-
PDF
- Split View
-
Views
-
Cite
Cite
Man-Jong Baek, Hyun Koo Kim, Cheol Woong Yu, Chan-Young Na, Mitral valve surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 1, January 2008, Pages 116–118, https://doi.org/10.1016/j.ejcts.2007.09.024
Close - Share Icon Share
Abstract
Acute myocardial infarction (AMI) complicated by septic coronary embolism due to active infective endocarditis is rare but usually fatal. We report a case of successful mitral valve surgery with surgical embolectomy in a 27-year-old man with an AMI complicated by septic coronary embolism due to mitral valve endocarditis. A chest radiograph revealed cardiomegaly and marked pulmonary edema. A transthoracic echocardiogram disclosed severe mitral regurgitation with highly mobile vegetations and hypokinesia of the left ventricular apex. The electrocardiographic findings of ST segment elevation in leads V2-4 and elevated cardiac enzyme levels were strongly suggestive of an acute anterolateral AMI. Nevertheless, emergent cardiac surgery was needed without selective coronary angiography because of intractable heart failure and life-threatening ventricular tachyarrhythmia requiring cardiopulmonary resuscitation. A total occlusion of the distal left anterior descending artery caused by embolic vegetation and thrombus, which was incidentally detected intraoperatively, was successfully recanalized by surgical embolectomy and thrombectomy using a direct coronary incision. The mitral valve endocarditis was managed with wide debridement and mechanical valve replacement. Three years after the surgery a follow-up echocardiogram showed no abnormalities of the regional wall, motion in the left ventricle and the patient is living an active life without any complications.
1 Introduction
Systemic embolization, with an incidence of 22–50% in cases of infective endocarditis, carries a high risk of morbidity and mortality [1,2]. Despite the high embolic event rates associated with endocarditis, acute myocardial infarction (AMI) caused by septic coronary embolism, from infective endocarditis, is rare but often fatal. For rapid restoration of coronary reperfusion, interventions including thrombolysis, percutaneous coronary interventions (PCI) and surgical embolectomy [3] have been performed. However, their outcomes have been disappointing. Furthermore, in this setting, rapid and adequate therapy for an AMI associated with endocarditis has a significant impact on patient prognosis; however, the appropriate treatment in this setting has not been fully determined. Here, we report a case of successful mitral valve surgery, with surgical embolectomy, using a direct coronary incision in a 27-year-old man with an AMI complicated by septic coronary embolism due to mitral valve endocarditis.
2 Case report
A 27-year-old male presented to our hospital 6 h after the sudden onset of severe chest pain. The patient had dyspnea on exertion (NYHA class IV), weight loss and persistent fever during the prior month. He had been treated with intravenous antibiotic agents and heart failure therapy for mitral valve endocarditis during the previous 4 days at another hospital. On admission, his blood pressure was 120/70 mmHg, respiratory rate 30 breaths/min, heart rate 138 beats/min, and temperature 38.5 °C. Chest auscultation revealed an III/IV systolic murmur at the apex and crackles and rales in both lung fields. The electrocardiogram (ECG) showed ST segment elevation in leads V2-4. The chest radiograph showed cardiomegaly and marked pulmonary edema. The white blood cell count was 24,380/mm3 with 92% neutrophils. The level of creatine kinase, creatine kinase isoenzyme MB and cardiac troponin I had increased to 2482 U/l, 58.4 U/l and 43.5 μg/l, respectively. The transthoracic echocardiogram showed highly mobile, variable sized and multiple vegetations on the chordae, annulus and both leaflets of the mitral valve and left atrial wall, with severe mitral regurgitation, moderate tricuspid regurgitation, hypokinesia of the left ventricular apex, and mild left ventricular systolic dysfunction (Fig. 1A and B ). Six hours after the onset of chest pain, the ECG findings and serum enzyme levels were strongly suggestive of an acute anterolateral AMI. Nevertheless, selective coronary angiography could not be performed because of the intractable heart failure due to mitral valve endocarditis and the life-threatening recurrent ventricular tachyarrhythmia related to the AMI requiring immediate cardiopulmonary resuscitation. Therefore, the patient proceeded to emergency cardiac surgery. The distal left anterior descending artery (LAD) was totally occluded by embolic vegetations and thrombi, detected at surgery and leading to the AMI (Fig. 2A ).

Preoperative echocardiographic findings showing the highly mobile, variable sized, multiple vegetations on the valvular and subvalvular regions of the mitral valve (A) and left atrial wall (B). Intraoperative photograph showing mitral valve endocarditis with large multiple vegetations and multiple chordal ruptures (C).
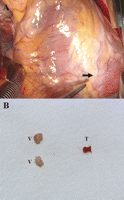
(A) The distal left anterior descending artery was totally occluded by septic embolic vegetations, which were located distal to the coronary incision and easily removed by pressing them out through the arterial wall; the thrombi located proximal to the vegetations were visualized as lesions with bluish discoloration (arrow). (B) Surgically removed embolic vegetations and a thrombus from the distal left anterior descending artery (V: vegetation; T: thrombus).
