-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Forteza, Jorge Centeno, Maria Jesus López, Violeta Sánchez, Enrique Perez, Beatriz López, Juan Jose Rufilanchas, Pedro Muñoz, Jose Cortina, Should aortic valve reimplantation be offered to patients with a large aorto-ventricular junction?, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages e130–e135, https://doi.org/10.1093/ejcts/ezt052
Close - Share Icon Share
Abstract
Large aortic root aneurysms might increase leaflet stress and compromise aortic valve durability after the reimplantation technique. We analysed the impact of the preoperative aorto-ventricular junction (AVJ) diameter on the durability of the valve.
Between March 2004 and January 2012, 150 patients underwent the David operation on the aortic root. We identified 47 patients with a preoperative AVJ >28 mm (Group A) and 103 patients with a diameter ≤28 mm (Group B). The mean follow-up was 44 ± 27 months. Both groups were compared regarding mortality, freedom from moderate or severe aortic valve regurgitation and freedom from reoperation.
Early mortality was 1.3%. Actuarial survival at 1, 3 and 5 years was 97 ± 2, 94 ± 3 and 94 ± 3% for Group A, and 99 ± 1, 97 ± 1 and 94 ± 3% for Group B, respectively (P = 0.3). Two patients in Group B were reoperated for severe aortic regurgitation (AR). Actuarial freedom from reoperation at 1, 3 and 5 years was 100% for Group A, and 98 ± 1, 98 ± 1 and 96 ± 2% for Group B, respectively (P = 0.3). During the follow-up, 6 patients (3 in each group) developed AR ≥Grade II. Therefore, actuarial freedom from AR grade II or greater at 1.3 and 5 years was 97 ± 2, 94 ± 4 and 87 ± 7% for Group A, and 99 ± 1, 97 ± 1 and 95 ± 2% for Group B (P = 0.3).
The reimplantation technique shows excellent results. Medium-term stability of the aortic valve repair was not influenced by the preoperative aorto-ventricular junction diameter.
INTRODUCTION
The reimplantation technique to treat aortic root aneurysms was introduced by David [1] in 1992 to avoid complications associated with prostheses and chronic anticoagulation treatment, particularly in young patients. David's technique yields great stabilization of all components of the aortic root, and therefore is currently considered to be the method of choice in patients with annuloaortic ectasia [2, 3].
Recently, many authors have [4–7] emphasized the importance of correcting the aorto-ventricular junction (AVJ) dilatation in valve-sparing operations, although there is no consensus about how we should normalize this diameter, and very few reports have been published analysing the influence of this dilatation in the mechanical leaflet stress and therefore in the durability of the valve after the repairing procedure. Tambeur et al. [8] speculated that valve-sparing procedures in patients with a preoperative aortic root diameter over 55 mm might lead to premature failure of the repair due to an increased mechanical leaflet stress. However, they did not analyse the effect of the AVJ dilatation on the aortic valve function. Leyh et al. [9] tried to analyse the influence of the preoperative diameter of the Valsalva sinuses (VS) on the durability of the valve, although they did not analyse the diameter of the AVJ.
Consequently, we conducted a descriptive study to analyse the influence of the preoperative AVJ on the durability of the valve in 150 patients.
MATERIALS AND METHODS
Between March 2004 and January 2012, 150 patients with aortic root aneurysms underwent valve-sparing surgery using the technique described by David [1]. The clinical characteristics of the cohort are summarized in Table 1. Conforming to the preoperative AVJ diameter, we obtained two groups: Group A (47 patients with an AVJ diameter >28 mm) and Group B (103 patients with an AVJ diameter ≤28 mm). Both groups were comparable in all the variables except for the incidence of Marfan syndrome (MFS). This condition was present in 29 patients in Group A (59%) vs 31 patients in Group B (30%), P = 0.001. Patients were diagnosed as MFS according to Ghent nosology [10], and in these cases, surgery was indicated when the aortic diameter at the VS was ≥50 mm (diameter was measured at end-diastole from the inner edge of the aortic wall to the opposite inner edge) or >45 mm when the progressive growth of the aorta was >2 mm per year or when there was a familiar history of acute aortic dissection. In non-MFS patients, the procedure was indicated when the diameter of the VS was >55 mm (50 mm in patients with bicuspid valve), except in 8 cases where the indication was due to the aortic regurgitation (AR).
