-
PDF
- Split View
-
Views
-
Cite
Cite
Nimrat Grewal, Adriana C. Gittenberger-de Groot, Marco C. DeRuiter, Robert J.M. Klautz, Robert E. Poelmann, Sjoerd Duim, Johannes H.N. Lindeman, Wilke M.C. Koenraadt, Monique R.M. Jongbloed, Salah A. Mohamed, Hans-Hinrich Sievers, Ad J.J.C. Bogers, Marie-José Goumans, Bicuspid aortic valve: phosphorylation of c-Kit and downstream targets are prognostic for future aortopathy, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 5, November 2014, Pages 831–839, https://doi.org/10.1093/ejcts/ezu319
Close - Share Icon Share
The clinical course of many patients with a bicuspid aortic valve (BAV) is complicated by ascending aortic dilatation. Currently, the indication for aortic surgery is solely based on the aortic diameter and subsequently only a small proportion of BAV patients undergoing valve surgery require concomitant ascending aortic replacement based on these recommendations. Unfortunately, a substantial number of BAV patients still develop aortic dilatation in the future and would potentially benefit from a more aggressive approach towards ascending aortic replacement. We, therefore, designed this study to identify molecular biological markers in the aortic wall predictive of aortopathy in BAV.
Ascending aortic wall specimen of BAV (n = 36) and tricuspid aortic valve (TAV) (n = 23), both without and with (>44 mm) dilatation were investigated histologically and immunohistochemically for the expression of markers for vascular remodelling [transforming growth factor (TGF)-β, phosphorylated Smad2, matrix metalloproteinase 9 (MMP9)], cellular differentiation [c-Kit, phosphorylated-c-Kit, hypoxia-inducable factor-1 alpha (HIF1α)] and haemodynamic influences on the aortic wall [endothelial nitric oxide (eNOS)].
All BAV patients showed significantly less inflammation (P < 0.001) and an altered intima/media ratio when compared with TAV patients. The expression of markers of a signalling pathway characteristic for cellular dedifferentiation, as exemplified by the marked expression of c-Kit, phosphorylated c-Kit and HIF1α; in the dilated BAV group was however completely comparable with only a subgroup of the non-dilated BAV (BAb), whereas the remainder of the non-dilated BAV group (BAa) was significantly distinct. This difference between the dilated BAV and BAa was further confirmed in the expression of TGF-β, phosphorylated Smad2, MMP9 and eNOS. Besides the expression pattern, similarity in the dilated BAV and BAb was also noted clinically in the most common variant of commissure position and conjoined raphe of the BAV. Based on these observations, we consider the BAb group a likely candidate for future dilatation as opposed to the BAa group.
Using a panel of molecular tissue markers, the non-dilated BAV patients can be divided into groups susceptible and non-susceptible to aortopathy.
INTRODUCTION
Bicuspid aortic valve (BAV) is the most common congenital cardiac defect, with a prevalence in the general population in the range 0.5–2% [1]. The clinical course of BAV is often complicated by aortic regurgitation and/or stenosis, infective endocarditis and aortic dilatation. In particular, the latter one forms a critical complication in individuals with BAV, as aortic dilatation carries an increased risk of dissection and rupture. Fifty-nine percent of patients with BAV, below the age of 30 have ascending aortic dilatation, which rises to 88% in people over 80 years old [2]. Currently, guidelines recommend ascending aortic replacement in patients with BAV and an ascending aortic diameter ≥50 mm, and concomitant aortic surgery if the diameter exceeds 45 mm [3]. Unfortunately, aortic diameter alone as a selection criterion is not sufficient for identifying patients with inherent aortic weakness before a life-threatening complication occurs [4, 5]. On the one hand, patients with a non-dilated aortic wall at the time of aortic valve surgery may still develop aortic dilatation in the future, while, on the other hand, some patients will never experience aortic wall dilatation. A preventive ascending aortic replacement would expose this last group of patients, not prone to develop dilatation of the aorta, unnecessarily to the risk of this procedure and ensuing postoperative complications. Therefore, there is an unmet need to identify genetic and/or molecular markers to improve a patient-tailored risk stratification for BAV individuals, applicable prior to or during surgery. Although histological and biochemical differences have been shown between dilated aortic walls of BAV and tricuspid aortic valve (TAV) patients, little is known about the difference in non-dilated aortas. We have previously shown marked histological differences and maturation defects in non-dilated aortas of BAV patients [6]. We were, however, not yet able to demonstrate differences within this group, as would be expected based on the fact that some patients with BAV never develop aortic dilatation. The aim of this study was therefore to identify patients with BAV and a non-dilated aorta who are susceptible for future aortopathy. To identify these patients, we compared dilated and non-dilated aortas of both BAV and TAV patients as controls with respect to biochemical markers of vascular remodelling, cellular differentiation and haemodynamic modifiers. Since developmental data [7] and recent clinical diagnostic data based on commissure position [8] suggest that the orientation of the conjoined raphe is of relevance, we also studied this aspect.
