | 33/57 | 38/62 | 0.42 |
| MI | 0.062 | 4/57 | 1/62 | Steroid | 11/57 | 6/62 | 0.23 |
| Hx of peripheral vascular disease | 0.423 | 6/57 | 4/62 | Chronic renal insufficiency | 6/57 | 16/62 | 0.19 |
| Hypertension | 0.55 | 28/57 | 27/62 | Left atrial enlargement | Mild = 2 Mod = 9 Norm = 43 | Mild = 2 Mod = 5 Norm = 56 | 0.33 |
| Induction chemotherapy | 0.42 | 6/57 | 4/62 | Extent of mitral valve dysfunction | Mild = 11 Mod = 0 Sev = 1 Norm = 42 | Mild = 4 Mod = 2 Sev = 0 Norm = 57 | 0.081 |
| Side | 0.23 | R/L 33/24 | R/L 32/30 |
Variables with P < 0.1 were entered in the multiple regression model. Italic values are statistically significant.
Age70: 70-year age cut-off; ASA3: American Society of Anesthesiology Classification; BMI 25: value of BMI cut-off; Hx: History; MI: myocardial infarction; FEV1%: percentage preoperative forced expiratory volume at 1 s; ST: standard treatment (see text); LS: LigaSure (see text); Mod: moderate; Sev: severe; Norm: normal; SD: standard deviation.
Comparison of surgical characteristics between patients treated with LS versus ST
| Outcome . | LS . | ST . | P . | Outcome . | LS . | ST . | P . |
|---|---|---|---|---|---|---|---|
| Categorical variables | Continuous variables | ||||||
| >3 resected nodal stations | 40/57 | 51/62 | 0.12 | Duration of node resection Median (range) | 8 min (5–15) | 10 min (3–15) | 0.017 |
| >1-day duration output | 52/57 | 60/62 | 0.19 | Resected nodal stations Median (range) | 3 (1–5) | 3 (2–5) | 0.32 |
| >Median output 0.895 L | 30/57 | 36/62 | 0.55 | N of nodes per station Median (range) | 4 (2–11) | 3 (1–20) | 0.81 |
| LOS >5 days | 51/57 | 57/62 | 0.64 | Day 1 Median (range) | 285 ml (0–1100) | 297 ml (20–785) | 0.80 |
| Tube duration >5 days | 28/57 | 38/62 | 0.18 | Day 2 Median (range) | 190 ml (0–640) | 200 ml (0–600) | 0.97 |
| Atrial fibrillation | 9/57 | 6/62 | 0.31 | Day 3 Median (range) | 154 ml (0–760) | 150 ml (0–650) | 0.97 |
| Flutter bag | 8/57 | 13/62 | 0.36 | Day 4 Median (range) | 100 ml (0–555) | 72.5 ml (0–400) | 0.80 |
| Intraoperative complications | 2/57 | 2/62 | 0.93 | Day 5 Median (range) | 85 ml (0–330) | 80 ml (0–410) | 0.098 |
| Day 6 Median (range) | 0 ml (0–460) | 65 ml (0–430) | 0.52 | ||||
| >8 min difference for nodal dissection | 31/57 | 43/62 | 0.0092 | Day 7 Median (range) | 0 ml (0–550) | 0 ml (0–400) | 0.42 |
| pT distribution | T1 = 22 T2 = 31 T3 = 3 T4 = 1 | T1 = 24 T2 = 32 T3 = 5 T4 = 1 | 0.99 | Day 8 Median (range) | 0 ml (0–470) | 0 ml (0–450) | 0.97 |
