-
PDF
- Split View
-
Views
-
Cite
Cite
Carlo Antona, Giulio Pompilio, Attilio A. Lotto, Silvia Di Matteo, Marco Agrifoglio, Paolo Biglioli, Video-assisted minimally invasive coronary bypass surgery without cardiopulmonary bypass, European Journal of Cardio-Thoracic Surgery, Volume 14, Issue Supplement_1, October 1998, Pages S62–S67, https://doi.org/10.1016/S1010-7940(98)00107-9
Close - Share Icon Share
Abstract
Background: There is a growing interest in cardiac surgery towards minimally invasive approach to coronary bypass operations without cardiopulmonary bypass. Patients and methods: From March 1995 to March 1997, 41 patients underwent a single left internal mammary artery (LIMA) to the left anterior descending artery (LAD) coronary grafting without cardiopulmonary bypass through a small left anterior thoracotomy (MIDCABG). The mean age was 61.2±8.7 years (range 43–77 years), 28 patients. were male (68.2%) and the redo rate was 4.8% (2/41). In all patients the coronary artery disease involved the LAD, which was occluded in seven patients (17.1%). Thirty-eight patients (96.2%) selected for MIDCABG had a monovascular disease on LAD not suitable for percutaneous coronary angioplasty; two (4.8%) a bivascular disease, and one (2.4%) a trivascular disease. Skin incision was performed in the 4th anterior intercostal space from the left parasternal line for a 10.5 cm length on average. The LIMA harvesting was partially video-assisted by thoracoscopy. Results: The LAD temporary occlusion was achieved with two double 5/0 polypropilene round-LAD sutures. The mean LAD ischemic time was 22±8 min (range 4–35 min). No thoracotomy procedure was changed into a sternotomy approach. We had one (2.4%) perioperative AMI; two patients (4.8%) were reoperated for bleeding. All patients underwent a postoperative angiographic reinvestigation within 1 month after surgery. All anastomoses were perfectly patent but two (4.8%). One patient was reoperated via a sternotomy access recycling the LIMA graft, the other one underwent successful PTCA. All patients also underwent an early and mid-term (6 months after surgery) echo-Doppler study of the LIMA flow and patency. At follow-up, performed at a mean of 8.7 months (range 1–23) after discharge, all patients were alive; no one experienced recurrence of angina. All patients also performed a mid-term negative treadmill stress test. Conclusions: MIDCABG is, in selected patients, reliable and safe, and offers encouraging early and mid-term clinical results.
1 Introduction
Minimally invasive procedures have recently become more and more popular in cardiac surgery. A great interest has arisen in new less invasive techniques of myocardial revascularization without cardiopulmonary bypass (CPB). Some interesting studies have been reported describing methods of direct coronary bypass grafting of the left anterior descending artery (LAD) with the left internal mammary artery (LIMA) through a small anterior thoracotomy without cardiopulmonary bypass (MIDCABG) [1–4]. Different techniques have been employed in an attempt to facilitate the LIMA harvesting and the anastomoses of LIMA to LAD on a beating heart, including the use of wound spreaders and coronary stabilizers [4], and the use of video-assisted thoracoscopy [5, 6]. The initial results seem promising in terms of reduction of surgical trauma, time of hospitalization and costs [1, 5].
In this paper, we report our 2-year single-institution surgical experience on 41 consecutive patients who underwent a video-assisted surgical revascularization of the LAD with LIMA through a small thoracotomy.
2 Materials and methods
2.1 Patient population
From March 1995 to March 1997, 41 patients (mean age 61.2±8.7 years; range 43–77 years) were selected for video-assisted myocardial revascularization on a beating heart through a small anterior thoracotomy. The procedures were performed by two surgeons (CA and PB). Preoperative data are shown in Table 1. Thirteen patients were female (31.7%); in two cases (4.8%) the procedure was a redo operation. Mean preoperative ejection fraction (EF) was 58±8%. Six patients (14.6%) were operated on with an unstable angina.

