-
PDF
- Split View
-
Views
-
Cite
Cite
Gonçalo F. Coutinho, Carlos Filipe Branco, Elisabete Jorge, Pedro M. Correia, Manuel J. Antunes, Mitral valve surgery after percutaneous mitral commissurotomy: is repair still feasible?, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages e1–e6, https://doi.org/10.1093/ejcts/ezu365
Close - Share Icon Share
Abstract
Due to progression of rheumatic disease, percutaneous mitral commissurotomy (PMC) is a palliative procedure. We aimed at evaluating the outcomes of patients requiring surgery for failure of PMC, focusing on the fate of the mitral valve (MV) (repair versus replacement).
From January 1993 through December 2012, 61 patients with previous PMC were submitted to MV surgery. Detailed operative findings were collected from all patients and an intraoperative anatomical score was introduced to predict reparability. Time to surgery, overall survival and freedom from reoperation were analysed.
The mean time to surgery after PMC was 6.9 ± 5.9 years and indications were restenosis in 25 patients (41%) and mitral regurgitation or mixed lesion in 36 (59%). Nine patients (14.8%) had more than one previous intervention. Intraoperative inspection of the valve revealed leaflet laceration outside the commissural area in 27 patients (44.3%). Valve repair was accomplished in 38 patients (62.3%). Pulmonary hypertension, calcification and intraoperative anatomical score were independently associated with the probability of valve replacement (OR 1.12, OR 7.03 and OR 4.49, respectively, P < 0.05). There was no hospital mortality. MV area increased on average 1.6 cm2 after surgery to 2.7 cm2; 5-, 10- and 20-year survival rates were 98.1 ± 1.9, 91 ± 5.2 and 82.7 ± 9.2%, respectively. The rate of freedom from mitral reoperation (for repaired cases) at 5, 10 and 15 years was 100, 95.8 ± 4.1 and 87.8 ± 8.5%, respectively. There was no difference in survival between repaired or replaced MVs, but the former had less valve-related events during follow-up.
The MV can be repaired after failed PMC, with very low complication rates and excellent long-term results. Hence, whenever possible, these patients should be sent to reference centres where repair can be successfully achieved.
INTRODUCTION
The incidence of rheumatic fever has dropped drastically in the last decades in Western countries. However, due to immigrant flows and to progression of the disease in previously affected patients, we still come across this valvular problem in our practice, especially in patients in the fifth to seventh decade of life, who have had rheumatic fever in their childhood and youth, four or five decades ago when the disease was still endemic in our country.
Mitral valve (MV) stenosis (MS) is the most common form of presentation of rheumatic disease and is characterized by leaflet thickening, commissural fusion and involvement of the subvalvular apparatus (thickening, shortening and fusion of the chordae tendineae) [1]. Occasionally, massive annular calcification may lead to MV obstruction [2].
Since MS is a consequence of a mechanical obstruction to diastolic flow, the only effective and definite treatment when the disease becomes clinically disabling [mitral valve area (MVA) ≤1.5 cm2] is the relief of the obstacle [3]. At the present time, there are three options to treat symptomatic MS: Percutaneous mitral commissurotomy (PMC), open mitral commissurotomy (OMC) and MV replacement (MVR). Although we believe that it still has a place in some circumstances, closed mitral commissurotomy was practically abandoned in developed countries some three decades ago [4]. PMC has become rapidly the procedure of choice in patients with favourable anatomical characteristics (Wilkins score ≤8) [5], for being a less invasive procedure and associated with a prompt recovery of the patient, in comparison to surgery [6].
A significant early and late failure rate after the percutaneous procedure has been documented, and the majority of studies predict that after PMC it is almost inevitable to replace the MV when surgery is required for mitral restenosis, regurgitation or mixed lesion [7]. However, our personal experience has shown very distinctive results. Hence, we have defined the following as aims to this study: the evaluation of the causes of immediate and long-term failure after PMC, the feasibility of MV repair, and survival and freedom from reoperation after MV surgery.
MATERIALS AND METHODS
Patient population
From January 1992 through December 2013, 1874 patients with rheumatic MV disease and no previous mitral intervention (closed or open commissurotomy or PMC) were submitted to MV surgery, of which 1514 (80.8%) had their valves repaired, independently of the valve lesion (stenosis, regurgitation or mixed). During the same period, 61 patients with previous PMC required MV surgery for technical failure of the procedure or evolution of the disease, and constitute the study population. For the purpose of this work, we have defined early failure of PMC as those cases that needed surgery up to 1 year after the percutaneous procedure and this occurred in 13 cases (21.3%). Nine patients (14.8%) had more than one previous PMC. Valve repair was accomplished in 38 patients (62.3%).
The mean age of this group was 51.7 ± 12.7 years and female sex prevailed (n = 56, 91.8%). The majority of patients were in NYHA functional class III–IV (n = 43, 70.5%), 8.2% had a previous history of cerebrovascular accident and 63.9% were in atrial fibrillation (AF) at the time of surgery. The mean EuroSCORE II was 1.98, which represents a low surgical risk population. There were significant differences in demographic characteristics and preoperative echocardiographic findings between patients who had a mitral prosthesis implanted and those who had their valves repaired (Table 1). Patients submitted to MVR were older, with a higher EuroSCORE II and with indicators of more advanced mitral disease (left atrial enlargement, pulmonary hypertension and marked calcium infiltration).