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Mean age ± SD | 39 ± 16 | 48 ± 17 | 0.213 |
| Gender (M/F) | 40/7 | 80/23 | 0.201 |
| NYHA I-II | 45 (95.7) | 100 (97) | 0.267 |
| Marfan syndrome | 28 (59) | 31 (30) | 0.001 |
| Bicuspid aortic valve | 8 (17) | 17 (16) | 0.313 |
| Acute aortic dissection | 2 (4) | 0 | 0.211 |
| Preoperative Valsalva sinus diameter | 0.285 | ||
| ≥60 mm | 7 (15) | 11 (11) | |
| 50–60 mm | 22 (47) | 48 (47) | |
| <50 mm | 18 (38) | 43 (42) | |
| Preoperative aortic insufficiency | 0.217 | ||
| Grade 0 | 25 (53.1) | 41 (39.8) | |
| Grade I | 3 (6.3) | 10 (9.7) | |
| Grade II | 7 (14.8) | 11 (10.6) | |
| Grade III–IV | 12 (25.5) | 41 (39.8) |
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Mean age ± SD | 39 ± 16 | 48 ± 17 | 0.213 |
| Gender (M/F) | 40/7 | 80/23 | 0.201 |
| NYHA I-II | 45 (95.7) | 100 (97) | 0.267 |
| Marfan syndrome | 28 (59) | 31 (30) | 0.001 |
| Bicuspid aortic valve | 8 (17) | 17 (16) | 0.313 |
| Acute aortic dissection | 2 (4) | 0 | 0.211 |
| Preoperative Valsalva sinus diameter | 0.285 | ||
| ≥60 mm | 7 (15) | 11 (11) | |
| 50–60 mm | 22 (47) | 48 (47) | |
| <50 mm | 18 (38) | 43 (42) | |
| Preoperative aortic insufficiency | 0.217 | ||
| Grade 0 | 25 (53.1) | 41 (39.8) | |
| Grade I | 3 (6.3) | 10 (9.7) | |
| Grade II | 7 (14.8) | 11 (10.6) | |
| Grade III–IV | 12 (25.5) | 41 (39.8) |
SD: standard deviation; M: male; NHYA: New York Heart Association; F: female; percentages are shown in parentheses.
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Mean age ± SD | 39 ± 16 | 48 ± 17 | 0.213 |
| Gender (M/F) | 40/7 | 80/23 | 0.201 |
| NYHA I-II | 45 (95.7) | 100 (97) | 0.267 |
| Marfan syndrome | 28 (59) | 31 (30) | 0.001 |
| Bicuspid aortic valve | 8 (17) | 17 (16) | 0.313 |
| Acute aortic dissection | 2 (4) | 0 | 0.211 |
| Preoperative Valsalva sinus diameter | 0.285 | ||
| ≥60 mm | 7 (15) | 11 (11) | |
| 50–60 mm | 22 (47) | 48 (47) | |
| <50 mm | 18 (38) | 43 (42) | |
| Preoperative aortic insufficiency | 0.217 | ||
| Grade 0 | 25 (53.1) | 41 (39.8) | |
| Grade I | 3 (6.3) | 10 (9.7) | |
| Grade II | 7 (14.8) | 11 (10.6) | |
| Grade III–IV | 12 (25.5) | 41 (39.8) |
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Mean age ± SD | 39 ± 16 | 48 ± 17 | 0.213 |
| Gender (M/F) | 40/7 | 80/23 | 0.201 |
| NYHA I-II | 45 (95.7) | 100 (97) | 0.267 |
| Marfan syndrome | 28 (59) | 31 (30) | 0.001 |
| Bicuspid aortic valve | 8 (17) | 17 (16) | 0.313 |
| Acute aortic dissection | 2 (4) | 0 | 0.211 |
| Preoperative Valsalva sinus diameter | 0.285 | ||
| ≥60 mm | 7 (15) | 11 (11) | |
| 50–60 mm | 22 (47) | 48 (47) | |
| <50 mm | 18 (38) | 43 (42) | |
| Preoperative aortic insufficiency | 0.217 | ||
| Grade 0 | 25 (53.1) | 41 (39.8) | |
| Grade I | 3 (6.3) | 10 (9.7) | |
| Grade II | 7 (14.8) | 11 (10.6) | |
| Grade III–IV | 12 (25.5) | 41 (39.8) |
SD: standard deviation; M: male; NHYA: New York Heart Association; F: female; percentages are shown in parentheses.