Inclusion criteria. (1) Elective video-assisted MIDCABG was mainly performed in case of isolated LAD stenosis or occlusion in which percutaneous transluminal coronary angioplasty was not advisable, not successful or not possible. As shown in Table 1, the largest part of our population (38/41 patients: 92.6%) had a monovascular coronary disease on LAD. (2) Patients with multivessel disease and severe contraindications to cardiopulmonary bypass or sternotomy were also considered suitable for MIDCABG off-pump. In fact, MIDCABG was performed in one trivascular and one bivascular patient (4.8%): the first one for a sternal radiation osteopathy, the second one for a previous stroke associated with a severe renal failure requiring dyalisis. In these patients, the presence of a multivessel disease was not considered as an exclusion criteria for isolated minimally invasive coronary bypass to LAD. (3) The presence of an associated diseased right coronary artery not eligible for surgical treatment was considered in one patient (2.4%) as an adjunctive indication for MIDCABG.
The presence of an intramyocardial or calcified LAD was an absolute contraindication for this operation.
2.2 Surgical technique
The patient is placed in a recumbent position, with the left arm placed upwards. Two back rolls are positioned under the left shoulder (see Fig. 1). A conventional single-lumen endotracheal tube was used in all patients. The chest is opened via a small left anterior toracothomy in the fourth intercostal space. At the beginning of our experience we used a conventional rib retractor. In the last 11 cases a specially designed wound spreader (Autosuture International, Norwalk, CT) was tested in order to better expose the operative field and avoid rib fractures. The pleural cavity is opened routinely and a moist sponge is used to partially collapse the left lung. The pericardial fat and the pericardium are opened and the LAD inspected, in order to make sure about the feasibility of the operation. A thoracoscope (Olympus, Hamburg, Germany) is inserted through an incision made in the fifth or fourth intercostal space, along the medial axillary artery. The LIMA was direct harvested through the thoracotomy for the distal segment, and indirectly through the thoracoscope for the proximal part. This combined approach allows the harvesting an adequate length of LIMA and permits a good visualization and ligation of LIMA branches also in the proximal segment of the artery. The branches are occluded with clips on the LIMA side, and are divided with electrocautery. The LIMA is harvested both pedicled and skeletonized. At the end of the LIMA harvesting, the thoracoscope also permits the hemostase of the proximal mammary bed. After systemic heparinization (1.5 mg/kg), the LIMA is distally clipped and prepared for the anastomoses. The edges of the opened pericardium are fixed to the chest wall with large silk sutures. The anastomotic site of the LAD is dissected. The LAD is then occluded proximally and distally using a 4/0 polypropylene suture passed twice to surround the vessel. The two sutures are gently snared to ensure an operative field as bloodless as possible. To test the tolerance to ischemia, the LAD is briefly occluded, with a constant monitoring by electrocardiogram and transesophageal echocardiography, in order to assess the degree of ischemia and regional wall motion impairments. Adjunctive 6/0 polypropylene sutures are eventually placed in the epicardium surrounding the LAD in order to better stabilize the artery. When the specially adapted spreader is used, it carries a LAD Stabilizer. The stabilizer is locked into place, with the LAD centered between the tines (Fig. 2). The LAD is opened, and the mammary–coronary artery anastomosis is performed with a 7/0 running polypropylene suture. After the anastomosis has been completed, a coronary sizer is passed through distally and proximally in order to verify the patency and to remodel the LAD segment occluded by the double sutures. The two looping sutures are then cut and the coronary stabilizer is removed. Protamine is given to reverse heparine. The LIMA is eventually secured to the epicardium with 6/0 polypropylene sutures, and the anastomoses is covered with the pericardial fat. The small thoracotomy is closed and one pleural drain is left in place.