Demographic and echocardiographic characteristics of patients submitted to mitral valve repair or replacement who had previous PMC
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Age (years) | 48.5 ± 13.0 | 57.2 ± 10.2 | <0.0001 |
| Female sex | 35 (92.1%) | 21 (91.3%) | 0.912 |
| NYHA III–IV | 27 (71.1%) | 16 (69.6%) | 0.902 |
| Atrial fibrillation | 21 (55.3%) | 18 (78.3%) | 0.070 |
| Tricuspid disease | 9 (23.7%) | 13 (56.5%) | 0.010 |
| Aortic valve disease | 6 (15.8%) | 9 (39.1%) | 0.040 |
| More than one PMC | 3 (7.9%) | 6 (26.1%) | 0.052 |
| EuroSCORE II | 1.37 ± 0.7 | 3.13 ± 2.60 | 0.004 |
| Echocardiographic | |||
| Pure stenosis | 15 (39.5%) | 10 (43.4%) | 0.608 |
| Mitral regurgitation (degree) | 2.76 ± 1.27 | 2.78 ± 0.90 | 0.950 |
| Ejection fraction (%) | 64.8 ± 15.3% | 65.3 ± 11.4% | 0.462 |
| Left ventricular systolic diameter (mm) | 34.4 ± 8.7 | 33.2 ± 7.6 | 0.664 |
| Left ventricular diastolic diameter (mm) | 52.5 ± 10.2 | 51.0 ± 6.5 | 0.592 |
| Left atrium diameter (mm) | 53.1 ± 9.2 | 57.0 ± 9.2 | 0.208 |
| MVA (mm) | 1.30 ± 0.46 | 1.09 ± 0.42 | 0.963 |
| Systolic pulmonary artery pressure (mmHg) | 44.1 ± 8.3 | 53.7 ± 12.6 | 0.003 |
| Mitral calcification | 6 (16.2%) | 14 (60.9%) | <0.0001 |
| Echocardiographic score | 7.4 ± 1.6 | 8.2 ± 1.8 | 0.760 |
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Age (years) | 48.5 ± 13.0 | 57.2 ± 10.2 | <0.0001 |
| Female sex | 35 (92.1%) | 21 (91.3%) | 0.912 |
| NYHA III–IV | 27 (71.1%) | 16 (69.6%) | 0.902 |
| Atrial fibrillation | 21 (55.3%) | 18 (78.3%) | 0.070 |
| Tricuspid disease | 9 (23.7%) | 13 (56.5%) | 0.010 |
| Aortic valve disease | 6 (15.8%) | 9 (39.1%) | 0.040 |
| More than one PMC | 3 (7.9%) | 6 (26.1%) | 0.052 |
| EuroSCORE II | 1.37 ± 0.7 | 3.13 ± 2.60 | 0.004 |
| Echocardiographic | |||
| Pure stenosis | 15 (39.5%) | 10 (43.4%) | 0.608 |
| Mitral regurgitation (degree) | 2.76 ± 1.27 | 2.78 ± 0.90 | 0.950 |
| Ejection fraction (%) | 64.8 ± 15.3% | 65.3 ± 11.4% | 0.462 |
| Left ventricular systolic diameter (mm) | 34.4 ± 8.7 | 33.2 ± 7.6 | 0.664 |
| Left ventricular diastolic diameter (mm) | 52.5 ± 10.2 | 51.0 ± 6.5 | 0.592 |
| Left atrium diameter (mm) | 53.1 ± 9.2 | 57.0 ± 9.2 | 0.208 |
| MVA (mm) | 1.30 ± 0.46 | 1.09 ± 0.42 | 0.963 |
| Systolic pulmonary artery pressure (mmHg) | 44.1 ± 8.3 | 53.7 ± 12.6 | 0.003 |
| Mitral calcification | 6 (16.2%) | 14 (60.9%) | <0.0001 |
| Echocardiographic score | 7.4 ± 1.6 | 8.2 ± 1.8 | 0.760 |
NYHA: New York Heart Association; MVA: mitral valve area; PMC: percutaneous mitral commissurotomy. Bold values indicate significance of P -values ≤0.05.
