-
PDF
- Split View
-
Views
-
Cite
Cite
Masanori Asakura, Shin Ito, Takahisa Yamada, Yoshihiko Saito, Kazuo Kimura, Akira Yamashina, Atsushi Hirayama, Youichi Kobayashi, Akihisa Hanatani, Mitsuru Tsujimoto, Satoshi Yasuda, Yukio Abe, Yorihiko Higashino, Yodo Tamaki, Hiroshi Sugino, Hiroyuki Niinuma, Yoshitaka Okuhara, Toshimi Koitabashi, Shin-Ichi Momomura, Kuniya Asai, Akihiro Nomura, Hiroya Kawai, Yasuhiro Satoh, Tsutomu Yoshikawa, Ken-Ichi Hirata, Yoshiaki Yokoi, Jun Tanaka, Yoshisato Shibata, Yasuhiro Maejima, Shunsuke Tamaki, Hiroyuki Kawata, Noriaki Iwahashi, Masatake Kobayashi, Yoshiharu Higuchi, Akiko Kada, Haruko Yamamoto, Masafumi Kitakaze, on behalf of the EARLIER investigators and study coordinators, Efficacy and Safety of Early Initiation of Eplerenone Treatment in Patients with Acute Heart Failure (EARLIER trial): a multicentre, randomized, double-blind, placebo-controlled trial, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 8, Issue 2, March 2022, Pages 108–117, https://doi.org/10.1093/ehjcvp/pvaa132
Close - Share Icon Share
Abstract
A mineralocorticoid receptor antagonist (MRA) is effective in patients with chronic heart failure; however, the effects of the early initiation of an MRA in patients with acute heart failure (AHF) have not been elucidated.
In this multicentre, randomized, double-blind, placebo-controlled, parallel-group study, we focused on the safety and effectiveness of the treatment with eplerenone, a selective MRA in 300 patients with AHF, that is, 149 in the eplerenone group and 151 in the placebo group in 27 Japanese institutions. The key inclusion criteria were (i) patients aged 20 years or older and (ii) those with left ventricular ejection fraction of ≤40%. The primary outcome was a composite of cardiac death or first re-hospitalization due to cardiovascular disease within 6 months. The mean age of the participants was 66.8 years, 27.3% were women, and the median levels of brain natriuretic peptide were 376.0 pg/mL. The incidences of the primary outcome were 19.5% in the eplerenone group and 17.2% in the placebo group [hazard ratio (HR): 1.09, 95% confidence interval (CI): 0.642–1.855]. In prespecified secondary outcomes, HR for the composite endpoint, cardiovascular death, or first re-hospitalization due to heart failure within 6 months was 0.55 (95% CI: 0.213–1.434). The safety profile for eplerenone was as expected.
The early initiation of eplerenone in patients with AHF could safely be utilized. The reduction of the incidence of a composite of cardiovascular death or first re-hospitalization for cardiovascular diseases by eplerenone is inconclusive because of inadequate power.
Introduction
The incidences of acute heart failure (AHF), as well as chronic heart failure (HF), have been steadily increasing because of various social and biological factors, including the ageing of society and improvements in the treatment for acute myocardial infarction (AMI).1 AHF is still a leading cause of hospitalization and mortality mainly following HF and has been the focus of critical treatments for cardiovascular diseases.2,3 Although the success of the treatment for HF with reduced ejection fraction (HFrEF) is attributable to the paradigm shift from therapies that target the symptomatic improvement to those that target organ protection as gauged by prognostic improvement,4,5 the current strategy and therapies for AHF are aimed to restore haemodynamic abnormalities at an acute phase by using diuretics, vasodilators, and inotropes, without explicit consideration of cardioprotection.2,3 This consideration has led us to shift a comparable paradigm with HFrEF treatment for AHF treatment that targets prognostic improvement.6,7 Indeed, the sub-analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) has recently demonstrated that, compared with therapy with positive inotropic agents in patients hospitalized with acute decompensated HF, therapy using a natriuretic peptide or vasodilator is associated with lower in-hospital mortality.8 A subsequent new clinical trial that evaluates the efficacy of AHF treatment on prognostic improvement has recently been reported.9–11 Controversial findings have been reported with nesiritide, a recombinant B-type natriuretic peptide (BNP) with vasodilatory properties; nesiritide was not associated with a change in death rate and re-hospitalization within 30 days in patients hospitalized for AHF.9 These results suggest that no established drugs are available to improve the prognosis of patients with AHF.
There was compelling evidence in earlier AHF therapy that aldosterone exerted different deleterious effects on the cardiovascular system via aldosterone receptors in the heart, brain, and blood vessels.12,13 Aldosterone has become a potential therapeutic target because of the increase in plasma aldosterone levels associated with the progression of myocardial damage during an acute phase of HF 14,15 and a poor prognosis of patients with AHF.16–18 Several clinical trials have elucidated the beneficial effects of mineralocorticoid receptor antagonists (MRAs), including eplerenone, on the prognosis of patients with HFrEF.19 Impeding activated aldosterone signalling during an acute phase of HF may provide a remedy for AHF treatment by protecting the heart and other organs, thus improving the prognosis of patients with AHF. The decision was taken to follow the aldosterone theory and conduct the EARLIER trial to prove the novel hypothesis that the early initiation of eplerenone treatment could be safely utilized and provide a better prognosis of patients who were hospitalized for AHF.
Methods
Study design
The EARLIER trial was a multicentre, randomized, double-blind, placebo-controlled, parallel-group study (JMACCT clinical trials registry identifier: JMA-IIA00127). The trial design was previously published,20 and the study aimed to compare and evaluate the effects of the early initiation of eplerenone on the occurrence of the composite endpoint (cardiovascular death or first re-hospitalization due to cardiovascular disease) in patients with AHF.
The academic committee designed the protocol, identified the participating institutions, and supervised the execution of the trial. The independent data monitoring committee reviewed safety data, having an access to unblinded data during the trial. The first author wrote the first draft of the manuscript. This study was conducted according to the Declaration of Helsinki and in compliance with the International Conference on Harmonization and Good Clinical Practice (GCP) guidelines. The institutional review board at each institution approved this trial, and all patients provided written informed consent.