MATERIALS AND METHODS
Aortic tissue samples
Ascending aortic wall biopsies were collected from individuals with BAV and TAV, with or without dilatation. Dilatation was clinically defined by surpassing an ascending aortic wall diameter of 44 mm, based on the American College of Cardiology/American Heart Association guidelines [3]. The institutional ethics committee at the Leiden University Medical Centre (LUMC) approved this study. The Heart Valve Bank, Thoraxcenter, Erasmus Medical Center, Rotterdam, provided six BAV samples without aortic dilatation as these were not suitable for transplantation, as approved by their Scientific Advisory Board. Furthermore, we received five BAV aortic specimens from both the convex and concave side of the aortic wall of patients with dilatation, from the Universitätsklinikum Schleswig-Holstein, Lübeck, Germany.
Patients were divided into four groups: (i) TAV without dilatation, termed TA (n = 11, mean age: 64.5 ± 9.0 years), obtained post-mortem, (ii) TAV with dilatation, termed TAD (n = 12, mean age: 72.3 ± 11.2 years), collected during elective repair, (iii) BAV without dilatation, termed BA (n = 17, mean age: 55.8 ± 9.8 years), representing the six BAV samples provided by the Heart Valve Bank Rotterdam and a group of patients who underwent elective stentless root replacement – our preferred technique – while they had no ascending aortic pathology and (iv) BAV with dilatation, termed BAD (n = 19, mean age: 60.7 ± 7.8 years). A small subgroup of BAD (n = 5, mean age: 52.6 ± 7.9 years, BAD2 group) were selectively studied for differences in the protein expression patterns of the aortic wall between the convex and concave site, obtained during elective repair. We excluded patients with a proven genetic disorder identified by genetic tests [e.g. Marfan's disease, familial thoracic aortic aneurysm and dissection (FTAAD)]. Characteristics of enrolled patients are presented in Table 1. All patients in BAV undergoing surgery had a stenotic valve with either mild or no regurgitation. The patients thus had comparable valve pathology. We also investigated the orientation of the conjoined leaflets in BAVs: the raphe was noted either between the right to non-coronary (RCC/NCC), right to left coronary (RCC/LCC) and left to non-coronary (LCC/NCC) cusp or was defined as unicuspid, with fusion at two commissure sites.
| Characteristics . | TA . | TAD . | BA . | BAD . | BAD2 . |
|---|---|---|---|---|---|
| N = 11 . | N = 12 . | N = 17 . | N = 19 . | N = 5 . | |
| Age (years) | 64.5 ± 9.0 | 72.3 ± 11.2 | 55.8 ± 9.8 | 60.7 ± 7.8 | 52.6 ± 7.9 |
| Males (%) | 54.5 | 33.3 | 70.1 | 84.2 | 40 |
| Females (%) | 45.5 | 66.7 | 29.4 | 15.8 | 60 |
| Ascending aortic diameter (mean) | a | 55.0 ± 10.7 | 36.5 ± 7.4b | 52.7 ± 6.2 | 56.2 ± 10.5 |
| Characteristics . | TA . | TAD . | BA . | BAD . | BAD2 . |
|---|---|---|---|---|---|
| N = 11 . | N = 12 . | N = 17 . | N = 19 . | N = 5 . | |
| Age (years) | 64.5 ± 9.0 | 72.3 ± 11.2 | 55.8 ± 9.8 | 60.7 ± 7.8 | 52.6 ± 7.9 |
| Males (%) | 54.5 | 33.3 | 70.1 | 84.2 | 40 |
| Females (%) | 45.5 | 66.7 | 29.4 | 15.8 | 60 |
| Ascending aortic diameter (mean) | a | 55.0 ± 10.7 | 36.5 ± 7.4b | 52.7 ± 6.2 | 56.2 ± 10.5 |
aData unavailable, clinically defined as non-dilated by the pathologist.
bData unavailable for 5 patients, clinically defined as non-dilated by the pathologist.