Demographic and echocardiographic characteristics of patients submitted to mitral valve repair or replacement who had previous PMC
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Age (years) | 48.5 ± 13.0 | 57.2 ± 10.2 | <0.0001 |
| Female sex | 35 (92.1%) | 21 (91.3%) | 0.912 |
| NYHA III–IV | 27 (71.1%) | 16 (69.6%) | 0.902 |
| Atrial fibrillation | 21 (55.3%) | 18 (78.3%) | 0.070 |
| Tricuspid disease | 9 (23.7%) | 13 (56.5%) | 0.010 |
| Aortic valve disease | 6 (15.8%) | 9 (39.1%) | 0.040 |
| More than one PMC | 3 (7.9%) | 6 (26.1%) | 0.052 |
| EuroSCORE II | 1.37 ± 0.7 | 3.13 ± 2.60 | 0.004 |
| Echocardiographic | |||
| Pure stenosis | 15 (39.5%) | 10 (43.4%) | 0.608 |
| Mitral regurgitation (degree) | 2.76 ± 1.27 | 2.78 ± 0.90 | 0.950 |
| Ejection fraction (%) | 64.8 ± 15.3% | 65.3 ± 11.4% | 0.462 |
| Left ventricular systolic diameter (mm) | 34.4 ± 8.7 | 33.2 ± 7.6 | 0.664 |
| Left ventricular diastolic diameter (mm) | 52.5 ± 10.2 | 51.0 ± 6.5 | 0.592 |
| Left atrium diameter (mm) | 53.1 ± 9.2 | 57.0 ± 9.2 | 0.208 |
| MVA (mm) | 1.30 ± 0.46 | 1.09 ± 0.42 | 0.963 |
| Systolic pulmonary artery pressure (mmHg) | 44.1 ± 8.3 | 53.7 ± 12.6 | 0.003 |
| Mitral calcification | 6 (16.2%) | 14 (60.9%) | <0.0001 |
| Echocardiographic score | 7.4 ± 1.6 | 8.2 ± 1.8 | 0.760 |
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Age (years) | 48.5 ± 13.0 | 57.2 ± 10.2 | <0.0001 |
| Female sex | 35 (92.1%) | 21 (91.3%) | 0.912 |
| NYHA III–IV | 27 (71.1%) | 16 (69.6%) | 0.902 |
| Atrial fibrillation | 21 (55.3%) | 18 (78.3%) | 0.070 |
| Tricuspid disease | 9 (23.7%) | 13 (56.5%) | 0.010 |
| Aortic valve disease | 6 (15.8%) | 9 (39.1%) | 0.040 |
| More than one PMC | 3 (7.9%) | 6 (26.1%) | 0.052 |
| EuroSCORE II | 1.37 ± 0.7 | 3.13 ± 2.60 | 0.004 |
| Echocardiographic | |||
| Pure stenosis | 15 (39.5%) | 10 (43.4%) | 0.608 |
| Mitral regurgitation (degree) | 2.76 ± 1.27 | 2.78 ± 0.90 | 0.950 |
| Ejection fraction (%) | 64.8 ± 15.3% | 65.3 ± 11.4% | 0.462 |
| Left ventricular systolic diameter (mm) | 34.4 ± 8.7 | 33.2 ± 7.6 | 0.664 |
| Left ventricular diastolic diameter (mm) | 52.5 ± 10.2 | 51.0 ± 6.5 | 0.592 |
| Left atrium diameter (mm) | 53.1 ± 9.2 | 57.0 ± 9.2 | 0.208 |
| MVA (mm) | 1.30 ± 0.46 | 1.09 ± 0.42 | 0.963 |
| Systolic pulmonary artery pressure (mmHg) | 44.1 ± 8.3 | 53.7 ± 12.6 | 0.003 |
| Mitral calcification | 6 (16.2%) | 14 (60.9%) | <0.0001 |
| Echocardiographic score | 7.4 ± 1.6 | 8.2 ± 1.8 | 0.760 |
NYHA: New York Heart Association; MVA: mitral valve area; PMC: percutaneous mitral commissurotomy. Bold values indicate significance of P -values ≤0.05.
Institutional review board authorization was obtained for this study.
Evaluation of the mitral valve
All patients had at least a preoperative transthoracic echocardiogram (TTE), an intraoperative 2D transoesophageal echocardiogram and a postoperative TTE. Severe stenosis was defined as MVA below 1 cm2 and/or mean transmitral gradient >10 mmHg. Preoperative MVA was determined anatomically (2D echocardiography, planimetry) and functionally (Doppler pressure half-time method); however, functional MVA determination was the reference method in evaluating patients after surgery [8].
The evaluation included calculation of an echocardiographic score (Wilkins) [5] and of an intraoperative morphological score derived from the accumulated experience of 2344 patients with rheumatic mitral disease submitted to MV surgery in our department. Briefly, the operative score consists of four factors (anterior leaflet mobility, degree of leaflet thickening, degree of chordae tendineae involvement and degree and/or location of mitral calcification), each with three possible degrees (1–3), adding to a total of 4–12 (Table 2). This model showed very good predictive ability, with an area under the curve (AUC) of the receiver operating characteristic (ROC) curve of 0.870 [95% confidence interval (CI) 0.744–0.996, P < 0.0001], and no evidence of lack of fit based on the Hosmer–Lemeshow statistic (χ2 = 5.8, P = 0.559). The higher the score, the more severe is the rheumatic involvement and less likely the probability to repair the valve.