Study patients
The key inclusion criteria were age of at least 20 years and clinical evidence of AHF demonstrated by at least one of the following events: (i) de novo AHF, (ii) acute exacerbation of HF, and (iii) post-AMI HF and left ventricular ejection fraction (LVEF) of no more than 40%. AHF was defined as the presence of one of following phenomena: pulmonary rales, chest radiography showing pulmonary venous congestion, or a third heart sound. The key exclusion criteria included the past history of receiving mineralocorticoid receptor antagonist therapy, a planned percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) surgery during the first hospitalization; a serum potassium level exceeding 5.0 mmol/L; an estimated glomerular filtration rate (eGFR) of less than 30 mL/min/1.73 m2; and any other clinically significant, coexisting conditions.
Randomization was conducted within 72 h after hospitalization with AHF and was eventually extended to 14 days because of the changes in the protocol at enrolment of approximately 180 patients, since obtaining informed consent in the acute phase did not proceed as expected.
Study procedures
Patients were stratified into three subgroups: (i) patients with post-AMI HF; (ii) those with AHF, but not post-AMI, having systolic blood pressure (SBP) exceeding 140 mmHg; and (iii) those with AHF, but not post-AMI, having SBP not greater than 140 mmHg. The patients in each subgroup were assigned equally at a 1:1 ratio to receive either eplerenone or placebo treatment using a minimization method with non-deterministic allocation probability based on a score derived from institutions and the onset/relapse. The investigators outsourced the randomization and allocation task to the independent contract research organization. Randomization and allocation were conducted automatically using the web system. The study drug allocation was concealed to the study subjects, investigator, sub-investigator, study coordinators, study drug provider, and the clinical research organization. The study drug allocation manager ensured the blinding in the following ways: (i) to ensure that the drugs cannot be visually identified and assign them randomly in a reproducible procedure and (ii) to create an emergency key and keep it sealed.
The administration of either eplerenone or placebo was started at a dose of 25 mg once daily just after randomization, and the dose was increased after 1 week–50 mg once daily (or started at 25 mg on alternate days and increased to 25 mg daily, if eGFR was 30 to <50 mL/min/1.73 m2), provided the serum potassium level was no more than 5.0 mmol/L. Investigators modified the doses of the study drug or withheld the study drug, according to both serum potassium level and eGFR measured at each visit. Investigators remeasured both serum potassium level and eGFR within 72 h after the reduction or the withdrawal of the study drug, and the study drug would restart if the serum potassium levels were below 5.0 mmol/L.
After randomization, the planned assessments were conducted at 48 h, 1 week, 2 weeks, and discharge (or 4 weeks), 8, 12, 16, 20, and 24 weeks. Adverse events were assessed at each follow-up visit or contact. All randomized subjects were investigated for clinical evaluation criteria until the end of the study, regardless if the study was continued or not.
Study outcomes
The primary outcome was a composite of cardiovascular death (including death due to exacerbation of HF, AMI, arrhythmia, stroke, or others related to cardiovascular diseases, or sudden death) or a first re-hospitalization due to any cardiovascular disease (including the exacerbation of HF, AMI, stroke, arrhythmia, and others related to cardiovascular diseases) within 6 months from enrolment. The prespecified secondary outcomes were a composite outcome of cardiovascular death or a first re-hospitalization due to HF; a composite outcome of cardiovascular death or non-fatal AMI; a first re-hospitalization due to any cardiovascular disease; death due to any cardiovascular disease; a first re-hospitalization due to HF; death due to any cause; a non-fatal AMI; a sudden cardiac death; and therapy using a medical device for HF such as a cardiac resynchronization therapy or a ventricular assist device. The outcomes were judged by an independent committee based on prespecified criteria. It was conducted with the drug allocation blinded. The safety outcome was the incidence of adverse events. An adverse event was defined as any untoward medical occurrence in a subject without regard to the possibility of a causal relationship with study drug.
Adherence to prescriptions of study drugs was evaluated. The subjects with the dose compliance rate of 80–120% at two visits were considered as adherent patients.
Statistical analysis
This trial was designed to acquire approval for extended indication for eplerenone in patients with AHF in Japan. After discussions with Japanese regulatory authority (PMDA), PMDA requested us to show the consistency with the EPHESUS trial. The consistency means that the point estimate of hazard ratio (HR) of eplerenone to placebo is <1.0 as in the EPHESUS trial. The sample size was established at 300, primarily based on feasibility. The present study aimed to confirm the above-mentioned consistency between the current trial and the EPHESUS trial. The ability of the present study was confirmed in the following settings by referring to the EPHESUS trial. For HR = 0.87,17 the probability of achieving consistency was calculated as 72.6% in 300 patients and 64.9% in 120 patients of post-AMI. For HR = 0.78,21 the probability was 85.2% in 300 patients and 74.8% of 120 patients of post-AMI.
We defined the intention to treat (ITT) analysis set as all enrolled patients. The safety analysis set was defined as patients who had taken the study drug at least once. The time to event of the primary and secondary endpoints, HR, and 95% confidence interval (CI) were estimated using the Cox proportional hazard model adjusted for the onset/relapse, stratified via the patient classification. For each subgroup category, HR and 95% CI were estimated using the Cox proportional hazards model without adjustment and stratification.
For each subgroup category, HR and 95% CI were estimated using the Cox proportional hazards model without stratification and covariates. Regarding the echocardiographic parameters and the plasma BNP levels, using a repeated measure mixed-effects model that included patient classification, onset/relapse, and baseline values, least squares estimates of the differences between treatment groups and 95% CI were calculated. The plasma BNP levels were utilized for calculation after the results to be presented in the original scale were log-transformed. For adverse event, Fisher’s exact test was utilized. The significance level was 0.05 in a two-tailed test. Using SAS Version 9 (SAS Institute Inc., Cary, NC, USA), these analyses were conducted.