TA: tricuspid valve, without dilatation; TAD: tricuspid valve, with dilatation; BA: bicuspid valve, without dilatation; BAD: bicuspid valve, with dilatation.
| Characteristics . | TA . | TAD . | BA . | BAD . | BAD2 . |
|---|---|---|---|---|---|
| N = 11 . | N = 12 . | N = 17 . | N = 19 . | N = 5 . | |
| Age (years) | 64.5 ± 9.0 | 72.3 ± 11.2 | 55.8 ± 9.8 | 60.7 ± 7.8 | 52.6 ± 7.9 |
| Males (%) | 54.5 | 33.3 | 70.1 | 84.2 | 40 |
| Females (%) | 45.5 | 66.7 | 29.4 | 15.8 | 60 |
| Ascending aortic diameter (mean) | a | 55.0 ± 10.7 | 36.5 ± 7.4b | 52.7 ± 6.2 | 56.2 ± 10.5 |
| Characteristics . | TA . | TAD . | BA . | BAD . | BAD2 . |
|---|---|---|---|---|---|
| N = 11 . | N = 12 . | N = 17 . | N = 19 . | N = 5 . | |
| Age (years) | 64.5 ± 9.0 | 72.3 ± 11.2 | 55.8 ± 9.8 | 60.7 ± 7.8 | 52.6 ± 7.9 |
| Males (%) | 54.5 | 33.3 | 70.1 | 84.2 | 40 |
| Females (%) | 45.5 | 66.7 | 29.4 | 15.8 | 60 |
| Ascending aortic diameter (mean) | a | 55.0 ± 10.7 | 36.5 ± 7.4b | 52.7 ± 6.2 | 56.2 ± 10.5 |
aData unavailable, clinically defined as non-dilated by the pathologist.
bData unavailable for 5 patients, clinically defined as non-dilated by the pathologist.
TA: tricuspid valve, without dilatation; TAD: tricuspid valve, with dilatation; BA: bicuspid valve, without dilatation; BAD: bicuspid valve, with dilatation.
Following excision, all specimens were fixed in formalin for 24 h, decalcified in Kristensen's solution (a formic acid–formate buffer) for 120 h and subsequently embedded in paraffin. Transverse sections (5 µm) were mounted on precoated Starfrost slides (Klinipath B.V., 3057-1, Duiven, Netherlands) comparing different stainings on consecutive sections.
Immunohistochemistry
In this article, we focused on the expression of proteins known to be involved in vascular remodelling [transforming growth factor (TGF)-β, phosphorylated Smad2 (pSmad2) and matrix metalloproteinase 9 (MMP9)] and cellular differentiation [alpha smooth muscle actin (αSMA) and c-Kit [3, 9]] and possible haemodynamic influences on the convex and concave area of the dilated BAV aortic wall [endothelial nitric oxide (eNOS)].
For immunohistochemical staining, sections were deparaffinated and rehydrated before antigen retrieval in citrate buffer (microwave, 92-98°C, 12 min). Inhibition of endogenous peroxidase was performed with 0.03% H2O2 in phosphate buffered saline (PBS) (20 min). Non-specific staining was reduced by blocking with PBS-Tween-20 (PBS-T) with 1% bovine serum albumin (1% BSA, Sigma-Aldrich, USA). Subsequently, the slides were incubated overnight at room temperature (20°C) with diluted primary antibodies against: eNOS 1/100 (Thermo Scientific, PA1037), MMP-9 1/100 (MCA2736), panTGF-β 1/1000 (MO-C40009E), pSmad2 1/250 (Cell Signaling, 3108), fibulin-1 1/100 (Santa Cruz, sc-20818), c-Kit 1/100 (DAKO, A4502) and phosphorylated c-Kit (pc-Kit) 1/100 (Abcam ab62154). All primary antibodies were dissolved in PBS-T with 1% BSA. Control staining was performed using PBS-T and 1% BSA as the primary step. Between subsequent incubation steps, all slides were rinsed with PBS (2×) and PBS-T (1×). The slides were incubated with secondary antibodies (45 min): for eNOS, c-Kit and pc-Kit with goat anti-rabbit biotin 1/200 (Vector Laboratories, USA, BA-1000) and goat serum 1/66 (Vector Laboratories, USA, S1000) in PBS-T; for MMP-9, panTGF-β and pSmad2 with horse anti-mouse biotin 1/200 (Santa Cruz Biotechnology, Inc., CA, USA, SC-9996-FITC) in horse serum 1/66 (Brunschwig Chemie, Switzerland, S-2000) in PBS-T. Subsequently, slides were incubated with ABC reagent (Vector Laboratories, USA, PK 6100) (45 min). The slides were incubated with 400 µg/ml 3–3'di-aminobenzidin tetrachloride (DAB, Sigma-Aldrich Chemie, USA, D5637) dissolved in Tris-maleate buffer (pH7.6) to which 20 µl of 30% H2O2 was added (10 min). Counterstaining was performed with 0.1% haematoxylin (Merck, Darmstadt, Germany) (10 s), followed by rinsing with tap water (10 min). Finally, slides were dehydrated and mounted with Entellan (Merck, Darmstadt, Germany).