The Hospital 12 de Octubre's ethics committee approved our study, and all patients gave informed consent to use their clinical information.
Measurement of the preoperative aorto-ventricular junction diameter
All patients underwent a complete 2D and colour Doppler echocardiographic study before surgery, using a digital ultrasonic device system with second harmonic imaging (VIVID 7, GE Vingmed Ultrasound, Horten, Norway). Images in standard planes were obtained preoperatively. Measurements of the preoperative AVJ diameter were performed off-line (Echopac GE Vingmed Ultrasound) according to the recommendations of the current guidelines [11]. The AVJ diameter was measured in systole, in a parasternal long-axis view, zoomed on the left ventricular outflow tract, between the hinge points of the aortic valve leaflets (inner edge–inner edge).
Surgical technique
Our technique has been described previously [12]. Briefly, we established extracorporeal circulation and induced moderate hypothermia (32°C). After clamping the aorta, the aneurysm was resected, leaving a 2–3 mm segment of the aortic wall for later reimplantation of the aortic valve. We used the David Type I technique in 29 patients (using a Gelweave Valsalva graft in 28 cases [Sulzer Vascutek; Renfrewshire, UK], and a straight tubular Dacron Hemashield graft [Medi-Tech, Boston Scientific Corp, Natick, MA, USA] in one case). In the other 121 patients, we used the modification described by Demers and Miller [13]. The AVJ was reduced to 22–23 mm in all patients. Prolapsed aortic leaflets were corrected after performing the valve reimplantation in 42 patients, which involved the following techniques: plication of the free edges at the Arantius' nodules in 34 patients using 6–0 polypropylene suture (Ethicon, LLC, San Lorenzo, PR, USA), free edge reinforcement using Gore-Tex 7/0 sutures (W.L. Gore and Associates; Flagstaff, AZ, USA) in one, decalcification of the leaflets and pericardial reconstruction in 3 and a combination of these techniques in 4. Sub commisural annuloplasty was performed in 14 patients (in all of them the AVJ was over 24 mm). Other procedures associated with aortic root surgery were mitral valve repair in 12 patients, myocardial revascularization in 6 and aortic arch replacement in 4. Intra- and perioperative data are presented in Table 2.
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Surgical technique | |||
| David V | 36 (77) | 85 (82) | 0.223 |
| David I | |||
| Straight graft | 1 (4) | 0 | |
| Valsalva graft | 11 (21) | 18 (18) | |
| CPB time (min ± SD) | 153 ± 41 | 158 ± 45 | 0.321 |
| Aortic cross-clamp time (min ± SD) | 132 ± 32 | 133 ± 33 | 0.224 |
| Size of Dacron prosthesis (mm) | 0.211 | ||
| 34 | 25 (53) | 53 (51) | |
| 32 | 10 (21) | 29 (28) | |
| 30 | 9 (19) | 9 (9) | |
| 28 | 3 (6) | 11 (11) | |
| 26 | 0 | 1 | |
| Concomitant procedures | 0.201 | ||
| Sub commisural annuloplasty | 2 (4) | 12 (12) | |
| Leaflets repair | 11 (23) | 31 (31) | |
| Free edge stitch | 8 | 26 | |
| Free edge Gore-Tex reinforcement | 1 | 0 | |
| Decalcification/pericardial reconstruction | 0 | 3 | |
| Several techniques | 2 | 2 | |
| Aortic arch replacement | 1 | 3 | |
| CABG | 1 | 5 | |
| Mitral valve surgery | 4 | 8 | |
| Operative complications | 0.211 | ||
| Re-exploration for bleeding | 1 | 3 | |
| Permanent CVA | 0 | 0 | |
| AMI | 0 | 4 | |
| Hospital mortality | 1 | 1 | |
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Surgical technique | |||