Results
Study population
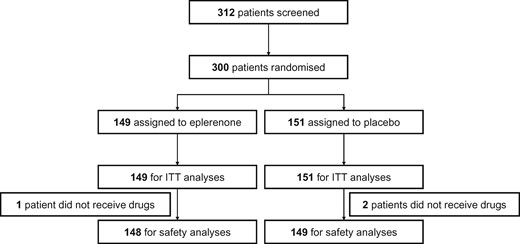
A total of 300 patients were randomized into 149 in the eplerenone group and 151 patients in the placebo group at 27 major institutions in Japan between June 2013 and April 2018 (Figure 1). All randomized participants were included for the ITT analysis and the primary efficacy analysis. All participants treated with the study drug at least once (148 and 149 in the eplerenone and placebo groups, respectively) were included in the safety analysis. There were no patients lost to follow-up.
Table 1 shows the baseline characteristics of the patients in both groups. Baseline characteristics in both groups regarding age, the median value of LVEF, the median level of BNP, and the percentage of eGFR were similar. The mean duration of treatment with the study drug was 142.5 and 147.9 days in the eplerenone and placebo groups, respectively. The number of patients who were adherent to prescriptions was 143 (95.9%) and 148 (98.0%) in the eplerenone and placebo groups, respectively.
| . | Eplerenone (N = 149) . | Placebo (N = 151) . |
|---|---|---|
| Male (%) | 67.1 | 78.1 |
| Age (years old) | 69.0 (63.0–76.0) | 66.0 (56.0–76.0) |
| BMI (kg/m2) | 23.0 (21.1–25.8) | 23.9 (20.7–28.2) |
| NYHA (I/II/III/IV) (%) | 4.7/37.6/43.6/13.4 | 7.9/33.8/38.4/18.5 |
| Cause of AHF ischaemic/non-ischaemic (%) | 37.6/62.4 | 34.4/65.6 |
| History of HF (%) | 12.1 | 12.6 |
| History of AF (%) | 36.9 | 33.1 |
| Hypertension (%) | 73.2 | 79.5 |
| Diabetes mellitus (%) | 39.6 | 40.4 |
| Dyslipidaemia (%) | 59.7 | 54.3 |
| Stroke (%) | 11.4 | 14.6 |
| Any cause of cancer (%) | 5.4 | 9.9 |
| COPD (%) | 5.4 | 5.3 |
| Bronchial asthma (%) | 6.0 | 9.3 |
| Chronic kidney disease (%) | 22.8 | 20.5 |
| eGFR (mL/min/1.73 m2) | 61.0 (48.5–73.0) | 63.0 (53.0–75.0) |
| Serum potassium (mEq/L) | 4.0 (3.7–4.3) | 3.9 (3.8–4.2) |
| Plasma BNP (pg/mL) | 374.5 (182.5–684.0) | 380.0 (198.0–597.0) |
| Plasma aldosterone (pg/mL) | 71.0 (47.0–114.0) | 82.0 (56.0–119.0) |
| LVEF (%) | 31.0 (25.0–35.9) | 30.0 (25.0–36.0) |
| . | Eplerenone (N = 149) . | Placebo (N = 151) . |
|---|---|---|
| Male (%) | 67.1 | 78.1 |
| Age (years old) | 69.0 (63.0–76.0) | 66.0 (56.0–76.0) |
| BMI (kg/m2) | 23.0 (21.1–25.8) | 23.9 (20.7–28.2) |
| NYHA (I/II/III/IV) (%) | 4.7/37.6/43.6/13.4 | 7.9/33.8/38.4/18.5 |
| Cause of AHF ischaemic/non-ischaemic (%) | 37.6/62.4 | 34.4/65.6 |
| History of HF (%) | 12.1 | 12.6 |
| History of AF (%) | 36.9 | 33.1 |
| Hypertension (%) | 73.2 | 79.5 |
| Diabetes mellitus (%) | 39.6 | 40.4 |
| Dyslipidaemia (%) | 59.7 | 54.3 |
| Stroke (%) | 11.4 | 14.6 |
| Any cause of cancer (%) | 5.4 | 9.9 |
| COPD (%) | 5.4 | 5.3 |
| Bronchial asthma (%) | 6.0 | 9.3 |
| Chronic kidney disease (%) | 22.8 | 20.5 |
| eGFR (mL/min/1.73 m2) | 61.0 (48.5–73.0) | 63.0 (53.0–75.0) |
| Serum potassium (mEq/L) | 4.0 (3.7–4.3) | 3.9 (3.8–4.2) |
| Plasma BNP (pg/mL) | 374.5 (182.5–684.0) | 380.0 (198.0–597.0) |
| Plasma aldosterone (pg/mL) | 71.0 (47.0–114.0) | 82.0 (56.0–119.0) |
| LVEF (%) | 31.0 (25.0–35.9) | 30.0 (25.0–36.0) |
| . | Eplerenone . | Placebo . |
|---|---|---|
| Median days from admission to randomization | 2 (1–5) | 2 (1–4) |
| Medical history (%) | ||
| Previous hospitalization for HF | 12.1 | 12.6 |
| Myocardial infarction | 19.5 | 19.2 |
| Angina pectoris | 16.1 | 15.2 |
| Atrial fibrillation/flutter | 36.9 | 33.1 |
| Ventricular tachycardia/fibrillation | 8.1 | 7.9 |
| PCI | 16.1 | 16.6 |
| Device therapy (%) | ||
| Pacemaker | 3.4 | 3.3 |
| Defibrillator | 1.3 | 1.3 |
| CRT | 0.7 | 0 |
| Medications (%) | ||
| ACE inhibitor or ARB | 89.9 | 89.4 |
| Beta-blocker | 62.4 | 65.6 |
| Diuretic | 91.9 | 89.4 |
| Aspirin | 46.3 | 35.1 |
| Statin | 43.6 | 45.0 |
| Other lipid lowering drugs | 46.3 | 45.0 |
| . | Eplerenone . | Placebo . |
|---|---|---|
| Median days from admission to randomization | 2 (1–5) | 2 (1–4) |
| Medical history (%) | ||
| Previous hospitalization for HF | 12.1 | 12.6 |
| Myocardial infarction | 19.5 | 19.2 |
| Angina pectoris | 16.1 | 15.2 |
| Atrial fibrillation/flutter | 36.9 | 33.1 |
| Ventricular tachycardia/fibrillation | 8.1 | 7.9 |
| PCI | 16.1 | 16.6 |
| Device therapy (%) | ||
| Pacemaker | 3.4 | 3.3 |
| Defibrillator | 1.3 | 1.3 |
| CRT | 0.7 | 0 |
| Medications (%) | ||
| ACE inhibitor or ARB | 89.9 | 89.4 |
| Beta-blocker | 62.4 | 65.6 |
| Diuretic | 91.9 | 89.4 |
| Aspirin | 46.3 | 35.1 |
| Statin | 43.6 | 45.0 |
| Other lipid lowering drugs | 46.3 | 45.0 |
Data are presented as % or median (interquartile range).