To differentiate c-Kit positive cells from mast cells expressing c-Kit, deparaffinated sections were stained with 0.1% toluidine blue (2 min), rinsed in distilled water, quickly dehydrated and cover-slipped. Toluidine blue staining excluded the possibility that the observed cells were mast cells, which appeared as metachromatic reaction granules containing heparin or histamine. The mast cells had a unique morphological appearance, and were rare among the c-Kit positive cells lining the adventitial–medial border.
Double immunofluorescence
To detect coexpression, we performed double immunofluorescent stainings. Sections were deparaffinated, rehydrated and subjected to antigen retrieval as described. Tissue sections were incubated with primary antibodies pc-Kit 1/100 and hypoxia-inducable factor-1 alpha (HIF1α) 1/50 overnight (4°C), followed by incubation with secondary antibody Cy3 donkey anti-mouse immunoglobulin G (IgG) (Jackson Immunoresearch, 715-165-150) for HIF1α and Alexa Fluor 647 donkey anti-rabbit IgG (Invitrogen, A-31573) for pc-Kit (1 h, 20°C). Cy3 and Alexa Fluor 647 were preferred secondary antibodies, because of green autofluorescence of the elastic lamellae. Nuclei were visualized with 4',6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). Finally, slides were mounted with ProlonGold (Invitrogen, P36930).
Histological parameters, immunohistochemical analyses and morphometry
To provide a basis for histopathological characteristics, we followed the techniques as previously described in our study on the differentiation differences between the aortic wall of BAV and TAV patients [6]. In addition, cytoplasmatic expression levels of MMP-9 and c-Kit, nuclear expression of pSmad2, pc-Kit and HIF1α, cytoplasmatic and extracellular expression of TGF-β, and cytoplasmatic and nuclear expression of eNOS were analysed using a BM500 microscope with plan achromatic objectives (Leica Microsystems, Wetzlar, Germany) on three predetermined locations (left, middle and right) of every section, which we refer to as ‘microscopic fields’ (MFs), and preserved in the evaluation of all stainings. In each MF, the level of expression was indexed on the three anatomical layers of the aortic wall (tunica intima, media and adventitia) as 0 (no expression in the respective layer), 2 (expression in less than one-third of the layer), 4 (expression in two-thirds of the layer) and 6 (expression in the whole layer). To determine the level of eNOS expression, the number of positively stained nuclei and cytoplasm was counted and analysed using ImageJ in the three fields for each stained section. A threshold was applied to filter background noise. The total number of cells (positively and negatively stained nuclei and cytoplasm) was not different between specimens. Therefore, in each MF the number of eNOS+ cells was normalized to the total number of cells per 105 µm2. Finally, the number of normalized positive cells for each staining was averaged between the three MFs. All specimens were re-evaluated by an independent, experienced histopathologist who was blinded to the clinical data.
Statistical analyses
All numerical data are presented as mean ± standard deviation of 3 fixed MFs on each stained slide. Statistical differences were evaluated with the Mann–Whitney U-test for comparison between the groups. Significance was assumed when P < 0.05 using the SPSS 20.0 software program (SPSS, Inc., Chicago, IL, USA). We have performed a one-, two- and three-way analysis of covariance test to correct for age and gender. The Graphpad software was used to create graphics of statistical analysis.