| Pathological stage | Ia = 22 Ib = 20 IIa = 6 IIb = 4 IIIa = 4 IIIb = 1 | Ia = 20 Ib = 12 IIa = 16 IIb = 6 IIIa = 7 IIIb = 1 | 0.11 | Output duration Median (range) | 4 days (1–13) | 6 days (1–19) | 0.025 |
| N1/2 vs N0 | 7/57 | 18/51 | 0.025 | Total output volume Median (range) | 930 ml (0–2965) | 980 ml (160–3025) | 0.52 |
| Tube duration Median (range) | 5 days (0–45) | 6 days (1–52) | 0.32 | ||||
| Outcome . | LS . | ST . | P . | Outcome . | LS . | ST . | P . |
|---|---|---|---|---|---|---|---|
| Categorical variables | Continuous variables | ||||||
| >3 resected nodal stations | 40/57 | 51/62 | 0.12 | Duration of node resection Median (range) | 8 min (5–15) | 10 min (3–15) | 0.017 |
| >1-day duration output | 52/57 | 60/62 | 0.19 | Resected nodal stations Median (range) | 3 (1–5) | 3 (2–5) | 0.32 |
| >Median output 0.895 L | 30/57 | 36/62 | 0.55 | N of nodes per station Median (range) | 4 (2–11) | 3 (1–20) | 0.81 |
| LOS >5 days | 51/57 | 57/62 | 0.64 | Day 1 Median (range) | 285 ml (0–1100) | 297 ml (20–785) | 0.80 |
| Tube duration >5 days | 28/57 | 38/62 | 0.18 | Day 2 Median (range) | 190 ml (0–640) | 200 ml (0–600) | 0.97 |
| Atrial fibrillation | 9/57 | 6/62 | 0.31 | Day 3 Median (range) | 154 ml (0–760) | 150 ml (0–650) | 0.97 |
| Flutter bag | 8/57 | 13/62 | 0.36 | Day 4 Median (range) | 100 ml (0–555) | 72.5 ml (0–400) | 0.80 |
| Intraoperative complications | 2/57 | 2/62 | 0.93 | Day 5 Median (range) | 85 ml (0–330) | 80 ml (0–410) | 0.098 |
| Day 6 Median (range) | 0 ml (0–460) | 65 ml (0–430) | 0.52 | ||||
| >8 min difference for nodal dissection | 31/57 | 43/62 | 0.0092 | Day 7 Median (range) | 0 ml (0–550) | 0 ml (0–400) | 0.42 |
| pT distribution | T1 = 22 T2 = 31 T3 = 3 T4 = 1 | T1 = 24 T2 = 32 T3 = 5 T4 = 1 | 0.99 | Day 8 Median (range) | 0 ml (0–470) | 0 ml (0–450) | 0.97 |
| Pathological stage | Ia = 22 Ib = 20 IIa = 6 IIb = 4 IIIa = 4 IIIb = 1 | Ia = 20 Ib = 12 IIa = 16 IIb = 6 IIIa = 7 IIIb = 1 | 0.11 | Output duration Median (range) | 4 days (1–13) | 6 days (1–19) | 0.025 |
| N1/2 vs N0 | 7/57 | 18/51 | 0.025 | Total output volume Median (range) | 930 ml (0–2965) | 980 ml (160–3025) | 0.52 |
| Tube duration Median (range) | 5 days (0–45) | 6 days (1–52) | 0.32 | ||||
All categorical values equal the median value for the correspondent continuous variable. Continuous variables are reported as median values. Variables with P < 0.1 were then entered in the multiple regression model. Italic values are statistically significant.
LOS: length of stay; L: litre; ST: standard treatment (see text); LS: LigaSure (see text).