Intraoperative morphological score based on the analysis of 2344 rheumatic mitral valves during mitral valve surgery (Grading 4–12)a
| Grade . | Anterior leaflet pliability . | Leaflet thickening . | Subvalvular thickening (chordae) . | Calcification (site) . |
|---|---|---|---|---|
| 1 | Pliable/minimal restriction | Minimal | Minimal | Absent/minimal |
| 2 | Mild–moderate restriction | Mild–moderate | Mild–moderate | Body of leaflet/commissures |
| 3 | Severe restriction (fixed) | Severe (all leaflets) | Severe (chordae shortening and/or fusion) | Free edge |
| Grade . | Anterior leaflet pliability . | Leaflet thickening . | Subvalvular thickening (chordae) . | Calcification (site) . |
|---|---|---|---|---|
| 1 | Pliable/minimal restriction | Minimal | Minimal | Absent/minimal |
| 2 | Mild–moderate restriction | Mild–moderate | Mild–moderate | Body of leaflet/commissures |
| 3 | Severe restriction (fixed) | Severe (all leaflets) | Severe (chordae shortening and/or fusion) | Free edge |
Minimal thickening implies a translucent leaflet/chordae, structurally almost normal. Severe thickening is considered when there is dense fibrous tissue with opaque or yellowish appearance. Mild-to-moderate leaflet/chordae thickening is in between those two grades.
ROC: receiver operating characteristic; AUC: area under the curve.
aThe higher the score, the less favourable is the feasibility to repair. ROC analysis determined an AUC of 0.896 (P < 0.001, 95% CI 0.867–0.926), showing a very good predictive ability of the score. The estimated cut-off point for replacement was equal to or greater than 9.
Intraoperative morphological score based on the analysis of 2344 rheumatic mitral valves during mitral valve surgery (Grading 4–12)a
| Grade . | Anterior leaflet pliability . | Leaflet thickening . | Subvalvular thickening (chordae) . | Calcification (site) . |
|---|---|---|---|---|
| 1 | Pliable/minimal restriction | Minimal | Minimal | Absent/minimal |
| 2 | Mild–moderate restriction | Mild–moderate | Mild–moderate | Body of leaflet/commissures |
| 3 | Severe restriction (fixed) | Severe (all leaflets) | Severe (chordae shortening and/or fusion) | Free edge |
| Grade . | Anterior leaflet pliability . | Leaflet thickening . | Subvalvular thickening (chordae) . | Calcification (site) . |
|---|---|---|---|---|
| 1 | Pliable/minimal restriction | Minimal | Minimal | Absent/minimal |
| 2 | Mild–moderate restriction | Mild–moderate | Mild–moderate | Body of leaflet/commissures |
| 3 | Severe restriction (fixed) | Severe (all leaflets) | Severe (chordae shortening and/or fusion) | Free edge |
Minimal thickening implies a translucent leaflet/chordae, structurally almost normal. Severe thickening is considered when there is dense fibrous tissue with opaque or yellowish appearance. Mild-to-moderate leaflet/chordae thickening is in between those two grades.
ROC: receiver operating characteristic; AUC: area under the curve.
aThe higher the score, the less favourable is the feasibility to repair. ROC analysis determined an AUC of 0.896 (P < 0.001, 95% CI 0.867–0.926), showing a very good predictive ability of the score. The estimated cut-off point for replacement was equal to or greater than 9.
Operative data and follow-up
The operative technique was standard for all patients and included cardiopulmonary bypass with mild hypothermia (30°C) and intermittent antegrade cold crystalloid cardioplegia infused in the aortic root. Most cases were done through a median sternotomy, but in 4 patients the access route was a right anterolateral thoracotomy. MV exposure was obtained by left atriotomy, posterior to Waterston's groove.
We were able to preserve the MV in 38 cases (62.3%). As expected, there were significant differences in the operative findings between the two surgical options (repair or replacement). Patients submitted to MVR had more intense calcium infiltration and significantly higher anatomical scores (Table 3).
Intraoperative findings of patients submitted to mitral valve repair or replacement after previous PMC
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Retraction of the posterior leaflet | 5 (13.2%) | 6 (26.1%) | 0.233 |
| Leaflet iatrogenic laceration | 18 (47.4%) | 9 (39.1%) | 0.530 |
| Chordae fusion/retraction | 23 (60.5%) | 18 (78.3%) | 0.055 |
| Commissural fusion | 36 (94.7%) | 23 (100%) | 0.274 |
| Commissural calcification | 5 (13.2%) | 10 (43.5%) | 0.008 |
| Leaflet calcification | 3 (7.9%) | 14 (60.9%) | <0.0001 |
| Anterior leaflet reduced mobilitity | 0 (0%) | 9 (40.9%) | <0.0001 |
| Morphological score | 7.0 ± 1.2 | 9.8 ± 1.5 | <0.0001 |
| Extracorporeal circulation time (min) | 49.5 ± 14.9 | 70.2 ± 26.1 | <0.0001 |
| Aortic clamping time (min) | 27.6 ± 11.3 | 45.8 ± 26.2 | <0.0001 |
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Retraction of the posterior leaflet | 5 (13.2%) | 6 (26.1%) | 0.233 |
| Leaflet iatrogenic laceration | 18 (47.4%) | 9 (39.1%) | 0.530 |
| Chordae fusion/retraction | 23 (60.5%) | 18 (78.3%) | 0.055 |
| Commissural fusion | 36 (94.7%) | 23 (100%) | 0.274 |
| Commissural calcification | 5 (13.2%) | 10 (43.5%) | 0.008 |
| Leaflet calcification | 3 (7.9%) | 14 (60.9%) | <0.0001 |
| Anterior leaflet reduced mobilitity | 0 (0%) | 9 (40.9%) | <0.0001 |
| Morphological score | 7.0 ± 1.2 | 9.8 ± 1.5 | <0.0001 |
| Extracorporeal circulation time (min) | 49.5 ± 14.9 | 70.2 ± 26.1 | <0.0001 |
| Aortic clamping time (min) | 27.6 ± 11.3 | 45.8 ± 26.2 | <0.0001 |
PMC: percutaneous mitral commissurotomy. Bold values indicate significance of P-values ≤0.05.