RESULTS
Histological observations
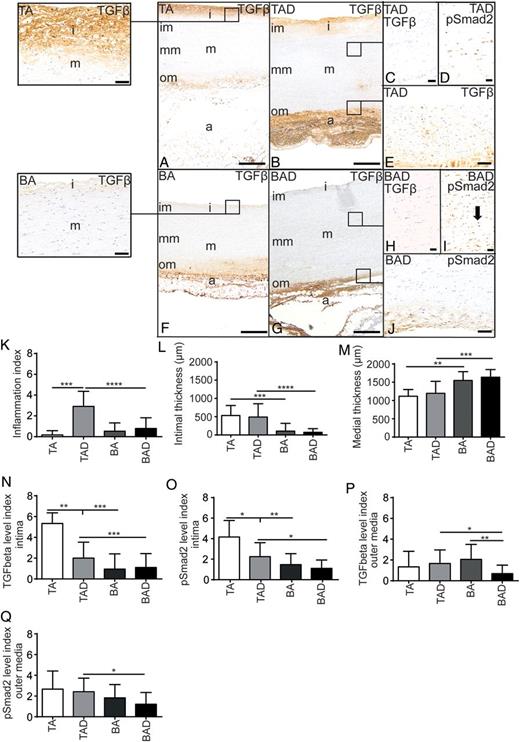
Transverse histological sections of the aortic wall (A–J), consisting of an intima (i), media (m), further subdivided in an inner (im), middle (mm), outer media (om) and adventitia (a). TGF-β: transforming growth factor-β; pSmad2: phosphorylated Smad2; TA: tricuspid valve without dilatation; TAD: tricuspid valve with dilatation; BA: bicuspid valve without dilatation; BAD: bicuspid valve with dilatation. *P < 0.05, **P < 0.01; ***P < 0.001 and ****P < 0.0001. Scale bars (A, B, F and G, 500 µm; E and J, 100 µm; C, D, H and I, 50 µm).
The adventitia consists of loose fibrous tissue containing nerve fibres, fibroblasts, adipocytes, a few quiescent resident inflammatory cells and vasa vasorum, lined by endothelial cells and vascular smooth muscle cells (VSMCs). Characteristics of the differences in histopathology between the TAV and BAV groups which we recently reported [6] are summarized in Fig. 1A, B, F, G, K–M.
Transforming growth factor-β, pSmad2 and endothelial nitric oxide
We examined two components of the TGF-β pathway, TGF-β and pSmad2, involved in vascular remodelling. TGF-β expression spread out from the intima to the inner media (Fig. 1A and B). Intimal expression was significantly lower in all specimens of the BAV (BA and BAD) when compared with all TAV groups (TA and TAD) (P = 0.0058 and P = 0.0076, respectively) (Fig. 1A, B, F, G and N). The middle media was completely devoid of TGF-β expression in both groups, but in the outer media, on the adjacent adventitial side, expression was visible as a gradient (Fig. 1A, B, E–G). In the outer media TGF-β expression was significantly lower in the BAD when compared with TAD and BA groups (P = 0.026 and P = 0.0035, respectively) (Fig. 1B, E–G, J and P).
The expression of the downstream mediator of the TGF-β signalling pathway, pSmad2, was in general comparable with its ligand TGF-β. pSmad2 expression was seen in the intima, and significantly lower expressed in all BAV when compared with all TAV groups (P = 0.0020 and P = 0.0179) (Fig. 1O). Expression of pSmad2 in the outer media was lower in the BAD compared with the TAD group (P = 0.012) (Fig. 1J and Q). Unlike TGF-β, which was only seen in a gradient in the inner and outer media, pSmad2 was observed in the complete media, including the middle media (Fig. 1C, D, H and I).

Transverse histological section of the aortic wall (A and D) with details (B and E). eNOS: endothelial nitric oxide; TA: tricuspid valve without dilatation; TAD: tricuspid valve with dilatation; BA: bicuspid valve without dilatation; BAD: bicuspid valve with dilatation. *P < 0.05, **P < 0.01, scale bar (A and D 50 µm; B and E, 10 µm).
Vascular smooth muscle cells expression of c-Kit
To further elucidate the pathological changes in the aortic wall, we stained the sections for c-Kit (Fig. 2D and E), while c-Kit is well known as a stem cell marker. We observed staining solely in the cytoplasm of a subset of VSMCs in the media and no expression in the intima. The level of expression was significantly higher in the BA group when compared with the TA (P = 0.0024, (Fig. 2F), which was inverted in the dilated aortic walls where there was a significantly lower expression in the BAD compared with the BA group (P = 0.028); whereas in the TAD compared with the TA group (P = 0.021) (Fig. 2F), a significant higher level was noted. Toluidine blue staining of consecutive sections excluded the possibility that the observed cells were mast cells. As we recently described that the aortic wall in all patients with BAV is less well differentiated compared with the TAV [6] and c-Kit was highly present in the less differentiated vascular wall when compared with the mature walls, we conclude that the c-Kit expressing cells are not stem cells but dedifferentiated VSMCs [9].