Comparison of surgical characteristics between patients treated with LS versus ST
| Outcome . | LS . | ST . | P . | Outcome . | LS . | ST . | P . |
|---|---|---|---|---|---|---|---|
| Categorical variables | Continuous variables | ||||||
| >3 resected nodal stations | 40/57 | 51/62 | 0.12 | Duration of node resection Median (range) | 8 min (5–15) | 10 min (3–15) | 0.017 |
| >1-day duration output | 52/57 | 60/62 | 0.19 | Resected nodal stations Median (range) | 3 (1–5) | 3 (2–5) | 0.32 |
| >Median output 0.895 L | 30/57 | 36/62 | 0.55 | N of nodes per station Median (range) | 4 (2–11) | 3 (1–20) | 0.81 |
| LOS >5 days | 51/57 | 57/62 | 0.64 | Day 1 Median (range) | 285 ml (0–1100) | 297 ml (20–785) | 0.80 |
| Tube duration >5 days | 28/57 | 38/62 | 0.18 | Day 2 Median (range) | 190 ml (0–640) | 200 ml (0–600) | 0.97 |
| Atrial fibrillation | 9/57 | 6/62 | 0.31 | Day 3 Median (range) | 154 ml (0–760) | 150 ml (0–650) | 0.97 |
| Flutter bag | 8/57 | 13/62 | 0.36 | Day 4 Median (range) | 100 ml (0–555) | 72.5 ml (0–400) | 0.80 |
| Intraoperative complications | 2/57 | 2/62 | 0.93 | Day 5 Median (range) | 85 ml (0–330) | 80 ml (0–410) | 0.098 |
| Day 6 Median (range) | 0 ml (0–460) | 65 ml (0–430) | 0.52 | ||||
| >8 min difference for nodal dissection | 31/57 | 43/62 | 0.0092 | Day 7 Median (range) | 0 ml (0–550) | 0 ml (0–400) | 0.42 |
| pT distribution | T1 = 22 T2 = 31 T3 = 3 T4 = 1 | T1 = 24 T2 = 32 T3 = 5 T4 = 1 | 0.99 | Day 8 Median (range) | 0 ml (0–470) | 0 ml (0–450) | 0.97 |
| Pathological stage | Ia = 22 Ib = 20 IIa = 6 IIb = 4 IIIa = 4 IIIb = 1 | Ia = 20 Ib = 12 IIa = 16 IIb = 6 IIIa = 7 IIIb = 1 | 0.11 | Output duration Median (range) | 4 days (1–13) | 6 days (1–19) | 0.025 |
| N1/2 vs N0 | 7/57 | 18/51 | 0.025 | Total output volume Median (range) | 930 ml (0–2965) | 980 ml (160–3025) | 0.52 |
| Tube duration Median (range) | 5 days (0–45) | 6 days (1–52) | 0.32 | ||||
| Outcome . | LS . | ST . | P . | Outcome . | LS . | ST . | P . |
|---|---|---|---|---|---|---|---|
| Categorical variables | Continuous variables | ||||||
| >3 resected nodal stations | 40/57 | 51/62 | 0.12 | Duration of node resection Median (range) | 8 min (5–15) | 10 min (3–15) | 0.017 |
| >1-day duration output | 52/57 | 60/62 | 0.19 | Resected nodal stations Median (range) | 3 (1–5) | 3 (2–5) | 0.32 |
| >Median output 0.895 L | 30/57 | 36/62 | 0.55 | N of nodes per station Median (range) | 4 (2–11) | 3 (1–20) | 0.81 |
| LOS >5 days | 51/57 | 57/62 | 0.64 | Day 1 Median (range) | 285 ml (0–1100) | 297 ml (20–785) | 0.80 |
| Tube duration >5 days | 28/57 | 38/62 | 0.18 | Day 2 Median (range) | 190 ml (0–640) | 200 ml (0–600) | 0.97 |
| Atrial fibrillation | 9/57 | 6/62 | 0.31 | Day 3 Median (range) | 154 ml (0–760) | 150 ml (0–650) | 0.97 |
| Flutter bag | 8/57 | 13/62 | 0.36 | Day 4 Median (range) | 100 ml (0–555) | 72.5 ml (0–400) | 0.80 |
| Intraoperative complications | 2/57 | 2/62 | 0.93 | Day 5 Median (range) | 85 ml (0–330) | 80 ml (0–410) | 0.098 |
| Day 6 Median (range) | 0 ml (0–460) | 65 ml (0–430) | 0.52 | ||||
| >8 min difference for nodal dissection | 31/57 | 43/62 | 0.0092 | Day 7 Median (range) | 0 ml (0–550) | 0 ml (0–400) | 0.42 |
| pT distribution | T1 = 22 T2 = 31 T3 = 3 T4 = 1 | T1 = 24 T2 = 32 T3 = 5 T4 = 1 | 0.99 | Day 8 Median (range) | 0 ml (0–470) | 0 ml (0–450) | 0.97 |
| Pathological stage | Ia = 22 Ib = 20 IIa = 6 IIb = 4 IIIa = 4 IIIb = 1 | Ia = 20 Ib = 12 IIa = 16 IIb = 6 IIIa = 7 IIIb = 1 | 0.11 | Output duration Median (range) | 4 days (1–13) | 6 days (1–19) | 0.025 |
| N1/2 vs N0 | 7/57 | 18/51 | 0.025 | Total output volume Median (range) | 930 ml (0–2965) | 980 ml (160–3025) | 0.52 |
| Tube duration Median (range) | 5 days (0–45) | 6 days (1–52) | 0.32 | ||||
All categorical values equal the median value for the correspondent continuous variable. Continuous variables are reported as median values. Variables with P < 0.1 were then entered in the multiple regression model. Italic values are statistically significant.