ACE, angiotensin-converting enzyme; AHF, acute heart failure; ARB, angiotensin-receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
| . | Eplerenone (N = 149) . | Placebo (N = 151) . |
|---|---|---|
| Male (%) | 67.1 | 78.1 |
| Age (years old) | 69.0 (63.0–76.0) | 66.0 (56.0–76.0) |
| BMI (kg/m2) | 23.0 (21.1–25.8) | 23.9 (20.7–28.2) |
| NYHA (I/II/III/IV) (%) | 4.7/37.6/43.6/13.4 | 7.9/33.8/38.4/18.5 |
| Cause of AHF ischaemic/non-ischaemic (%) | 37.6/62.4 | 34.4/65.6 |
| History of HF (%) | 12.1 | 12.6 |
| History of AF (%) | 36.9 | 33.1 |
| Hypertension (%) | 73.2 | 79.5 |
| Diabetes mellitus (%) | 39.6 | 40.4 |
| Dyslipidaemia (%) | 59.7 | 54.3 |
| Stroke (%) | 11.4 | 14.6 |
| Any cause of cancer (%) | 5.4 | 9.9 |
| COPD (%) | 5.4 | 5.3 |
| Bronchial asthma (%) | 6.0 | 9.3 |
| Chronic kidney disease (%) | 22.8 | 20.5 |
| eGFR (mL/min/1.73 m2) | 61.0 (48.5–73.0) | 63.0 (53.0–75.0) |
| Serum potassium (mEq/L) | 4.0 (3.7–4.3) | 3.9 (3.8–4.2) |
| Plasma BNP (pg/mL) | 374.5 (182.5–684.0) | 380.0 (198.0–597.0) |
| Plasma aldosterone (pg/mL) | 71.0 (47.0–114.0) | 82.0 (56.0–119.0) |
| LVEF (%) | 31.0 (25.0–35.9) | 30.0 (25.0–36.0) |
| . | Eplerenone (N = 149) . | Placebo (N = 151) . |
|---|---|---|
| Male (%) | 67.1 | 78.1 |
| Age (years old) | 69.0 (63.0–76.0) | 66.0 (56.0–76.0) |
| BMI (kg/m2) | 23.0 (21.1–25.8) | 23.9 (20.7–28.2) |
| NYHA (I/II/III/IV) (%) | 4.7/37.6/43.6/13.4 | 7.9/33.8/38.4/18.5 |
| Cause of AHF ischaemic/non-ischaemic (%) | 37.6/62.4 | 34.4/65.6 |
| History of HF (%) | 12.1 | 12.6 |
| History of AF (%) | 36.9 | 33.1 |
| Hypertension (%) | 73.2 | 79.5 |
| Diabetes mellitus (%) | 39.6 | 40.4 |
| Dyslipidaemia (%) | 59.7 | 54.3 |
| Stroke (%) | 11.4 | 14.6 |
| Any cause of cancer (%) | 5.4 | 9.9 |
| COPD (%) | 5.4 | 5.3 |
| Bronchial asthma (%) | 6.0 | 9.3 |
| Chronic kidney disease (%) | 22.8 | 20.5 |
| eGFR (mL/min/1.73 m2) | 61.0 (48.5–73.0) | 63.0 (53.0–75.0) |
| Serum potassium (mEq/L) | 4.0 (3.7–4.3) | 3.9 (3.8–4.2) |
| Plasma BNP (pg/mL) | 374.5 (182.5–684.0) | 380.0 (198.0–597.0) |
| Plasma aldosterone (pg/mL) | 71.0 (47.0–114.0) | 82.0 (56.0–119.0) |
| LVEF (%) | 31.0 (25.0–35.9) | 30.0 (25.0–36.0) |
| . | Eplerenone . | Placebo . |
|---|---|---|
| Median days from admission to randomization | 2 (1–5) | 2 (1–4) |
| Medical history (%) | ||
| Previous hospitalization for HF | 12.1 | 12.6 |
| Myocardial infarction | 19.5 | 19.2 |
| Angina pectoris | 16.1 | 15.2 |
| Atrial fibrillation/flutter | 36.9 | 33.1 |
| Ventricular tachycardia/fibrillation | 8.1 | 7.9 |
| PCI | 16.1 | 16.6 |
| Device therapy (%) | ||
| Pacemaker | 3.4 | 3.3 |
| Defibrillator | 1.3 | 1.3 |
| CRT | 0.7 | 0 |
| Medications (%) | ||
| ACE inhibitor or ARB | 89.9 | 89.4 |
| Beta-blocker | 62.4 | 65.6 |
| Diuretic | 91.9 | 89.4 |
| Aspirin | 46.3 | 35.1 |
| Statin | 43.6 | 45.0 |
| Other lipid lowering drugs | 46.3 | 45.0 |
| . | Eplerenone . | Placebo . |
|---|---|---|
| Median days from admission to randomization | 2 (1–5) | 2 (1–4) |
| Medical history (%) | ||
| Previous hospitalization for HF | 12.1 | 12.6 |
| Myocardial infarction | 19.5 | 19.2 |
| Angina pectoris | 16.1 | 15.2 |
| Atrial fibrillation/flutter | 36.9 | 33.1 |
| Ventricular tachycardia/fibrillation | 8.1 | 7.9 |
| PCI | 16.1 | 16.6 |
| Device therapy (%) | ||
| Pacemaker | 3.4 | 3.3 |
| Defibrillator | 1.3 | 1.3 |
| CRT | 0.7 | 0 |
| Medications (%) | ||
| ACE inhibitor or ARB | 89.9 | 89.4 |
| Beta-blocker | 62.4 | 65.6 |
| Diuretic | 91.9 | 89.4 |
| Aspirin | 46.3 | 35.1 |
| Statin | 43.6 | 45.0 |
| Other lipid lowering drugs | 46.3 | 45.0 |
Data are presented as % or median (interquartile range).