Bicuspid valve without dilatation variability and vascular wall remodelling
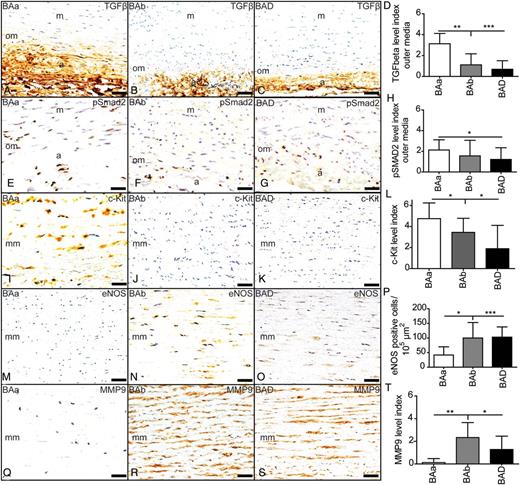
Subdivision of the group patients with a bicuspid aortic valve, without dilatation (BA) in a BAa and BAb group, compared with bicuspid aortic valve with dilatation (BAD). a: adventitia; m: media; om: outer media; mm: middle media; TGF-β: transforming growth factor-β; pSmad2: phosphorylated Smad2; eNOS: endothelial nitric oxide; MMP-9: matrix metalloproteinase-9. *P < 0.05, **P < 0.01 and ***P < 0.001, scale bar: 50 µm.
To further analyse whether the differences found between the BAa and Bab groups could be associated with differences in vascular wall remodelling, we analysed the expression of αSMA and MMP9. While αSMA expression was significantly lower in both dilated and non-dilated BAVs when compared with all TAVs (P = 0.015 and P = 0.044, respectively) [6], its expression pattern was not variable within the BA group. This is in contrast to the expression of MMP9, for which the two BA subgroups could be distinguished. The BAa group showed almost absent expression of MMP9, whereas significant expression was seen in the BAD, comparable with the BAb group (Fig. 3Q–T).
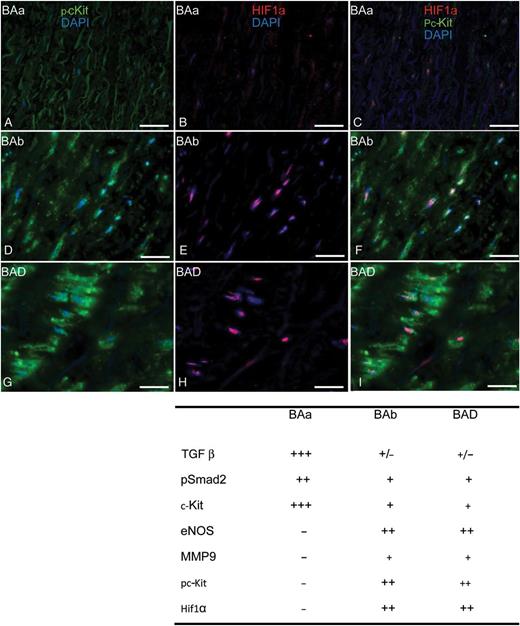
Transverse histological sections of the aortic wall (A–I). Immunofluorescent staining of pc-Kit (green), HIF1α (red) and DAPI (blue). Scale bar: 25 µm.
Commissure position in bicuspid aortic valve
| Commissure position . | BA susceptible . | BA non-susceptible . |
|---|---|---|
| N = 9 . | N = 8 . | |
| Unicuspid, n (%) | 1 (13%) | |
| RCC/LCC, n (%) | 6 (67%) | 2 (25%) |
| RCC/NCC, n (%) | 4 (50%) | |
| LCC/NCC, n (%) | 1 (11%) | |
| Unknown, n (%) | 2 (22%) | 1 (13%) |
| Commissure position . | BA susceptible . | BA non-susceptible . |
|---|---|---|
| N = 9 . | N = 8 . | |
| Unicuspid, n (%) | 1 (13%) | |
| RCC/LCC, n (%) | 6 (67%) | 2 (25%) |
| RCC/NCC, n (%) | 4 (50%) | |
| LCC/NCC, n (%) | 1 (11%) | |
| Unknown, n (%) | 2 (22%) | 1 (13%) |
Commissure position in subdivided groups with bicuspid aortic valve, without dilatation: BA, susceptible and BA, non-susceptible.