LOS: length of stay; L: litre; ST: standard treatment (see text); LS: LigaSure (see text).
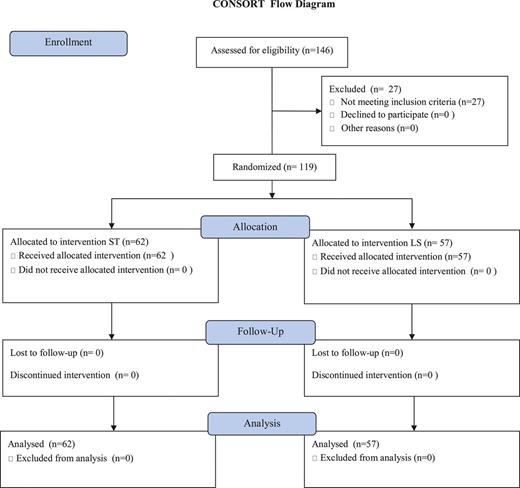
CONSORT flow diagram (see text). ST: standard treatment; LS: LigaSure.
Echocardiographic assessment of the left atrium size was performed by measuring its anteroposterior dimension by M mode or 2D echocardiography in the parasternal long-axis view (Table 1). The median ejection fraction was 60% (range, 49–69) with no difference between ST and LS groups (P = 0.6). In 116 patients, the atrial size was quantified according to the recommendations of the American Society of Echocardiography as mild (left atrial diameter 4.1–4.6 cm in men or 3.9–4.2 cm in women), moderate (4.7–5.1 cm in men or 4.3–4.6 cm in women) or severe (≥5.2 cm in men or ≥4.7 cm in women) [13]. Normal atrial size was observed in 98 patients (ST = 56; LS = 42). While mild enlargement was seen in 2 patients each group, moderate enlargement was measured in 5 and 9 patients treated with ST and LS, respectively (P = 0.33, Table 1). As to mitral valve dysfunction, echocardiography did not show a statistically significant difference between the ST and the LS groups (P = 0.08, Table 1). In particular, the mitral valve was defined normal in 99 patients (ST = 57; LS = 42). While mild insufficiency was quantified in 4 and 10 patients in the ST and LS groups, respectively, a moderate-to-severe insufficiency was detected in 2 in the ST and 1 in the LS group (Table 1).
The median percent preoperative FEV1 was 91 (range, 49–168); no difference in the percent preoperative FEV1 was noted between the two groups (P = 0.4, Table 1).
The study was approved by the Ethics Committee of the Istituto Nazionale Tumori, Fondazione Pascale IRCCS (21/2010—18 January 2010) and was supported by an unrestricted educational grant from Covidien.
Power calculation and statistical analysis
The primary study end-point was incidence of atrial fibrillation. Secondary end-points were the cumulative chest drain volume and chest tube duration. The test of two proportions had been used to determine if the patients who undergo dissection with standard techniques experience significantly more events in terms of atrial fibrillation than patients who undergo dissection with the LS device. We considered the LS technique to be superior to standard electrocautery in terms of the proportion of patients who experience postoperative atrial fibrillation if we were to observe a reduction of 20% in the rate of postoperative atrial fibrillation compared with reported literature's highest figure of 30–40% [9, 12]. For an α = 0.05 and a β = 0.20 (a power of 80%), it was deemed necessary to include 62 subjects per group, or 124 patients total for a study of superiority. In order to account for patient fall out, we decided to increase the enrolment to 70 patients in each arm, or 140 patients in total. Candidates for lobectomy were entered in the study following a standardized preoperative work-up according to the European Respiratory Society- European Society of Thoracic Surgeons' recommendations [14]. Randomization was via a closed envelope system in permuted blocks of 6 and took place in the operating theatre before induction. Data were prospectively collected and filed in an electronic format.