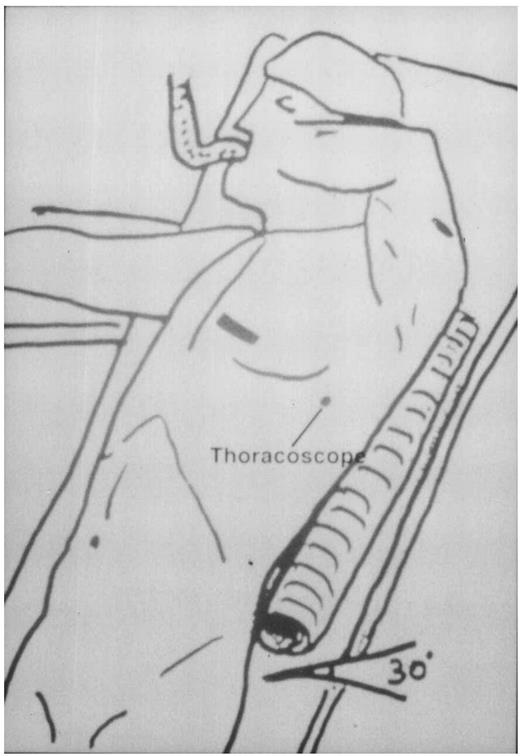
The patient is placed in a recumbent position, with the left arm placed upwards. Two back rolls are positioned under the left shoulder. The left thoracotomy is represented at the fourth intercostal space. In the drawing, the thoracoscope is inserted at the fifth intercostal space along the median axillary line.
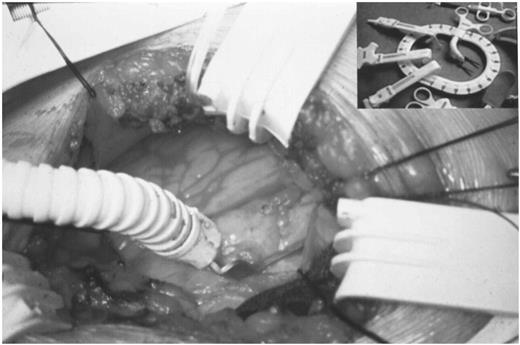
The LIMA, coming from the right, is anastomised to the left anterior descending artery. The LAD stabilized is locked into place, with the LAD centered between the tines. In the table above is shown the wound spreader.
2.3 Angiographic study and follow-up
All patients underwent, within 1 month after discharge, a postoperative angiographic reinvestigation of LIMA to LAD grafts. An echo-Doppler study of LIMA patency was performed within 1 week and 6 months postoperatively (Fig. 3). To study the LIMA flow with echo-Doppler we have used the following parameters: maximum velocity (Vmax; cm/s), minimum velocity (Vmin; cm/s), mean velocity (Vmean; cm/s); vessel diameter and flow, calculated by multiplying Vmean×area. Patients also underwent a mid-term (6 months after the operation) treadmill stress test. The mean follow-up period was 8.7 months (range 1–23).
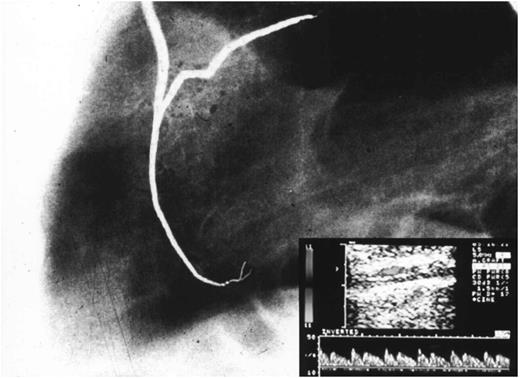
Postoperative angiographic control of LIMA graft to LAD. In the table below is shown the early echo-Doppler study.
3 Results
3.1 Surgical data
Surgical data are shown in Table 2. The LAD occlusion time was 22.1±8 min (range 15–35 min). The mean anastomotic time was 18.4±6 min (range 10–31 min). The mean operative time was 2.3±0.5 h and the wound length was on average 10.5 cm. No toracothomy procedure was converted into sternotomy. In one case (2.4%) the toracothomy was enlarged because of the difficulty to reach an abnormal lateral LAD. In one patient (2.4%) we observed intraoperatively a LIMA intramural hematoma, without LIMA flow impairment. In seven cases (17%), transient ST-segment changes were recorded during LAD occlusion, completely relieved after reperfusion. Ventricular arrhytmias never occurred during the LAD occlusion.