| David V | 36 (77) | 85 (82) | 0.223 |
| David I | |||
| Straight graft | 1 (4) | 0 | |
| Valsalva graft | 11 (21) | 18 (18) | |
| CPB time (min ± SD) | 153 ± 41 | 158 ± 45 | 0.321 |
| Aortic cross-clamp time (min ± SD) | 132 ± 32 | 133 ± 33 | 0.224 |
| Size of Dacron prosthesis (mm) | 0.211 | ||
| 34 | 25 (53) | 53 (51) | |
| 32 | 10 (21) | 29 (28) | |
| 30 | 9 (19) | 9 (9) | |
| 28 | 3 (6) | 11 (11) | |
| 26 | 0 | 1 | |
| Concomitant procedures | 0.201 | ||
| Sub commisural annuloplasty | 2 (4) | 12 (12) | |
| Leaflets repair | 11 (23) | 31 (31) | |
| Free edge stitch | 8 | 26 | |
| Free edge Gore-Tex reinforcement | 1 | 0 | |
| Decalcification/pericardial reconstruction | 0 | 3 | |
| Several techniques | 2 | 2 | |
| Aortic arch replacement | 1 | 3 | |
| CABG | 1 | 5 | |
| Mitral valve surgery | 4 | 8 | |
| Operative complications | 0.211 | ||
| Re-exploration for bleeding | 1 | 3 | |
| Permanent CVA | 0 | 0 | |
| AMI | 0 | 4 | |
| Hospital mortality | 1 | 1 | |
CPB: cardiopulmonary bypass; SD: standard deviation; CABG: coronary artery bypass grafting; CVA: cerebrovascular accident; AMI: acute myocardial infarct.
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Surgical technique | |||
| David V | 36 (77) | 85 (82) | 0.223 |
| David I | |||
| Straight graft | 1 (4) | 0 | |
| Valsalva graft | 11 (21) | 18 (18) | |
| CPB time (min ± SD) | 153 ± 41 | 158 ± 45 | 0.321 |
| Aortic cross-clamp time (min ± SD) | 132 ± 32 | 133 ± 33 | 0.224 |
| Size of Dacron prosthesis (mm) | 0.211 | ||
| 34 | 25 (53) | 53 (51) | |
| 32 | 10 (21) | 29 (28) | |
| 30 | 9 (19) | 9 (9) | |
| 28 | 3 (6) | 11 (11) | |
| 26 | 0 | 1 | |
| Concomitant procedures | 0.201 | ||
| Sub commisural annuloplasty | 2 (4) | 12 (12) | |
| Leaflets repair | 11 (23) | 31 (31) | |
| Free edge stitch | 8 | 26 | |
| Free edge Gore-Tex reinforcement | 1 | 0 | |
| Decalcification/pericardial reconstruction | 0 | 3 | |
| Several techniques | 2 | 2 | |
| Aortic arch replacement | 1 | 3 | |
| CABG | 1 | 5 | |
| Mitral valve surgery | 4 | 8 | |
| Operative complications | 0.211 | ||
| Re-exploration for bleeding | 1 | 3 | |
| Permanent CVA | 0 | 0 | |
| AMI | 0 | 4 | |
| Hospital mortality | 1 | 1 | |
| Variables . | Group A (n = 47) . | Group B (n = 103) . | P-value . |
|---|---|---|---|
| Surgical technique | |||
| David V | 36 (77) | 85 (82) | 0.223 |
| David I | |||
| Straight graft | 1 (4) | 0 | |
| Valsalva graft | 11 (21) | 18 (18) | |
| CPB time (min ± SD) | 153 ± 41 | 158 ± 45 | 0.321 |
| Aortic cross-clamp time (min ± SD) | 132 ± 32 | 133 ± 33 | 0.224 |
| Size of Dacron prosthesis (mm) | 0.211 | ||
| 34 | 25 (53) | 53 (51) | |
| 32 | 10 (21) | 29 (28) | |
| 30 | 9 (19) | 9 (9) | |
| 28 | 3 (6) | 11 (11) | |
| 26 | 0 | 1 | |
| Concomitant procedures | 0.201 | ||
| Sub commisural annuloplasty | 2 (4) | 12 (12) | |
| Leaflets repair | 11 (23) | 31 (31) | |
| Free edge stitch | 8 | 26 | |
| Free edge Gore-Tex reinforcement | 1 | 0 | |
| Decalcification/pericardial reconstruction | 0 | 3 | |
| Several techniques | 2 | 2 | |
| Aortic arch replacement | 1 | 3 | |
| CABG | 1 | 5 | |
| Mitral valve surgery | 4 | 8 | |
| Operative complications | 0.211 | ||
| Re-exploration for bleeding | 1 | 3 | |
| Permanent CVA | 0 | 0 | |
| AMI | 0 | 4 | |
| Hospital mortality | 1 | 1 | |
CPB: cardiopulmonary bypass; SD: standard deviation; CABG: coronary artery bypass grafting; CVA: cerebrovascular accident; AMI: acute myocardial infarct.