Intraoperative findings of patients submitted to mitral valve repair or replacement after previous PMC
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Retraction of the posterior leaflet | 5 (13.2%) | 6 (26.1%) | 0.233 |
| Leaflet iatrogenic laceration | 18 (47.4%) | 9 (39.1%) | 0.530 |
| Chordae fusion/retraction | 23 (60.5%) | 18 (78.3%) | 0.055 |
| Commissural fusion | 36 (94.7%) | 23 (100%) | 0.274 |
| Commissural calcification | 5 (13.2%) | 10 (43.5%) | 0.008 |
| Leaflet calcification | 3 (7.9%) | 14 (60.9%) | <0.0001 |
| Anterior leaflet reduced mobilitity | 0 (0%) | 9 (40.9%) | <0.0001 |
| Morphological score | 7.0 ± 1.2 | 9.8 ± 1.5 | <0.0001 |
| Extracorporeal circulation time (min) | 49.5 ± 14.9 | 70.2 ± 26.1 | <0.0001 |
| Aortic clamping time (min) | 27.6 ± 11.3 | 45.8 ± 26.2 | <0.0001 |
| Variables . | Repair, n = 38 (62.3%) . | Replacement, n = 23 (37.7%) . | P-value . |
|---|---|---|---|
| Retraction of the posterior leaflet | 5 (13.2%) | 6 (26.1%) | 0.233 |
| Leaflet iatrogenic laceration | 18 (47.4%) | 9 (39.1%) | 0.530 |
| Chordae fusion/retraction | 23 (60.5%) | 18 (78.3%) | 0.055 |
| Commissural fusion | 36 (94.7%) | 23 (100%) | 0.274 |
| Commissural calcification | 5 (13.2%) | 10 (43.5%) | 0.008 |
| Leaflet calcification | 3 (7.9%) | 14 (60.9%) | <0.0001 |
| Anterior leaflet reduced mobilitity | 0 (0%) | 9 (40.9%) | <0.0001 |
| Morphological score | 7.0 ± 1.2 | 9.8 ± 1.5 | <0.0001 |
| Extracorporeal circulation time (min) | 49.5 ± 14.9 | 70.2 ± 26.1 | <0.0001 |
| Aortic clamping time (min) | 27.6 ± 11.3 | 45.8 ± 26.2 | <0.0001 |
PMC: percutaneous mitral commissurotomy. Bold values indicate significance of P-values ≤0.05.
MV repair consisted mainly of bilateral commissurotomy (97.4%) with a commissuroplasty/annuloplasty procedure (posterior or complete in 79%). Chordae tendineae and papillary muscle division was performed in 52.6% of patients, secondary chordae cutting and chordal transposition in 5.3% each, and mitral decalcification (commissures and/or leaflets) in 10.5% of the cases.
Since this was a young population, most patients who had their valve replaced received a mechanical valve (82.6%). The mean valve size was 26.3 ± 1.3 mm (25–29 mm) and posterior leaflet and Subvalvular apparatus preservation was achieved in 41.9% of the patients, despite the intense fibrosis.
Tricuspid valve repair by a modified DeVega technique [9] and aortic valve replacement were performed in 36.1% and 14.8% of patients, respectively.
Follow-up was done through a mailed questionnaire or by telephone interview with surviving patients, family members or the patient's personal physician. Follow-up data included information about level of activity, current symptoms and occurrence of late valve-related events. The total duration of follow-up for the entire cohort was 518.5 patient-years (range 0.9–22 years; median 8.0 ± 5.6 years; 25–75 IQR 4.0–12.4) and was complete for 98.3% of the patients.
Mortality and morbidity were reported according to the latest guidelines [10].
Statistical analysis
Continuous variables were reported as mean + standard deviation (SD) and compared by Student's t-test. Values obtained from pre- and postoperative data were compared by a paired t-test. Categorical variables were reported as percentages and were compared using the χ2 test. Actuarial survival rates were plotted using the Kaplan–Meier method, and the two groups (repair and replacement) were compared using a log-rank analysis. Multivariate analysis to identify risk factors for survival was performed using Cox regression models. For each patient included in the study, the corresponding average age- and gender-specific annual mortality of the Portuguese general population was obtained (National Institute of Statistics, 2011 census). On the basis of these mortality data, the probability of cumulative expected survival was ascertained and an expected survival curve was built. Comparison was made using a one-sample log-rank test.