ACE, angiotensin-converting enzyme; AHF, acute heart failure; ARB, angiotensin-receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Study outcomes
The primary composite outcome of cardiovascular death or first re-hospitalization due to cardiovascular disease within 6 months were not significantly different and occurred in 29 patients (19.5%) in the eplerenone group and 26 patients (17.2%) in the placebo group (HR = 1.09; 95% CI, 0.64–1.86). (Table 2 and Figure 2A). The effect of eplerenone on the primary outcome was generally consistent across the prespecified subgroups (Figure 3). The composite outcome of cardiovascular death or initial re-hospitalization due to HF within 6 months occurred in seven patients (4.7%) in the eplerenone group and 11 patients (7.3%) in the placebo group (HR = 0.55; 95% CI, 0.21–1.43) (Figure 2B). The need for and use of medical devices for HF occurred in four patients (2.7%) in the eplerenone group and nine patients (6.0%) in the placebo group (HR = 0.39; 95% CI, 0.12–1.28). The rate of death due to cardiovascular causes was 1.3% and 0% in the eplerenone and placebo groups, respectively. Ventricular arrhythmia was observed in two events (1.3%) in the eplerenone group and in one event (0.7%) in the placebo group.
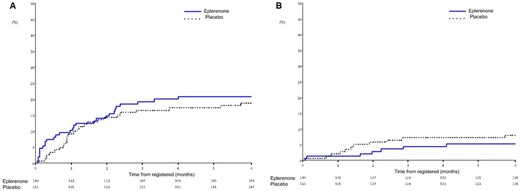
Kaplan–Meier plots for (A) the primary composite outcome of cardiovascular death or first re-hospitalization due to cardiovascular disease and (B) the composite outcome of cardiovascular death or initial re-hospitalization due to heart failure.
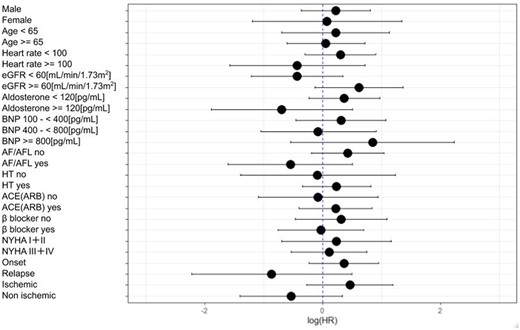
Hazard ratios for the composite outcome of cardiovascular death or initial re-hospitalization due to heart failure according to subgroups. ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin-receptor blocker; BNP, brain natriuretic peptide; eGFR, mean estimated glomerular filtration rate; HR, hazard ratio; HT, hypertension; NYHA, New York Heart Association.
| . | Eplerenone (N = 149) . | Placebo (N = 151) . | Hazard ratio . |
|---|---|---|---|
| Primary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Components of primary endpoint | |||
| HF | 6 (4.0%) | 11 (7.3%) | |
| STEMI | 0 | 1 (0.7%) | |
| Valvular diseases | 1 (0.7%) | 1 (0.7%) | |
| Stroke | 2 (1.3%) | 2 (1.3%) | |
| Ventricular arrhythmia | 2 (1.3%) | 1 (0.7%) | |
| Atrial fibrillation | 2 (1.3%) | 0 | |
| Left ventricular thrombus | 0 | 1 (0.7%) | |
| Coronary artery disease | 16 (10.7%) | 9 (6.0%) | |
| Secondary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Cardiovascular death or non-fatal AMI | 2 (1.3%) | 1 (0.7%) | 1.88 (0.170–20.747) |
| First re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Death from any cardiovascular disease | 2 (1.3%) | 0 (0.0%) | |
| First re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Death from any cause | 2 (1.3%) | 0 (0.0%) | |
| Non-fatal AMI | 0 (0.0%) | 1 (0.7%) | |
| Cardiac sudden death | 0 (0.0%) | 0 (0.0%) | |
| Therapy with medical device for HF | 4 (2.7%) | 9 (6.0%) | 0.39 (0.120–1.278) |
| . | Eplerenone (N = 149) . | Placebo (N = 151) . | Hazard ratio . |
|---|---|---|---|
| Primary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Components of primary endpoint | |||
| HF | 6 (4.0%) | 11 (7.3%) | |
| STEMI | 0 | 1 (0.7%) | |
| Valvular diseases | 1 (0.7%) | 1 (0.7%) | |
| Stroke | 2 (1.3%) | 2 (1.3%) | |
| Ventricular arrhythmia | 2 (1.3%) | 1 (0.7%) | |
| Atrial fibrillation | 2 (1.3%) | 0 | |
| Left ventricular thrombus | 0 | 1 (0.7%) | |
| Coronary artery disease | 16 (10.7%) | 9 (6.0%) | |
| Secondary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Cardiovascular death or non-fatal AMI | 2 (1.3%) | 1 (0.7%) | 1.88 (0.170–20.747) |
| First re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Death from any cardiovascular disease | 2 (1.3%) | 0 (0.0%) | |
| First re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Death from any cause | 2 (1.3%) | 0 (0.0%) | |
| Non-fatal AMI | 0 (0.0%) | 1 (0.7%) | |
| Cardiac sudden death | 0 (0.0%) | 0 (0.0%) | |
| Therapy with medical device for HF | 4 (2.7%) | 9 (6.0%) | 0.39 (0.120–1.278) |
AMI, acute myocardial infarction; HF, heart failure; STEMI, ST-segment elevation myocardial infarction.