As to the statistical analysis, descriptive and regression statistics were calculated using NCSS statistical software (version 8; NCSS, LLC, Kaysville, UT, USA). Student's t-test or the χ2 test as appropriate was used for univariate analysis. Given the limited size of the population sample, multivariate analysis could not be performed through the logistic regression model because of complete separation. However, we applied cut-points (i.e. median values) to categorize continuous variables, which were then entered in a multiple regression model based on Huber's robust regression method with bootstrap analysis using the Monte–Carlo simulation. Probability values less than 0.05 were considered statistically significant.
Informed consent
Before surgery, the details of the patients who received of the informed consent form were recorded in two chapters. In Chapter 1, the Information Sheet with the following subheaders was included: Introduction; Purpose; Type of research intervention; Participant selection; Voluntary participation; Information on the Product/Device; Procedures and protocol; Randomization; Description of the Process: Duration, Side Effects, Risks, Discomforts, Benefits, Confidentiality, Sharing the Results, Right to Refuse or Withdraw, Alternatives to Participating, Who to contact; and IRB details. In Chapter 2, the Certificate of Consent with dates and printed names as well as signatures of the patient, witness and the researcher was included. One copy of the signed informed consent was provided to the patient who was blinded to the type of electrosurgical modality used during surgery.
RESULTS
Surgical technique and intraoperative data
Standard open approach was used in all 119 cases and it consists of an 8- to 10-cm muscle-sparing thoracotomy in the auscultatory triangle. In order to avoid inadvertent fractures, a 1-cm posterior segment of either the fifth or the sixth rib is removed. The intercostal muscles are incised for 2 cm anteriorly in excess of the cutaneous wound. The Finocchietto spreader is inserted and a maximum of three turns are used to reach an 8 cm × 8 cm opening. If necessary, pneumonolysis is performed and the hilar structures are dissected to be stapled and divided according to the vein–artery–bronchus sequence. Only in the event of incomplete fissures for right upper lobectomies, the bronchus is stapled before the ascending posterior artery. Otherwise, the artery is dissected into the fissure until a plane is developed where a stapler can be used to complete the fissure. During these manoeuvres, nodes at the lobar hilum and in the fissure are removed. Upon lung specimen removal, the mediastinal lymphadenectomy is performed by dissecting all tissue in the relevant anatomical areas according to the European Society of Thoracic Surgeons' guidelines on intraoperative mediastinal nodal dissection [15]. The use of sealants was permitted as per the surgeons' preferences only for persistent air leakage. Two chest tubes were placed at the end of the procedures, one in the apex and one in the basal location. The apical tube would come out when air leak subsided. The basal chest tube was removed when the 24-h output was 0 ml.
There was no difference between the two groups as to the side of surgery (60 patients received a left thoracotomy compared with 59 right thoracotomies; P = 0.23). The median number of resected mediastinal nodal stations was 3 (range, 1–5) with no statistically significant difference between ST and LS (P = 0.32, Table 2). Also, there was no difference between the two groups in the median number of resected nodes, which was 3 (range, 1–20; P = 0.81, Table 2).
Overall, atrial fibrillation complicated the postoperative course of 13 patients (11%). In spite of a significantly higher percentage of patients with ASA3–4 score and N+ disease in the LS group, the incidence of atrial fibrillation in the LS group was not different from that in the ST group (6 patients in the ST group and 7 in the LS group; P = 0.55, Table 2). All patients remained haemodynamically stable and underwent successful chemical cardioversion with intravenous amiodarone followed by oral amiodarone within a mean of 2 days of the intravenous load [9]. Anticoagulation was maintained for the entire duration of the administration of amiodarone and up to 4 weeks after return to sinus rhythm [9]. Preoperative and continuous outcome and categorical variables with a P ≤ 0.1 on univariate analysis were entered on robust multiple regression model using the Huber's method. As a result, predictors of the postoperative onset of atrial fibrillation were ASA3–4 score (P = 0.032) and evidence of severe mitral valve dysfunction (P = 0.050, Table 3).