Univariate and multivariate predictors for MVR were identified using logistic regression models. Criteria for entry and retention into multivariable models were set at the 0.1 and 0.05 confidence level, respectively. The discriminatory performance of the model (including the intraoperative score) to distinguish between patients who will have their valve replaced or repaired was verified by constructing ROC curves to calculate the AUC with 95% CIs. Calibration was tested with the Hosmer–Lemeshow goodness-of-fit test, which compares observed with predicted values by decile of predicted probability. Statistical significance was defined as a two-tailed probability value <0.05. The data were analysed using the statistical package program SPSS (version 19, SPSS, Inc., Chicago, IL, USA).
RESULTS
The median time from PMC to MV surgery was 5.3 ± 5.9 years (25–75 IQR: 1.7–10.6).
Preoperative echocardiographic data revealed MV calcification in 20 patients (32.8%); mitral stenosis in 25 (41%) and moderate-to-severe mitral regurgitation (MR) in 36 (59.1%). The MVA was 1.21 ± 0.5 cm2 and the left atrial and left ventricular (systolic) dimensions were 49.5 ± 10 and 33.9 ± 8 mm, respectively. The estimated pulmonary artery pressure was 48 ± 11 mmHg and left ventricular function was preserved in 52 cases (85.2%). The average echocardiographic score (Wilkins) was 7.0 ± 2.2.
Intraoperative findings showed leaflet laceration outside the commissural area in 27 patients (44.3%), mostly localized in the posterior leaflet. Nevertheless, that event did not imply MVR, and when we were able to preserve the valve, suture of the laceration was done and a proper commissurotomy was made. Interestingly, in the cases of early failure, we were able to repair nearly 77% of the valves; in contrast, the rate of repair dropped to 58% when MV surgery was performed several years after PMV (probably related to disease progression and not technical failure).
There was no mortality (30 days) in this specific population and postoperative morbidity was low, with acute renal failure (creatinine level ≥2.0 mg/dl) in 9.8% of patients (no patient required renal replacement therapy) and atrial tachyarrhythmia in 18%, which included new-onset AF/atrial flutter and patients with previous AF who required intravenous antiarrhythmics for ventricular rate control, emerging as the most frequent postoperative complications.
Postoperative echocardiogram after surgery revealed a mean MVA of 2.7 ± 0.6 cm2, with a mean increase of 1.6 cm2 between the pre- and postoperative values, mean MR of 0.74 ± 1.01 (only 1 patient with MR greater than mild), mean left atrial size of 46.7 ± 10.8 mm and a mean systolic pulmonary pressure of 39.9 ± 16.3 mmHg (all with P < 0.05 in comparison with the preoperative).
Factors associated to MVR were investigated; in the univariate analysis, high EuroSCORE II values, concomitant aortic or tricuspid valve disease, more than one PMV, mitral calcification (leaflets and/or commissures), anterior leaflet hypomobility, age, high preoperative creatinine level, elevated pulmonary artery pressure and high morphological scores were associated with valve replacement (P < 0.05). However, in multivariate analysis, only pulmonary hypertension (OR: 1.12; 95% CI 1.09–1.21, P = 0.012), mitral calcification (OR: 7.03; 95% CI 1.6–30.2, P = 0.018) and the morphological score (OR: 4.49; 95% CI 1.7–11.6, P = 0.003) were identified as independent risk factors for MVR.
Overall survival at 5, 10 and 18 years was 98.1 ± 1.9% (95% CI 88.9–99.8%), 91 ± 5.2% (95% CI 78.4–97.7%) and 82.7 ± 9.2% (95% CI 64.3–95.4%), respectively, and comparable with the Portuguese age- and sex-matched population (Fig. 1). Age was the only independent risk factor for late mortality (HR: 1.25; 95% CI 1.041–1.50, P = 0.017). Although we did not find differences regarding survival between patients who had repair or replacement (P = 0.843), the latter had more adverse valve-related events, especially thromboembolic events (13.0 vs 5.3%, P = 0.029), bleeding accidents (8.7 vs 3.1%, P = 0.049) and endocarditis (4.3 vs 0%, P = 0.060).
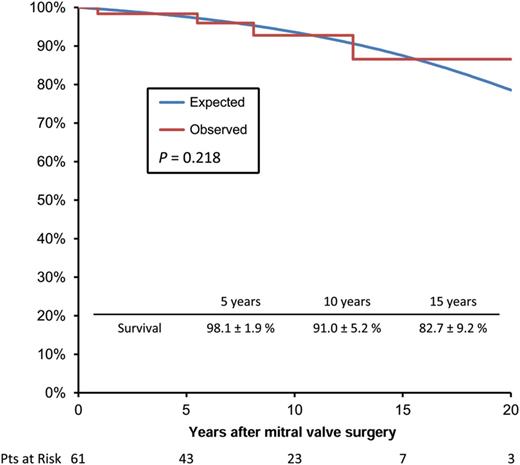
Overall survival curve of the study population compared with an age- and sex-adjusted general population (National Institute of Statistics, census 2011). Pts: patients.