The surgical procedure is briefly described here. Routine cardiopulmonary bypass with moderate hypothermia was used. After complete cardiac arrest was induced and both atrial incisions were made, the mitral and tricuspid valves were evaluated. The highly mobile and variable sized vegetations with multiple chordal ruptures were noted in the mitral valve and left atrial wall (Fig. 1C). The mitral leaflets and its native chordae, of the A2-3 and P2-3 portions, invaded by endocarditis were completely resected, but the remaining mitral leaflets were preserved. The multiple vegetations on the left atrial endocardium were also widely debrided. Then two pairs of new artificial chordae, using a 4-0 expanded polytetrafluoroethylene, were formed from the posteromedial papillary muscle to the mitral annulus in the 2 and 5 o’clock directions (from the surgeon’s view), respectively. The mitral annulus at the 2 to 7 o’clock directions was reinforced with a double-fold felt strip using a bovine pericardium. Finally, 16 everting mattress sutures of 4-0 polypropylene with bovine pericardial pledgets were placed along the entire mitral annulus and the mitral valve was replaced with a 29-mm St. Jude Medical (St. Paul, MN) prosthesis. Next, a tricuspid valve annuloplasty was performed with the DeVega technique.
Subsequently, a small transverse incision was made to the occluded portion of the LAD. The vegetative emboli located distal to the incision were easily removed by pressing them out through the arterial wall, and the thrombus located proximal to the incision was removed by the same technique (Fig. 2B). The coronary incision was repaired with three simple 8-0 polypropylene interrupted sutures. The patient had an uneventful postoperative course. The culture of the vegetations removed from the coronary artery grew Streptococcus viridans. After the surgery, the patient was treated with vancomycin and gentamicin intravenously for 4 weeks. His symptoms significantly improved and he had negative repeat blood cultures. The postoperative echocardiogram revealed normal function of the prosthetic mitral valve and hypokinesia of the LV apex. However, 3 years after the surgery, the follow-up echocardiogram showed no abnormalities in the regional wall motion of the LV and he has been living an active life without any complications.
3 Discussion
Acute myocardial infarction (AMI) complicated by a septic coronary embolism from infective endocarditis is a rare but usually fatal condition. Accordingly, urgent and appropriate coronary reperfusion is required for patient management. As the main therapeutic options for restoring the coronary reperfusion, the use of thrombolytics [4], percutaneous transluminal coronary angioplasty (PTCA) [5,6], intracoronary stent [7], aspiration catheter [8], and surgical embolectomy [3] have been documented in some case reports. However, no intervention has been recommended as the definitive therapeutic management because of the potential risks and the rarity of such an event, despite the theoretical advantages.
The potential role of thrombolytic therapy is uncertain for endocarditis-associated AMIs, while its use for AMIs in patients with atherothrombosis is widely accepted. Septic coronary vegetations due to endocarditis, which consist mainly of inflammatory cells and microorganisms [9], are pathologically different from atherosclerotic coronary disease and lead to decreased coronary patency with thrombolytic therapy. PTCA is widely accepted as a major intervention for atherothrombotic coronary occlusions; however, there is limited information on the use of PTCA in AMIs due to septic embolisms [5–7]. Moreover, potential problems, such as the development of mycotic aneurysms at the ballooning site of the coronary artery, failure to achieve coronary patency and distal mobilization of vegetative emboli and thrombi, may result in an unsuccessful intervention. Intracoronary stent implantations, for coronary vegetative occlusions from endocarditis, may cause a greater risk for mycotic aneurysm formation than PTCA alone, resulting in complications such as rupture or sudden death [10]. There are a few reports on the use of aspiration catheters for AMIs caused by a septic coronary embolism. Catheter manipulation in this situation may result in problems such as the inability to pass the catheter beyond the lesion, angiographically identified residual stenosis due to the vegetative embolus itself and a potential risk for dissection or perforation as observed in the PCI.
Surgical embolectomy, using a direct coronary incision, has been successfully used in many cases of AMIs due to thrombosis or embolism related to cardiac surgery, but its use in the setting of AMIs caused by a septic coronary embolism has very rarely been reported [5]. This method would present a higher operative risk, in patients with active endocarditis, and an additional risk for an AMI, resulting in some hesitation when considering this modality. However, in the case of an AMI complicated by acute septic emboli from active endocarditis with severe valvular dysfunction, in the unfavorable clinical setting of intractable heart failure and life-threatening ventricular tachyarrhythmia requiring immediate cardiopulmonary resuscitation (as in our case), an emergency operation without selective coronary angiography is needed despite the higher operative risk with the active endocarditis and AMI.
The appropriate surgical option, such as bypass surgery, embolectomy or no coronary intervention, should be selected intraoperatively based on restoration of coronary reperfusion in the case of septic embolic occlusion of the coronary artery. The surgeon must also consider the history of the patient’s illness, the clinical picture at the time of surgery and the surgical in situ evaluations. In a chronic case of coronary occlusion with septic embolism, coronary bypass surgery may be preferred because of the difficulty in the removal of the vegetations due to the tight adhesion of the vegetations to the arterial wall. However, with life-threatening abnormalities including intractable heart failure due to severe valvular dysfunction and AMI-related ventricular arrhythmia complicated by septic coronary embolism in a patient with active infective endocarditis, a surgical embolectomy using a direct coronary incision may be the best surgical option.
References
- myocardial infarction, acute
- cardiopulmonary resuscitation
- electrocardiogram
- coronary angiography
- mitral valve insufficiency
- cardiomegaly
- tachycardia, ventricular
- echocardiography
- bacterial endocarditis
- cardiac enzymes
- embolectomy
- vegetation
- st segment elevation
- cardiac surgery procedures
- anterior descending branch of left coronary artery
- pulmonary edema
- heart failure
- left ventricle
- debridement
- coronary artery embolism
- follow-up
- hypokinesia
- chest x-ray
- surgical procedures, operative
- thrombectomy
- thrombus
- embolism
- echocardiography, transthoracic
- mitral valve procedures
- mitral valve endocarditis