Intraoperative transoesophageal echocardiography was performed after discontinuation of cardiopulmonary bypass to assess the valve function and the aortic root diameters.
Follow-up
Clinical follow-up started 2 months after surgery and on a yearly basis thereafter. All patients were followed clinically and echocardiographically and were last seen between May and December 2011. AR was assessed as 0, none; I, minimal; II, mild; III, moderate; IV, severe. Valve performance and outcome analysis are reported as suggested by the guidelines of the American Association for Thoracic Surgery and the Society of Thoracic Surgeons [14]. Follow-up consultations were made over the telephone with patients from outside of Madrid, and we requested their corresponding echocardiogram reports for analysis. Three patients were missed during the follow-up period (98% of the cohort), which lasted a mean of 44 ± 27 months (range: 2–99).
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Normally distributed continuous variables were presented as means ± standard error of the media. Categoric variables were presented as frequencies and percentages.
The Kaplan–Meier method was used to calculate the actuarial probability of survival, freedom from reoperation or AR, and compared using the log-rank test. We performed univariate and multivariate analysis in the Cox proportional hazards model to identify significant predictors for reoperation or late AR grade II or greater. Variables introduced into the models included those from univariate analysis with P < 0.20. A value of P < 0.05 was considered significant.
RESULTS
Perioperative outcome
The intra- and perioperative data are presented in Table 2. The intraoperative echocardiography study showed a normally functioning aortic valve in all the patients (AR grade 0 or I). There were two hospital deaths, one in each group (P = 0.2). The first, a 7-year old male with Beals syndrome, died from refractory low cardiac output following the operation. In the postoperative coronary angiography, we observed a pharmacological irreversible spasm of the descending anterior and right coronary arteries. The second case was a 60-year old male with no initial postoperative complications, who was discharged from the hospital 9 days after the operation: he was readmitted 5 days later with fever. The transoesophageal echocardiogram indicated a normally functioning aortic valve, with no evidence of infectious endocarditis or pericardial effusion. Six days later, the patient suffered cardiac arrest of an unknown cause. The family did not give authorization for a necropsy.
The operative morbidity in each subgroup is specified in Table 2.
Follow-up
Survival
The mean follow-up was 44 ± 27 months. There were no differences between both groups (the mean follow-up for Group A was 48 ± 29 months and 44 ± 27 months for Group B, P = 0.2).
Four patients died during the follow-up (2 in each group) and none of the deaths was valve related. Two patients died during the first year: one from a ruptured aneurysm of the abdominal aorta and one due to pulmonary thromboembolism. Another patient died 5 years afterward, due to a neoplasia, and the last patient died during the seventh year (she was a young MFS patient and she died on a complicated postoperative course after an abdominal surgery). Actuarial survival at 1, 3 and 5 years was 97 ± 2, 94 ± 3 and 94 ± 3% for Group A, and 99 ± 1, 97 ± 1 and 94 ± 3% for Group B, respectively (P = 0.3) (Fig. 1). We did not find any predictors for late death in univariate analysis.
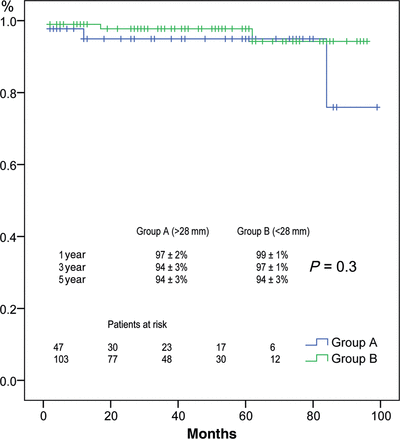
Survival in patients stratified by AVJ diameter >28 mm (blue) or less (green).