| . | Eplerenone (N = 149) . | Placebo (N = 151) . | Hazard ratio . |
|---|---|---|---|
| Primary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Components of primary endpoint | |||
| HF | 6 (4.0%) | 11 (7.3%) | |
| STEMI | 0 | 1 (0.7%) | |
| Valvular diseases | 1 (0.7%) | 1 (0.7%) | |
| Stroke | 2 (1.3%) | 2 (1.3%) | |
| Ventricular arrhythmia | 2 (1.3%) | 1 (0.7%) | |
| Atrial fibrillation | 2 (1.3%) | 0 | |
| Left ventricular thrombus | 0 | 1 (0.7%) | |
| Coronary artery disease | 16 (10.7%) | 9 (6.0%) | |
| Secondary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Cardiovascular death or non-fatal AMI | 2 (1.3%) | 1 (0.7%) | 1.88 (0.170–20.747) |
| First re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Death from any cardiovascular disease | 2 (1.3%) | 0 (0.0%) | |
| First re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Death from any cause | 2 (1.3%) | 0 (0.0%) | |
| Non-fatal AMI | 0 (0.0%) | 1 (0.7%) | |
| Cardiac sudden death | 0 (0.0%) | 0 (0.0%) | |
| Therapy with medical device for HF | 4 (2.7%) | 9 (6.0%) | 0.39 (0.120–1.278) |
| . | Eplerenone (N = 149) . | Placebo (N = 151) . | Hazard ratio . |
|---|---|---|---|
| Primary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Components of primary endpoint | |||
| HF | 6 (4.0%) | 11 (7.3%) | |
| STEMI | 0 | 1 (0.7%) | |
| Valvular diseases | 1 (0.7%) | 1 (0.7%) | |
| Stroke | 2 (1.3%) | 2 (1.3%) | |
| Ventricular arrhythmia | 2 (1.3%) | 1 (0.7%) | |
| Atrial fibrillation | 2 (1.3%) | 0 | |
| Left ventricular thrombus | 0 | 1 (0.7%) | |
| Coronary artery disease | 16 (10.7%) | 9 (6.0%) | |
| Secondary endpoint | |||
| Cardiovascular death or a first re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Cardiovascular death or non-fatal AMI | 2 (1.3%) | 1 (0.7%) | 1.88 (0.170–20.747) |
| First re-hospitalization due to any cardiovascular disease | 29 (19.5%) | 26 (17.2%) | 1.09 (0.642–1.855) |
| Death from any cardiovascular disease | 2 (1.3%) | 0 (0.0%) | |
| First re-hospitalization due to HF | 7 (4.7%) | 11 (7.3%) | 0.55 (0.213–1.434) |
| Death from any cause | 2 (1.3%) | 0 (0.0%) | |
| Non-fatal AMI | 0 (0.0%) | 1 (0.7%) | |
| Cardiac sudden death | 0 (0.0%) | 0 (0.0%) | |
| Therapy with medical device for HF | 4 (2.7%) | 9 (6.0%) | 0.39 (0.120–1.278) |
AMI, acute myocardial infarction; HF, heart failure; STEMI, ST-segment elevation myocardial infarction.
Safety outcomes
Three patients (one in the eplerenone group and two in the placebo group) were excluded from the safety analyses because they did not receive either eplerenone or placebo. The incidence of adverse events was 79.1% and 79.2% in the eplerenone and placebo groups, respectively. The incidence of an adverse drug reaction was 24.3% and 18.8% in the eplerenone and placebo groups, respectively (Table 3). The incidence of serious adverse events was 23.0% and 20.8% in the eplerenone and placebo groups, respectively. There was no significant difference in the incidence of adverse events or adverse drug reactions between the two groups. The incidence of hyperkalaemia and hypokalaemia was 4.1% and 2.7% in the eplerenone group and 2.0% and 4.7% in the placebo group. Two patients in the eplerenone group died; both were considered as non-drug-related deaths, whereas none died in the placebo group.