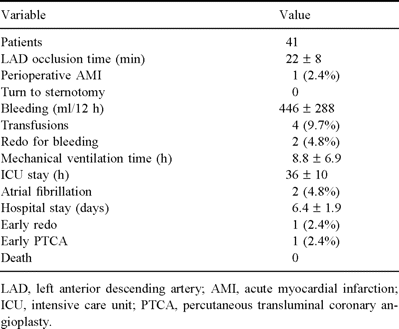
Early clinical results (Table 2). No deaths occurred. One patient (2.4%) experienced a perioperative myocardial septal infarction, without hemodynamic deterioration. Two patients (4.8%) were reoperated for bleeding. The mean ventilatory support time and the mean ICU stay were 8.8±6.9 and 36±10 h, respectively. There was not any other non-cardiac complications. The mean hospitalization time was 6.4±1.9 days.
3.2 Angiographic reinvestigation
All patients underwent an angiographic reinvestigation within 1 month from the operation. All anastomoses were perfectly patent but two (4.8%), that belonged to the series without coronary stabilizer. In the patient with a postoperative myocardial infarction, the early angiography showed an occluded LIMA–LAD anastomosis. The patient was reoperated via a sternotomic access recycling the LIMA graft. In a second patient, that experienced recurrent angina and a positive stress test 2 weeks after the operation, the angiographic reinvestigation showed a severe stenosis of the LIMA–LAD anastomosis. A percutaneous coronary angioplasty was then successfully performed. In the patient with intraopeartive evidence of a LIMA intramural hematoma, the early angiographic reinvestigation showed a LIMA graft perfectly patent.
3.3 Echo-Doppler study
All patients underwent an echo-Doppler study of the LIMA graft patency 1 week after the operation (see Table 3). Twenty-nine patients (70.7%) repeated the echo-Doppler study 6 months after discharge. At early stage, the echo-Doppler evaluation showed a severe impairment of LIMA flow in both patients who required an anastomotic revision, with a systolic velocity in the LIMA graft of less than 10 cm/s and a loss of the diastolic component.
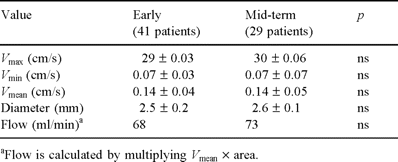
Early (1 week) and mid-term (6 months) postoperative echo-Doppler evaluation of LIMA grafts to LAD.
The mid-term study showed an anterograde flow with a well defined systolic peak followed by a shorter and wider diastolic wave in all patients, but one, asymptomatic patient, in which a mild impairment of the LIMA flow was observed. The patient underwent a negative treadmill stress test. In this patient, the preoperative coronary angiography showed a narrow LAD, and the early angiographic reinvestigation was normal, so that we decided not to perform a second angiographic reinvestigation.
3.4 Clinical follow-up and exercise stress test
At clinical follow-up, performed at a mean of 8.7 months after discharge (median 6.8; range 1–24 months), all patients were alive and no one experienced recurrence of angina and other cardiac complications. At the same period of follow-up, patients also underwent a negative exercise stress test without drugs.
In particular, the patient that had intraoperative finding of LIMA intramural hematoma, was asymptomatic 7 months after the operation, with negative echo-Doppler and exercise test results.
4 Discussion
Recently, the interest towards minimally invasive procedures in cardiac surgery has dramatically increased. The great interest on new trends of coronary revascularization has been mainly focused on minimally invasive coronary bypass surgery without cardiopulmonary bypass (MIDCABG) [1–6]. This operation seems to well correlate the benefits both of the avoidance of CPB and of a minimally invasive approach.
The initial reports seem promising in terms of early clinical results, hospital stay and costs [1, 5, 6]. However, a word of caution has been suggested [7]with respect both to the selection of patients candidates to this operation and to the amounts of anastomotic failure, which is to date superior to conventional techniques of myocardial revascularization on pump [1].
We have performed a series of 41 patients in a 2-year period. Early and mid-term results (with a mean follow-up of 8.4 months) are encouraging. All patients tolerated the operation very well and were asymptomatic at mid-term follow-up. Necessity of anastomotic revision (surgical or with PTCA) occurred in two patients (4.8%), one for the occlusion of the anastomoses and one for a severe stenosis. Global AMI rate was 2.4%, and no patient died.