Aortic valve regurgitation
Actuarial freedoms from AR grade II or greater at 1.3 and 5 years were 97 ± 2, 94 ± 4 and 87 ± 7% for Group A, and 99 ± 1, 97 ± 1 and 95 ± 2% for Group B, respectively (P = 0.3) (Fig. 2). AR ≥ Grade II developed in 6 patients during the follow-up, 3 in each group (P = 0.4). In univariate analysis, we studied predictors of late aortic valve stability but we did not find statistical significance (Table 3).
| Variables . | Univariate, P-value . | |
|---|---|---|
| . | Group A (n = 47) . | Group B (n = 103) . |
| Aortic valve regurgitation > Grade II | ||
| AVJ diameter >28 mm | 0.177 | 0.986 |
| VS diameter >55 mm | 0.115 | 0.114 |
| Preoperative AoI ≥ Grade II | 0.142 | 0.976 |
| Bicuspid aortic valve | 0.188 | 0.173 |
| Marfan syndrome | 0.124 | 0.165 |
| Cusp repair | 0.117 | 0.123 |
| David I | 0.175 | 0.177 |
| Reoperation | ||
| AVJ diameter >28 mm | 0.121 | 0.186 |
| VS diameter >55 mm | 0.101 | 0.124 |
| Preoperative AoI ≥ Grade II | 0.112 | 0.876 |
| Bicuspid aortic valve | 0.121 | 0.773 |
| Marfan syndrome | 0.124 | 0.135 |
| Cusp repair | 0.116 | 0.122 |
| David I | 0.114 | 0.165 |
| Variables . | Univariate, P-value . | |
|---|---|---|
| . | Group A (n = 47) . | Group B (n = 103) . |
| Aortic valve regurgitation > Grade II | ||
| AVJ diameter >28 mm | 0.177 | 0.986 |
| VS diameter >55 mm | 0.115 | 0.114 |
| Preoperative AoI ≥ Grade II | 0.142 | 0.976 |
| Bicuspid aortic valve | 0.188 | 0.173 |
| Marfan syndrome | 0.124 | 0.165 |
| Cusp repair | 0.117 | 0.123 |
| David I | 0.175 | 0.177 |
| Reoperation | ||
| AVJ diameter >28 mm | 0.121 | 0.186 |
| VS diameter >55 mm | 0.101 | 0.124 |
| Preoperative AoI ≥ Grade II | 0.112 | 0.876 |
| Bicuspid aortic valve | 0.121 | 0.773 |
| Marfan syndrome | 0.124 | 0.135 |
| Cusp repair | 0.116 | 0.122 |
| David I | 0.114 | 0.165 |
AVJ: aorto-ventricular junction; VS: Valsalva sinus; AoI: aortic valve insufficiency.
| Variables . | Univariate, P-value . | |
|---|---|---|
| . | Group A (n = 47) . | Group B (n = 103) . |
| Aortic valve regurgitation > Grade II | ||
| AVJ diameter >28 mm | 0.177 | 0.986 |
| VS diameter >55 mm | 0.115 | 0.114 |
| Preoperative AoI ≥ Grade II | 0.142 | 0.976 |
| Bicuspid aortic valve | 0.188 | 0.173 |
| Marfan syndrome | 0.124 | 0.165 |
| Cusp repair | 0.117 | 0.123 |
| David I | 0.175 | 0.177 |
| Reoperation | ||
| AVJ diameter >28 mm | 0.121 | 0.186 |
| VS diameter >55 mm | 0.101 | 0.124 |
| Preoperative AoI ≥ Grade II | 0.112 | 0.876 |
| Bicuspid aortic valve | 0.121 | 0.773 |
| Marfan syndrome | 0.124 | 0.135 |
| Cusp repair | 0.116 | 0.122 |
| David I | 0.114 | 0.165 |
| Variables . | Univariate, P-value . | |
|---|---|---|
| . | Group A (n = 47) . | Group B (n = 103) . |
| Aortic valve regurgitation > Grade II | ||
| AVJ diameter >28 mm | 0.177 | 0.986 |
| VS diameter >55 mm | 0.115 | 0.114 |
| Preoperative AoI ≥ Grade II | 0.142 | 0.976 |
| Bicuspid aortic valve | 0.188 | 0.173 |
| Marfan syndrome | 0.124 | 0.165 |
| Cusp repair | 0.117 | 0.123 |
| David I | 0.175 | 0.177 |
| Reoperation | ||
| AVJ diameter >28 mm | 0.121 | 0.186 |
| VS diameter >55 mm | 0.101 | 0.124 |
| Preoperative AoI ≥ Grade II | 0.112 | 0.876 |
| Bicuspid aortic valve | 0.121 | 0.773 |
| Marfan syndrome | 0.124 | 0.135 |
| Cusp repair | 0.116 | 0.122 |
| David I | 0.114 | 0.165 |
AVJ: aorto-ventricular junction; VS: Valsalva sinus; AoI: aortic valve insufficiency.