| Variable . | Coefficient . | Standard error . | P . |
|---|---|---|---|
| ASA score 3 | 0.4997 | 0.1722 | 0.032 |
| Fluid duration | 0.0000 | 0.0019 | 0.98 |
| Hx of MI | −0.0097 | 0.0315 | 0.75 |
| Presence of mitral valve dysfunction | −0.4892 | 0.1734 | 0.050 |
| N+ stage | 0.0112 | 0.0196 | 0.83 |
| Time for node resection | 0.0001 | 0.0022 | 0.95 |
| Variable . | Coefficient . | Standard error . | P . |
|---|---|---|---|
| ASA score 3 | 0.4997 | 0.1722 | 0.032 |
| Fluid duration | 0.0000 | 0.0019 | 0.98 |
| Hx of MI | −0.0097 | 0.0315 | 0.75 |
| Presence of mitral valve dysfunction | −0.4892 | 0.1734 | 0.050 |
| N+ stage | 0.0112 | 0.0196 | 0.83 |
| Time for node resection | 0.0001 | 0.0022 | 0.95 |
ASA score 3 and mitral valve dysfunction were significant predictors of atrial fibrillation after lobectomy and lymphadenectomy. Italic values are statistically significant.
| Variable . | Coefficient . | Standard error . | P . |
|---|---|---|---|
| ASA score 3 | 0.4997 | 0.1722 | 0.032 |
| Fluid duration | 0.0000 | 0.0019 | 0.98 |
| Hx of MI | −0.0097 | 0.0315 | 0.75 |
| Presence of mitral valve dysfunction | −0.4892 | 0.1734 | 0.050 |
| N+ stage | 0.0112 | 0.0196 | 0.83 |
| Time for node resection | 0.0001 | 0.0022 | 0.95 |
| Variable . | Coefficient . | Standard error . | P . |
|---|---|---|---|
| ASA score 3 | 0.4997 | 0.1722 | 0.032 |
| Fluid duration | 0.0000 | 0.0019 | 0.98 |
| Hx of MI | −0.0097 | 0.0315 | 0.75 |
| Presence of mitral valve dysfunction | −0.4892 | 0.1734 | 0.050 |
| N+ stage | 0.0112 | 0.0196 | 0.83 |
| Time for node resection | 0.0001 | 0.0022 | 0.95 |
ASA score 3 and mitral valve dysfunction were significant predictors of atrial fibrillation after lobectomy and lymphadenectomy. Italic values are statistically significant.
The distribution of final pathological stage between the two groups was not statistically different (P = 0.17, Table 2). In fact, pIa was found in 42 patients (ST = 20; LS = 22), pIb in 32 (ST = 12;LS = 20), pIIa in 22 (ST = 16; LS = 6), pIIb in 10 (ST = 6; LS = 4), pIIIa in 11 (ST = 7; LS = 4) and pIIIb in 2 (ST = 1; LS = 1). In addition, there was no difference in the distribution of the T component between the two groups (P = 0.94) since 46 patients had T1 (ST = 24; LS = 22); 63 had T2 (ST = 32; LS = 31); 8 had T3 (ST = 5; LS = 3); and T4 in 2 (ST = 1; LS = 1). As to the N component, final pathology showed a difference between the two groups (P = 0.037) since N+ (i.e. N1 and N2) disease was found in 18 patients in the ST group compared with 7 in the LS one.
The total median duration of lymphadenectomy was different in the ST and the LS group (10 vs 8 min; P = 0.017, Table 2). Although there was no difference as to volume of postoperative fluid drainage from Day 1 through 8 and total volume of drainage, a significant shorter duration of fluid drainage was noted in the LS compared with the ST group (Table 2). The median postoperative length of stay (LOS) in the ST and LS groups was similar (8 days) with range values of 3–52 and 4–45, respectively (P = 0.82). Also, when the LOS greater than 5 days was considered, no significant difference was detected between the two groups (P = 0.64, Table 2).
An uneventful postoperative course was recorded in 86 patients (ST = 45; LS = 41). One patient in the LS group died (0.84%) 33 days after left upper lobectomy for synchronous lung cancer after previous contralateral sublobar resection; he was a 74-year old patient diagnosed with moderate left atrial enlargement due to mild mitral valve stenosis. The patient had been discharged home with a flutter bag for prolonged air leak (PAL) on Day 16 after having been successfully treated with amiodarone for the sudden onset of atrial fibrillation. Final pathology demonstrated a stage IB adenocarcinoma.