| Event . | Adverse event . | Adverse event leading to study-drug withdrawal . | ||||
|---|---|---|---|---|---|---|
| . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . |
| All events | 117 (79.1%) | 118 (79.2%) | 1.000 | 10 (6.8%) | 6 (4.0%) | 0.433 |
| Hyperkalaemia | 6 (4.1%) | 4 (2.7%) | 0.741 | 1 (0.7%) | 0 | 0.997 |
| Hypokalaemia | 3 (2.0%) | 7 (4.7%) | 0.341 | 0 | 0 | — |
| Renal impairment | 10 (6.8%) | 9 (6.0%) | 0.988 | 1 (0.7%) | 0 | 0.997 |
| Liver impairment | 4 (2.7%) | 2 (1.3%) | 0.678 | 1 (0.7%) | 0 | 0.997 |
| Hypotension | 3 (2.0%) | 0 (0.0%) | 0.245 | 0 | 0 | — |
| Event . | Adverse event . | Adverse event leading to study-drug withdrawal . | ||||
|---|---|---|---|---|---|---|
| . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . |
| All events | 117 (79.1%) | 118 (79.2%) | 1.000 | 10 (6.8%) | 6 (4.0%) | 0.433 |
| Hyperkalaemia | 6 (4.1%) | 4 (2.7%) | 0.741 | 1 (0.7%) | 0 | 0.997 |
| Hypokalaemia | 3 (2.0%) | 7 (4.7%) | 0.341 | 0 | 0 | — |
| Renal impairment | 10 (6.8%) | 9 (6.0%) | 0.988 | 1 (0.7%) | 0 | 0.997 |
| Liver impairment | 4 (2.7%) | 2 (1.3%) | 0.678 | 1 (0.7%) | 0 | 0.997 |
| Hypotension | 3 (2.0%) | 0 (0.0%) | 0.245 | 0 | 0 | — |
| Event . | Adverse event . | Adverse event leading to study-drug withdrawal . | ||||
|---|---|---|---|---|---|---|
| . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . |
| All events | 117 (79.1%) | 118 (79.2%) | 1.000 | 10 (6.8%) | 6 (4.0%) | 0.433 |
| Hyperkalaemia | 6 (4.1%) | 4 (2.7%) | 0.741 | 1 (0.7%) | 0 | 0.997 |
| Hypokalaemia | 3 (2.0%) | 7 (4.7%) | 0.341 | 0 | 0 | — |
| Renal impairment | 10 (6.8%) | 9 (6.0%) | 0.988 | 1 (0.7%) | 0 | 0.997 |
| Liver impairment | 4 (2.7%) | 2 (1.3%) | 0.678 | 1 (0.7%) | 0 | 0.997 |
| Hypotension | 3 (2.0%) | 0 (0.0%) | 0.245 | 0 | 0 | — |
| Event . | Adverse event . | Adverse event leading to study-drug withdrawal . | ||||
|---|---|---|---|---|---|---|
| . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . | Eplerenone (N = 148) . | Placebo (N = 149) . | P-value . |
| All events | 117 (79.1%) | 118 (79.2%) | 1.000 | 10 (6.8%) | 6 (4.0%) | 0.433 |
| Hyperkalaemia | 6 (4.1%) | 4 (2.7%) | 0.741 | 1 (0.7%) | 0 | 0.997 |
| Hypokalaemia | 3 (2.0%) | 7 (4.7%) | 0.341 | 0 | 0 | — |
| Renal impairment | 10 (6.8%) | 9 (6.0%) | 0.988 | 1 (0.7%) | 0 | 0.997 |
| Liver impairment | 4 (2.7%) | 2 (1.3%) | 0.678 | 1 (0.7%) | 0 | 0.997 |
| Hypotension | 3 (2.0%) | 0 (0.0%) | 0.245 | 0 | 0 | — |
Effects on echocardiographic parameters and the plasma BNP levels
Changes in the plasma BNP levels at 6 months after admission were 64.0 and 60.8 pg/mL in the eplerenone and placebo groups, respectively (Table 4). In echocardiographic analyses, the decreases in diastolic left ventricular dimensions were 4.0% and 2.6%, and the increases in LVEF were 18.1% and 13.0% in the eplerenone and placebo groups, respectively (Table 4).
| Parameter . | Eplerenone . | Placebo . | Eplerenone–placebo . | ||||
|---|---|---|---|---|---|---|---|
| . | Baseline . | 6 months . | Change . | Baseline . | 6 months . | Change . | Difference (95% CI) . |
| LVDd (mm) | N = 143 | N = 95 | N = 147 | N = 99 | −0.95 (−2.21 to 0.31) | ||
| Median | 57.0 | 52.5 | −4.0 | 58.0 | 54.8 | −2.6 | |
| (IQR) | (51.0 to 63.0) | (46.0 to 59.0) | (−8.25 to 0) | (53.0 to 63.2) | (50.0 to 60.0) | (−7.0 to 1.5) | |
| LVDs (mm) | N = 145 | N = 95 | N = 147 | N = 99 | −1.07(−2.45 to 0.31) | ||
| Median | 48.0 | 38.3 | −8.8 | 50.0 | 41.0 | −8.0 | |
| (IQR) | (43.0 to 56.0) | (33.0 to 47.8) | (−13.0 to −4.0) | (43.5 to 56.0) | (34.0 to 49.0) | (−14.0 to −1.8) | |
| LVEF (%) | N = 147 | N = 95 | N = 147 | N = 99 | 1.56 (−0.70 to 3.82) | ||
| Median | 31.0 | 49.1 | 18.1 | 30.0 | 45.0 | 13.0 | |
| (IQR) | (25.0–35.9) | (37.3 to 58.0) | (8.0 to 26.8) | (25.0 to 36.0) | (37.0 to 54.0) | (6.0 to 22.0) | |
| Plasma BNP (pg/mL) | N = 147 | N = 95 | N = 147 | N = 99 | 0.9 (0.8–1.1) | ||
| Geometric mean | 332.0 | 84.8 | 64.0 | 340.4 | 86.0 | 60.8 | |
| (Geometric CV, %) | (125.6) | (176.0) | (211.5) | (110.2) | (177.2) | (347.5) | |
| Parameter . | Eplerenone . | Placebo . | Eplerenone–placebo . | ||||
|---|---|---|---|---|---|---|---|
| . | Baseline . | 6 months . | Change . | Baseline . | 6 months . | Change . | Difference (95% CI) . |
| LVDd (mm) | N = 143 | N = 95 | N = 147 | N = 99 | −0.95 (−2.21 to 0.31) | ||
| Median | 57.0 | 52.5 | −4.0 | 58.0 | 54.8 | −2.6 | |
| (IQR) | (51.0 to 63.0) | (46.0 to 59.0) | (−8.25 to 0) | (53.0 to 63.2) | (50.0 to 60.0) | (−7.0 to 1.5) | |
| LVDs (mm) | N = 145 | N = 95 | N = 147 | N = 99 | −1.07(−2.45 to 0.31) | ||
| Median | 48.0 | 38.3 | −8.8 | 50.0 | 41.0 | −8.0 | |