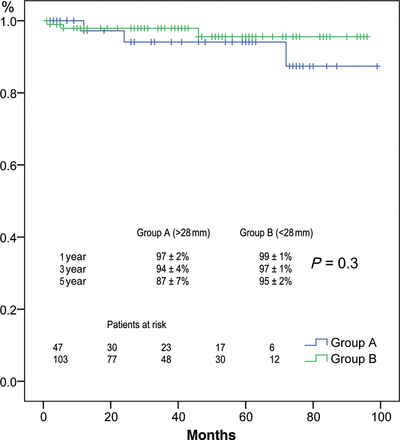
Freedom from AR >Grade II stratified by AVJ diameter >28 mm (blue) or less (green).
Reoperation
Two patients in Group B were reoperated on for severe AR during the follow-up, 1 and 5 years after the surgery. In the first patient, we found two-leaflets prolapse while the other patient showed one-leaflet retraction. In both cases, the diameter of the AVJ was 23 mm. Another patient was reoperated on in the third year due to severe mitral regurgitation. No subsequent operations were required for other causes. Actuarial freedom from reoperation on the aortic valve at 1, 3 and 5 years was 100, 100 and 100% for Group A, and 98 ± 1, 98 ± 1 and 96 ± 2% for Group B, respectively (P = 0.3) (Fig. 3). In univariate analysis, we studied the predictors of reoperation on the aortic valve, but did not find statistical significance (Table 3).
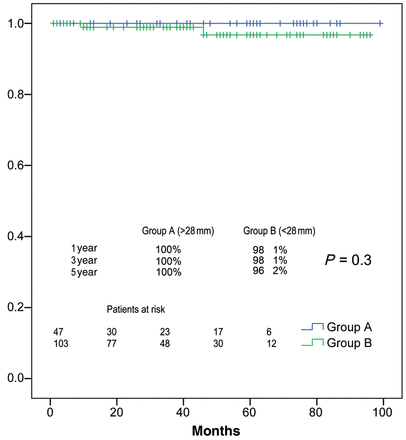
Freedom from reoperation on the aortic valve stratified by AVJ diameter >28 mm (blue) or less (green).
In univariate analysis, we did not find any predictors of reoperation or AR over Grade II during follow-up in the entire cohort. Variables with P < 0.20 were included in the multivariate model and we did not find statistical significance.
DISCUSSION
Reimplantation technique has shown to be adequate to preserve aortic valve function at long-term follow-up in patients with aortic root aneurysms [2, 15–17]. However, there is still controversy about the durability of the valve in conditions such as Marfan syndrome (MS) or in patients with large AVJ [7, 15, 18]. Different data have been presented on long-term valve stability depending on preoperative aortic root geometry, although few reports have been published analysing the influence of AVJ dilatation on mechanical leaflet stress and therefore on the durability of the valve after the repairing procedure. Leyh et al. [9] analysed the outcomes in 123 patients who underwent aortic valve-sparing reimplantation. They showed that the preoperative diameter of the aortic root has no impact on the longevity of the repair, although they did not evaluate the diameter of the AVJ.
Kunihara et al. [7] reported long-term outcomes after valve-sparing aortic root repair in 430 patients (remodelling in 401, reimplantation in 29). They found that an AVJ diameter >28 mm proved to be the strongest predictor for reoperation on the aortic valve, and this was observed also for patients operated on with the reimplantation technique. Hanke et al. [18] showed, in 191 patients, (remodelling in 108, reimplantation in 83) that a larger preoperative annulus seems to favour later AR after remodelling procedures while smaller preoperative aortic diameters facilitate the development of AR in reimplantation techniques.