RCC/LCC: right to left coronary leaflet fusion; RCC/NCC: right to non-coronary leaflet fusion; LCC/NCC: left to non-coronary leaflet fusion.
| Commissure position . | BA susceptible . | BA non-susceptible . |
|---|---|---|
| N = 9 . | N = 8 . | |
| Unicuspid, n (%) | 1 (13%) | |
| RCC/LCC, n (%) | 6 (67%) | 2 (25%) |
| RCC/NCC, n (%) | 4 (50%) | |
| LCC/NCC, n (%) | 1 (11%) | |
| Unknown, n (%) | 2 (22%) | 1 (13%) |
| Commissure position . | BA susceptible . | BA non-susceptible . |
|---|---|---|
| N = 9 . | N = 8 . | |
| Unicuspid, n (%) | 1 (13%) | |
| RCC/LCC, n (%) | 6 (67%) | 2 (25%) |
| RCC/NCC, n (%) | 4 (50%) | |
| LCC/NCC, n (%) | 1 (11%) | |
| Unknown, n (%) | 2 (22%) | 1 (13%) |
Commissure position in subdivided groups with bicuspid aortic valve, without dilatation: BA, susceptible and BA, non-susceptible.
RCC/LCC: right to left coronary leaflet fusion; RCC/NCC: right to non-coronary leaflet fusion; LCC/NCC: left to non-coronary leaflet fusion.
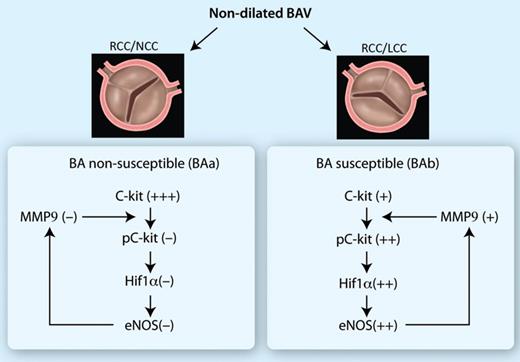
The observed differences in expression of molecular biologic markers are presented in a cascade. BAa: non-susceptible group, BAb: susceptible group.
DISCUSSION
In this study, we found two distinct expression patterns in patients with BAV without apparent dilatation. One pattern is completely comparable with the expression pattern seen in BAV patients with aortic dilatation, suggestive of a different state of vulnerability. To date, decision-making for preventive aortic root replacement in patients with BAV is solely based at the population level on the aortic diameter. However, although thoracic aortic dilatation forms the most critical complication, not all BAV patients are at risk for dissection and rupture. Additional criteria are needed to identify patients at risk as early as possible. Earlier, clinical parameters, such as the morphological appearance of the commissure position of BAV, have been considered [8, 11]; however, these tools are not conclusive. Therefore we searched for additional morphological features of the aortic wall, suggestive of vulnerability for future complications, which could either alone or in combination with the commissure position be applied as a patient-tailored risk stratification.
When analysing the aortic wall histologically, we could not substantiate the observation made by many thoracic surgeons of a thinner aortic wall in BAV. Although this is a striking discrepancy with the clinical findings, other studies also reported that the total wall thickness, excluding the adventitia, is not different between BAV and TAV groups [12]. The difference in the ratio between the intimal and medial thickness might therefore have been interpreted as difference in the wall thickness.
We previously confirmed that inflammation was much more pronounced in the dilated TAV groups than in any other group [6]. We also showed that the aortic wall in BAV patients is intrinsically different from those in TAV patients, as in TAV inflammation and accelerated ageing led to aortic pathology [6]. In all cases with BAV, in both the dilated and non-dilated groups, there is a maturation defect of the aortic wall, showing less well-differentiated VSMCs and a low lamin A/C and progerin expression [6]. As the inflammatory status and the intima/medial thickness ratio are similar in all BAVs, these factors could not serve as a marker to identify the subset of BAV patients susceptible to future aortopathy. We, therefore, then focused on differences in the expression of markers/proteins involved in vascular remodelling (TGF-β, phosphorylated Smad2, MMP9), cellular differentiation (c-Kit [9], phosphorylated-c-Kit,MMP9, HIF1α) and eNOS as a marker of possible haemodynamic influences.