Only 3 patients required a further MV operation (7.9%) during follow-up; 1 patient had a mitral prosthesis thrombosis and the other 2, who had the MV repaired (6.3%) in the first surgery, were reoperated for severe MR and mitral restenosis, respectively. Freedom from mitral reoperation after repair at 5, 10 and 18 years was 100%, 95.8 ± 4.1% (95% CI 76.8–98.4%) and 87.8 ± 8.5% (95% CI 60.2–95.9%), respectively (Fig. 2).
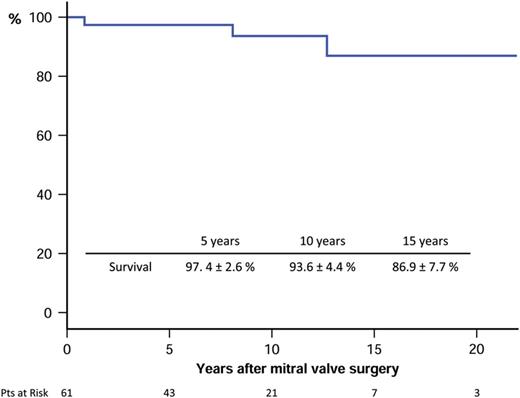
Survival free from mitral reoperation of patients submitted to mitral valve surgery after previous percutaneous mitral commissurotomy. Pts: patients.
At the end of follow-up, the majority of patients (89.5%) were in NYHA class I or II (mean NYHA class 1.58 ± 0.8).
DISCUSSION
Closed mitral commissurotomy, the classic treatment of rheumatic MS, has been almost completely abandoned in favour of PMC or open MV surgery (MVR or OMC) in developed countries. The field of PMC has expanded rapidly in the last two decades [11, 12] and has become the first-line treatment of MS, advocated in the international guidelines [3]. This therapeutic shift, in conjunction with the decline of the incidence of rheumatic fever, has resulted in a swift reduction of OMC and, consequently, left young surgeons ‘unaware’ of this procedure. The lack of exposure to this particular technique has resulted in the situation that the majority of patients who require MV surgery for MS (either as a primary procedure or after failed PMC) will receive a mitral prosthesis.
The absence of large-scale randomized trials that demonstrate unequivocally the superiority of PMC over OMC, save for a couple of small studies [13, 14], is notorious. It is indisputable that PMC is less invasive, therefore more appealing, but the surgical procedure has several methodological advantages, the most obvious being the direct inspection of the MV, enabling the surgeon to appropriately identify the lesions and correct them. In this way, the stenotic subvalvular component, which is one of the Achilles' heel of the PMC, can be approached and relieved. In this regard, the term valvuloplasty, usually used by the intervention cardiologists is not appropriate, because the balloon only treats the fused commissures. But it is not uncommon to find incomplete separation of the commissures or lacerations outside the commissural line in failed PMCs (44.3% in our series) after the procedure. In contrast, surgical repair can achieve a complete splitting of the fused commissures, of the chordae tendineae and of the papillary muscle in the correct place.
We have previously published our experience with OMC [15]. The mean valve area achieved was 2.89 ± 0.49 cm2 immediately after surgery and 2.37 ± 0.42 cm2 (range: 1.6–3.6 cm2) at the 10-year follow-up. The rate of freedom from reoperation was 98% (only 2 patients required reoperation). The 9-year rate of mitral reintervention-free survival after PMC in our institution is 85.5 ± 4.5%, the mean valve area immediately after the procedure was 2.3 ± 0.4 cm2 and the median time to mitral reintervention around 6.2 years. In a 99-month follow-up study after PMC, 16.5% of patients were referred to MV surgery [16].
In our opinion, these data clearly demonstrate the superiority of OMC in obtaining higher MVA and prolonged time of freedom from mitral reintervention. Others have also shown the adequacy of the procedure [17, 18], meaning that experienced centres can accomplish excellent long-lasting results.
The rate of mitral valve reintervention after PMC, either for restenosis or new-onset MR, is not negligible and widely variable, even among centres with a high volume of percutaneous interventions. The rate of freedom from PMC or surgery at 10 years can range from 36 to 88% [6, 19]. Treatment of mitral restenosis is not consensual, with experienced centres claiming for a redo percutaneous procedure whenever there are anatomically favourable conditions. However, careful analysis of reports on repeat PMC indicate that outcomes are far from perfect.
Iung et al. [20] evaluated the results of repeat PMC in 53 patients with symptomatic restenosis (average time to repeat PMC was 6.2 years; 2–11 years). MVA increased on average from 1.03 to 1.82 cm2, a good result being defined as MVA obtained after PMC equal or greater than 1.5 cm2. In this particular study, the rate of freedom from surgery, mainly mitral replacement, at 5 years was only 69%. Pathan et al. [21] also evaluated their results of repeat PMC (n = 36) and obtained an immediate success rate of 75%, and a survival rate free from events (including MVR) at 3 years of only 47%. The mean MVA achieved was 1.9 cm2. Finally, a very recent report from a reference centre evaluated mitral reinterventions after PMC and the efficacy of repeat PMC [22]. Almost half of patients after primary PMC required another intervention during a 20-year period. Surgery was performed in 76% of the reinterventions, but only 9.1% of patients had their valves repaired, which is very low compared to our series (62.3%). The authors conclude that repeat PMC allowed postponement of surgery in a quarter of patients.