Our study, with 150 patients, is the largest published cohort that analyses the influence of the preoperative AVJ diameter on the stability of the aortic valve during the follow-up in valve-sparing reimplantation patients. We divided the patients into two groups depending on the preoperative AVJ diameter (47 patients in Group A with AVJ diameter >28 mm, 103 patients in Group B with AVJ diameter <28 mm). We chose a 28-mm cut-off first, because we considered that an AVJ over 28 mm is large enough to induce changes in leaflets morphology and consequently the valve affection could modify the durability of the aortic valve repair and second, because we took into consideration the data reported by Kunihara et al. [7], who found that over this diameter there was an increased risk for reoperation.
We achieved results similar to those reported by other authors [2, 15–18]. Remarkably, we did not find any differences between both groups in the incidence of reoperation on the aortic valve, in freedom from AR grade II or greater or in survival. We consider that this finding is due in part to a greater stabilization of all the components of the aortic root with the reimplantation technique and to our efforts to reduce AVJ to 22–23 mm. Moreover, we performed a sub commisural annuloplasty in 14 patients because AVJ remained over 24 mm after tying the graft. We believe that this technique allows reduction of the AVJ and improves leaflet coaptation.
The remodelling technique, first described by Yacoub [19], is the other aortic valve-sparing operation to treat patients with aortic root aneurysms. Although there is still controversy, remodelling could be a factor in later aortic valve failure due to AVJ dilatation, mainly in MS patients. Cameron et al. [3] found that 7 of the 40 patients with MS who had a remodelling procedure experienced late severe AR due to annular dilatation, and 5 of these patients required late aortic valve replacement. In fact, several authors [4–7] consider recently that with remodelling, the dilated AVJ should be stabilized separately and different techniques have been proposed to treat this condition. An aortic annuloplasty using an external ring was performed by Lansac et al. [6]. Kazui et al. [4] suggested a sub-valvular circular annuloplasty using a polytetrafluoroethylene (PTFE) ring, and a subannular PTFE suture was reported by Svensson et al. [5].
There are data that MFS represents a risk factor for reoperation after a valve-sparing procedure, probably due in part to the involvement of the AVJ. Kallenbach et al. [15] found the presence of MFS to be a risk factor for reoperation in their cohort of patients operated on with the reimplantation technique. Other authors [3, 18] report this finding, but mainly in patients with MFS operated on with the remodelling technique. In our study, we observed a higher proportion of patients with MFS in the group with AVJ over 28 mm, and this finding is consistent with the typical diagnostic features in MFS previously reported [10]. However, the presence of MFS in our overall cohort of patients operated on with the reimplantation technique, as previously reported [20], does not represent a risk factor for reoperation or an increased aortic valve insufficiency during the follow-up.
Cusp intervention plays an essential role in valve-sparing operations and an important question is whether concomitant leaflet repair influence the long-term aortic valve function. We have performed cusp repair in 23% of the patients of Group A and in 31% of the patients of Group B (central plication in most of the cases), and in both groups, leaflet repair was not a predictor for late aortic valve failure. Moreover, no patient undergoing surgery for cusp repair suffered from AR greater than Grade II during the follow-up period. We recommend operating on the leaflets after completing the valve reimplantation, as the altered geometry of the aortic root could cause prolapse of previously normal leaflets. Kunihara et al. [7] emphasize the importance of cusp repair and they consider that correction of prolapse improves the durability of the valve. By contrast, Hanke et al. [18] found that AR increased by 1.5 grade after 10 years if cusps interventions, mostly a plication of the free edge of 1 or 2 cusps, were performed. They suggest that the reasons could be an unsuitable valve for sparing, secondary fibrotic retraction of the cusps margins in the long-term or improper surgical techniques.
Study limitations
First, this is a retrospective design study. Second, as the number of patients in Group A is small and there is a higher incidence of MS patients in this group, it would be desirable to have a larger cohort. Third, the size of the AVJ was determined only by echo. This fact may lead to underestimation of true AVJ size if compared with direct intubation. Finally, the mean follow-up was 44 months, consequently longer follow-up is necessary to evaluate whether a large preoperative AVJ is a predictor of late aortic valve failure.
Most of the cases were operated on by a single surgeon, and this circumstance has minimized the differences in the surgical techniques. In almost all the patients, VS were reconstructed, and there have been small technical modifications over time.
CONCLUSIONS
Medium-term stability of the aortic valve repair was not influenced by the preoperative AVJ diameter. This study provides some evidence that patients with large preoperative AVJ diameter and/or with MS may benefit from the reimplantation technique.
Conflict of interest: none declared.