We could distinguish a marked variability in the expression pattern of the above-indicated set of differentiation markers within the non-dilated BAV group. We see a comparable expression pattern in the BAD and BAb subgroups, different from that in the BAa group. We, therefore, postulate that the BAb is susceptible to dilatation, while the BAa group is non-susceptible to aortopathy. We hypothesize that the enhanced expression of the dedifferentiation markers and eNOS induces a cascade that will lead to a less stable aortic wall which is accompanied by a decrease in the expression of TGF β and pSmad2 correlating with (future) aortic wall dilatation. The precise mechanism causing the decrease in expression of the vascular remodelling markers in the BAb and BAD groups is not yet identified, and is a focus for future research. Further study also is necessary to determine the developmental and environmental factors that initiate the cascade and thus the distinction in a susceptible and non-susceptible group. The findings and hypothesis are summarized in Fig. 5.
Finally, we considered the morphological appearance of the commissure position as a determinant of susceptibility, since commissure position, which can clinically be analysed echocardiographically, is reported to be important in predicting future aortopathy [8, 11]. Previously, several studies reported that BAVs with an RCC/LCC BAV, which is the most common variant, are associated with more aortic dilatation in adults, whereas BAVs with fusion of the RCC/NCC are responsible for valve dysfunction at a younger age [13–18]. Although the groups were small in our study, the orientation of the commissure and position of the raphe was in line with previous findings: RCC/NCC was most apparent in the BAa (the considered non-susceptible group), while the RCC/LCC was seen more often in the BAb group (the considered susceptible group) and in BAD patients (Table 2). Recent preclinical studies showed that RCC/NCC and RCC/LCC likely have a different pathogenesis [19, 20]. RCC/NCC BAVs are observed in eNOS −/− mouse embryos [19, 21, 22], which is comparable with our BAa-non-susceptible group, as this group also shows almost absent expression of eNOS and has the RCC/NCC BAV type as the most common variant.
Although more research is needed, we suggest that identifying patients with an RCC/LCC commissure type could be the first step in selecting patients for a preventive aortic root replacement; however, as some variation in commissure position is apparent, patient selection could not solely be based on valve morphology. The proposed activation cascade should also be taken into account and the combination is a possible protocol for decision-making. To choose the best markers for clinical applications, we first need to question whether decision-making for aortic replacement surgery will be possible before or only during surgery, as this influences the choice of markers. The clearest difference in expression level between the susceptible and non-susceptible BA group was seen for MMP9, phosphorylated c-Kit and Hif1α, and these would be most appropriate to serve as clinical biomarkers. Analysis of these markers would be relatively easy to perform using a quick histological frozen section during surgery. A disadvantage is that a biopsy from the aortic wall is only possible after the patient is on extracorporeal circulation and the aorta is clamped. Immunohistochemical analyses are time consuming and increases the time the patient is on bypass. Preoperative decision making would be preferable, and we will therefore be concentrating in future studies on factors detectable in blood. Not only will the decision be formed preoperatively, a blood test is also less invasive. Therefore, we will explore if any of the markers identified in this study can be measured, is sensitive enough to distinguish the susceptible patients and specific enough to recognize patients without expression and thus being non-susceptible.
Study limitations
We designed our study by comparing the expression of a panel of markers distinguishing cases of non-dilated aorta of BAV with BAV that will progress to dilatation over time. A limitation of our study is that we did not have frozen tissue samples of all the aortic wall specimens we received fixed in formalin from the various groups. Therefore, we could not perform a western blot to correlate this to our findings of immunohistochemistry. To secure greater confidence in the obtained results, an animal model that recapitulates the c-Kit phosphorylation and downstream HIF1α biology, using knock-down or c-Kit inhibition strategies is needed.
ACKNOWLEDGEMENTS
We thank the Heart Valve Bank Rotterdam, Netherlands, for providing the described cryopreserved valves.
Conflict of interest: none declared.
REFERENCES
- aortic valve
- nitric oxide
- hemodynamics
- fibrinogen
- bicuspid aortic valve
- dilatation of aorta
- inflammation
- vascular remodeling
- hypoxia
- anaplasia
- biological markers
- dilatation, pathologic
- endothelium
- gelatinase b
- nitric oxide synthase
- phosphorylation
- proto-oncogene protein c-kit
- transforming growth factors
- tunica intima
- patient prognosis
- signal pathway
- signal transduction pathways
- aortic surgery
- replacement of ascending aorta
- aortic diameter
- aortic wall