This conclusion points to surgery as a dire complication to be avoided at all cost, which, in our opinion, is a misconception. These patients are usually young, with little comorbidity, therefore representing a low surgical risk group. Operative mortality can be close to zero, and higher MVAs and long-term freedom from mitral reoperation demonstrate the superiority of surgery. Furthermore, in all above-mentioned studies, an important number of patients remained with at least mild MS (MVA <1.9 cm2), which is an MVA significantly lower than in our surgically repaired patients (mean MVA = 2.7 cm2). Additionally, none of our patients required mitral reoperation in the first 5 years after surgery, and at 20 years 88% were free from mitral surgery.
There are no doubts regarding how to deal with patients who develop severe MR during follow-up after PMC (around 10%). When they become symptomatic or with signs of left ventricular deterioration, MV surgery is the only effective treatment. MR can be limited to the commissural level (57%) or outside the commissures (43%). It has been suggested that the former could be associated with a lesser probability of mitral replacement (15 vs 70%) [23]. In our experience, 44.3% of patients had a laceration induced by PMC outside the commissures (mainly in the posterior leaflet). However, this lesion did not require mitral replacement and we were able to preserve the valve in 66.7% of those patients.
The introduction of a new method to evaluate the MV during surgery (morphological intraoperative score) appears to be of great utility. The majority of available scores were designed to predict the feasibility of PMC and not surgical repair. These are two distinct procedures and a possible negative factor for one method may not be so for another. For instance, commissural calcification is associated with a worse prognosis and most groups would consider it a contraindication for PMC. We were able to preserve the valve in one-third of patients who had commissural calcification, and considered this finding as a moderate risk factor for the success rate of repair (2 of 3 points in the intraoperative score). In comparison, echocardiographic scores have limitations, such as the limited ability to differentiate nodular fibrosis from calcification, inability to account for uneven distribution of pathological abnormalities and to assess commissural involvement, and frequent underestimation of subvalvular disease.
The available operative classification schemes for OMC are either over simplistic or too complex. Ghosh et al. [24] introduced a scoring system to assist the decision to repair the MV, but included six variables and all sorts of measurements involving the chordae tendineae and the papillary muscles, which makes it less intuitive and practical. We believe that our scoring system, more ‘user-friendly’ and of rapid execution, allows a more straightforward analysis. Beyond that, there is a numerical correspondence with the Wilkins score (score >9 has a poor prognosis as to the feasibility of surgical repair), which facilitates their interpretation among surgeons and cardiologists.
Finally, we also did not find significant differences in overall survival between repair and replacement of the MV, and the study population had a life expectancy similar to the general population (age- and sex-adjusted). However, patients submitted to MVR had more adverse events during follow-up. Hence, whenever possible, MV repair should remain as the procedure of choice. Interestingly, Kim et al. [7] compared PMC and MVR in the setting of restenosis after PMC, demonstrating the superiority of the latter. They came to the conclusion that the surgical method should be the procedure of choice in this context. These results showed that even MVR does not jeopardize patient's survival compared with PMC.
STUDY LIMITATIONS
This study has several shortcomings that deserve mention. Firstly, it is a relatively small population from a single centre, which raises some word of caution with generalizing these results to other populations. However, in the literature, there are few studies analysing specifically this subject and the numbers do not vary much from ours.
Secondly, this is a retrospective analysis with a long time frame; therefore, the presence of unknown confounding factors cannot be ruled out. The small number of events is also a limitation, but can also be viewed as an excellent outcome of MV surgery after PMV.
Finally, we have to acknowledge that freedom from reoperation is far from being a perfect method to evaluate the durability of MV repair, despite the fact that the patients submitted to repair were very young and probably would have been sent to surgery if significant MR or stenosis developed.
CONCLUSIONS
Patients with previous PMC who develop restenosis seem to be better treated with MV surgery than with repeat PMC. Surgical MV repair is feasible in the majority of patients, independently of the type of lesion encountered (stenosis, regurgitation or mixed lesion); hence, it appears to be the preferable solution since it carries a lesser rate of complications in the future. For this reason, these patients should be sent to reference centres with experience in repairing these valves.
Conflict of interest: none declared.
REFERENCES
- mitral valve insufficiency
- percutaneous balloon mitral valvuloplasty
- restenosis
- rheumatic disorders
- mitral valve
- pulmonary hypertension
- follow-up
- objective (goal)
- hospital mortality
- intraoperative care
- lacerations
- repeat surgery
- surgical procedures, operative
- survival rate
- palliative care
- surgery specialty
- mitral valve procedures
- inspection
- calcification