Postoperative complications of different severity developed in 32 patients (27%; ST = 19; LS = 13; P = 0.45). A PAL (i.e. >5 days) developed in 22 patients (18%). The air leak resolved during the same hospitalization in 6 patients and 1 patient in the ST and LS group, respectively. In addition, 5 patients in the ST group and 1 in the LS group were discharged home on a flutter bag (P = 0.36). These patients underwent removal of the chest tube on an outpatient basis within a mean of 6 days of discharge. One patient with PAL was reoperated for aerostasis on Day 4 and subsequently discharged 3 days later with full lung re-expansion.
Major postoperative morbidity such as chylothorax and pancreatitis developed in 1 patient in each group and only in 1 patient in the ST group, respectively. In all patients, conservative management and medical treatment were successfully used. Furthermore, in the ST group, pulmonary embolism and sinus tachycardia developed in 1 patient each and were successfully treated during the same hospitalization.
Comment
Several risk factors have been recognized to justify the onset of atrial fibrillation after non-cardiac thoracic surgical procedures [12]. From a pathophysiological standpoint, the dissection around the pulmonary hila and mediastinal lymphadenectomy could induce electric activation of atrial tissue triggered by bursts originating in the myocardial sleeves, i.e. extensions of the left atrium onto the pulmonary veins [16]. In this setting, the high temperatures from significant current dispersion in the surrounding mediastinal structures and the thermal damage caused by monopolar energy devices may represent a triggering event for the onset of atrial arrhythmias [17]. Conversely, bipolar radiofrequency energy devices are actually preferred in the surgical treatment of atrial fibrillation due to the total transmural block obtained with the passage of current between the two electrodes [17]. Bipolar energy devices are frequently used for either sealing blood vessels or for the division of fissures carried out either in open or during thoracoscopic procedures [6, 18]. With the current trial, we aimed at investigating a possible protective effect against atrial fibrillation achieved by using the LS bipolar device during dissection and we failed to demonstrate significant differences compared with ST. However, risk factors such as ASA3–4 score and the presence of hilar or mediastinal nodal disease (N+) were unevenly distributed, being more represented in the LS group. Larger powered studies conducted in a multicentric setting may be needed to further investigate this issue.
As to our secondary end-points, LS was associated to significant reduction of duration of both the mediastinal nodal dissection (P = 0.017) and the cumulative chest tube drainage (P = 0.025). Conversely, no differences between LS and ST were noted as to the other parameters related to drainage output. These findings seem to be in contrast with the ones reported by other authors who have justified an increased output after the use of LS during parenchymal resection based on the thermal damage generated by the bipolar device used to develop interlobar fissures [6]. Our data seem to support the use of LS for hilar and mediastinal nodal dissection in minimally invasive hybrid or thoracoscopic surgery since it is related to a more rapid performance of mediastinal lymphadenectomy and a reduced time to drainage cessation; however, we speculate that the latter did not translate into shorter chest tube duration and hospitalization due to non-clinical reasons [19]. In fact, cultural and socioeconomic factor may play a significant role in determining the discharge date from the hospital and may be investigated in the future to better evaluate postoperative outcomes after lung cancer surgery.
ACKNOWLEDGEMENTS
The authors thank Massimo Di Maio, from our Clinical trial Unit, for his invaluable help in data analysis.
Conflict of interest: Gaetano Rocco discloses to have served in the speakers bureau of and to have received honoraria from Covidien (Mansfield, MA, USA).
REFERENCES
- atrial fibrillation
- non-small-cell lung carcinoma
- chest tubes
- tissue dissection
- objective (goal)
- length of stay
- lymph node excision
- surgical procedures, operative
- thoracoscopy
- hemostasis procedures
- mediastinum
- surgery specialty
- thoracic surgery procedures
- lung volume reduction
- lymph node dissection
- lung cancer
- lobectomy
- pulmonary lobectomy
- medical devices
- surrogate endpoints