However, there are some questions about this operation that should be addressed in the near future. First of all, the indications. We have selected patients in which a PTCA on LAD was not feasible, so that we have not had a certain overlap with indications to PTCA, as previously described [5, 8]. Further comparative studies should better define the alternative or complementary role of MIDCABG versus PTCA on LAD. We have extended the indication to MIDCABG from monovascular to bivascular patients only in case of an associated diseased right coronary artery not eligible for surgical treatment. We don't think that a combined minimally invasive surgical and PTCA approach to multivessel patients [1]is advisable. Conversely, it is likely that this procedure could be useful in bivascular or trivascular patients at very high risk for cardiopulmonary bypass or for a median sternotomy, when the need of a complete revascularization is less important then the inherent risk of the conventional operation on-pump.
Another concern is the rate of anastomotic failure. The necessity of anastomotic revision in this study (two cases: 4.8%) correlates with the large experience of Calafiore et al. [1]. These patients, however, belonged to the initial experience without coronary stabilizer. It is likely, therefore, that improvements in the surgical skill and the use of new devices will lower the rate of anastomotic failure.
The possibility of coronary stenosis at the snare site proximal and distal to the anastomosis, caused by the occlusive sutures around the coronary artery, has been reported by Pagni et al. [9]. We have observed the same phenomenon in patients who underwent CABG without CPB through sternotomy. For this reason, we stress the importance to remodel the LAD with a coronary sizer proximally and distally to the anastomotic site.
Another point of discussion is the rate of conversion into sternotomy. In our series, no patients were converted into sternotomy, but in one patient the thoracotomy was enlarged, because of an abnormal lateral LAD. An appropriate selection of patients may considerably reduce the rate of failed MIDCABG procedures.
The LIMA harvesting can represent a phase of the operation very demanding from a technical point of view. Thoracoscopy has been described by Nataf et al. [10]and Benetti et al. [5]as an important tool to ease LIMA harvesting, mainly in its proximal segment, to reduce the length of the left thoracothomy, and to allow a careful hemostasis of the mammary artery bed. In this study, the LIMA was harvested directly through the thoracotomy for the distal part and indirectly with video-assist for the proximal part. With this technique, we have had only one LIMA hematoma, without flow impairment, and the length of LIMA harvested was in all patients far enough to perform the anastomosis avoiding LIMA kinkings and without the need to extend the LIMA length with other conduits [1].
In two patients of this study, the MIDCABG was a coronary redo operation. As suggested by Boonstra et al. [11], MIDCABG can be a promising technique also in selected cases of reoperative coronary revescularization, because it dramatically reduces the surgical trauma if compared with the conventional sternotomic approach.
Finally, the echo-Doppler study is a simple and quick method to check on the graft flow in the postoperative period of MIDCABG patients. Although the echocolor-Doppler examination has not yet reached the same attendibility of coronarography, it seems very useful to select patients who show at their tracing an abnormal flow, requiring an angiographic reinvestigation.
In conclusion, despite some issues that need to be further investigated, we are convinced that this new approach to coronary bypass operations has, in selected cases, the potential to be included in the array of the cardiac surgeon.
References
Presented at the World Congress on Minimally Invasive Cardiac Surgery, Paris, May 30-31, 1997.
- angina pectoris
- angiogram
- percutaneous coronary intervention
- ischemia
- coronary artery bypass surgery
- cardiopulmonary bypass
- coronary arteriosclerosis
- echocardiography
- cardiac surgery procedures
- internal thoracic artery
- anterior descending branch of left coronary artery
- hemorrhage
- minimally invasive direct coronary artery bypass surgery
- percutaneous transluminal coronary angioplasty
- exercise stress test
- anastomosis, surgical
- follow-up
- surgical procedures, operative
- sutures
- thoracoscopy
- thoracotomy
- skin
- transplantation
- coronary artery bypass-left internal mammary artery (lima) graft
- patents
- anterior thoracotomy
- treadmills
- intercostal space
- fluid flow