| (IQR) | (43.0 to 56.0) | (33.0 to 47.8) | (−13.0 to −4.0) | (43.5 to 56.0) | (34.0 to 49.0) | (−14.0 to −1.8) | |
| LVEF (%) | N = 147 | N = 95 | N = 147 | N = 99 | 1.56 (−0.70 to 3.82) | ||
| Median | 31.0 | 49.1 | 18.1 | 30.0 | 45.0 | 13.0 | |
| (IQR) | (25.0–35.9) | (37.3 to 58.0) | (8.0 to 26.8) | (25.0 to 36.0) | (37.0 to 54.0) | (6.0 to 22.0) | |
| Plasma BNP (pg/mL) | N = 147 | N = 95 | N = 147 | N = 99 | 0.9 (0.8–1.1) | ||
| Geometric mean | 332.0 | 84.8 | 64.0 | 340.4 | 86.0 | 60.8 | |
| (Geometric CV, %) | (125.6) | (176.0) | (211.5) | (110.2) | (177.2) | (347.5) | |
BNP, brain natriuretic peptide; CV, coefficient of variation; IQR, interquartile range; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
| Parameter . | Eplerenone . | Placebo . | Eplerenone–placebo . | ||||
|---|---|---|---|---|---|---|---|
| . | Baseline . | 6 months . | Change . | Baseline . | 6 months . | Change . | Difference (95% CI) . |
| LVDd (mm) | N = 143 | N = 95 | N = 147 | N = 99 | −0.95 (−2.21 to 0.31) | ||
| Median | 57.0 | 52.5 | −4.0 | 58.0 | 54.8 | −2.6 | |
| (IQR) | (51.0 to 63.0) | (46.0 to 59.0) | (−8.25 to 0) | (53.0 to 63.2) | (50.0 to 60.0) | (−7.0 to 1.5) | |
| LVDs (mm) | N = 145 | N = 95 | N = 147 | N = 99 | −1.07(−2.45 to 0.31) | ||
| Median | 48.0 | 38.3 | −8.8 | 50.0 | 41.0 | −8.0 | |
| (IQR) | (43.0 to 56.0) | (33.0 to 47.8) | (−13.0 to −4.0) | (43.5 to 56.0) | (34.0 to 49.0) | (−14.0 to −1.8) | |
| LVEF (%) | N = 147 | N = 95 | N = 147 | N = 99 | 1.56 (−0.70 to 3.82) | ||
| Median | 31.0 | 49.1 | 18.1 | 30.0 | 45.0 | 13.0 | |
| (IQR) | (25.0–35.9) | (37.3 to 58.0) | (8.0 to 26.8) | (25.0 to 36.0) | (37.0 to 54.0) | (6.0 to 22.0) | |
| Plasma BNP (pg/mL) | N = 147 | N = 95 | N = 147 | N = 99 | 0.9 (0.8–1.1) | ||
| Geometric mean | 332.0 | 84.8 | 64.0 | 340.4 | 86.0 | 60.8 | |
| (Geometric CV, %) | (125.6) | (176.0) | (211.5) | (110.2) | (177.2) | (347.5) | |
| Parameter . | Eplerenone . | Placebo . | Eplerenone–placebo . | ||||
|---|---|---|---|---|---|---|---|
| . | Baseline . | 6 months . | Change . | Baseline . | 6 months . | Change . | Difference (95% CI) . |
| LVDd (mm) | N = 143 | N = 95 | N = 147 | N = 99 | −0.95 (−2.21 to 0.31) | ||
| Median | 57.0 | 52.5 | −4.0 | 58.0 | 54.8 | −2.6 | |
| (IQR) | (51.0 to 63.0) | (46.0 to 59.0) | (−8.25 to 0) | (53.0 to 63.2) | (50.0 to 60.0) | (−7.0 to 1.5) | |
| LVDs (mm) | N = 145 | N = 95 | N = 147 | N = 99 | −1.07(−2.45 to 0.31) | ||
| Median | 48.0 | 38.3 | −8.8 | 50.0 | 41.0 | −8.0 | |
| (IQR) | (43.0 to 56.0) | (33.0 to 47.8) | (−13.0 to −4.0) | (43.5 to 56.0) | (34.0 to 49.0) | (−14.0 to −1.8) | |
| LVEF (%) | N = 147 | N = 95 | N = 147 | N = 99 | 1.56 (−0.70 to 3.82) | ||
| Median | 31.0 | 49.1 | 18.1 | 30.0 | 45.0 | 13.0 | |
| (IQR) | (25.0–35.9) | (37.3 to 58.0) | (8.0 to 26.8) | (25.0 to 36.0) | (37.0 to 54.0) | (6.0 to 22.0) | |
| Plasma BNP (pg/mL) | N = 147 | N = 95 | N = 147 | N = 99 | 0.9 (0.8–1.1) | ||
| Geometric mean | 332.0 | 84.8 | 64.0 | 340.4 | 86.0 | 60.8 | |
| (Geometric CV, %) | (125.6) | (176.0) | (211.5) | (110.2) | (177.2) | (347.5) | |
BNP, brain natriuretic peptide; CV, coefficient of variation; IQR, interquartile range; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
Discussion
Evidence from the EARLIER trial indicated that eplerenone did not decrease the incidence of a primary composite outcome of cardiovascular death or first re-hospitalization due to cardiovascular disease in patients with AHF. We hypothesized the beneficial cardiovascular effects of early exposure to eplerenone in patients with AHF and hence administered eplerenone during the early phase of AHF; however, we did not statistically present beneficial effects against cardiovascular events. The effects of MRA treatment on AHF is difficult to be evaluated with only this trial because there are well-established lines of evidence that MRAs, as well as eplerenone, are beneficial to the pathophysiology of HFrEF.
Deep insight into the present results
The EPHESUS trial provided the present hypothesis of the EARLIER trial, showing that eplerenone in patients with systolic left ventricular dysfunction and HF post-myocardial infarction (MI) had a 15% relative risk reduction in all-cause mortality and a 13% relative risk reduction in cardiovascular mortality/cardiovascular complications compared with patients on current standard therapy. We estimated the sample size of the present study on the basis of the results of the previous trial. We could not show the benefit of early treatment with eplerenone regarding a primary composite outcome of cardiovascular death or first hospitalization for cardiovascular disease because the present result is inconsistent with that in the EPHESUS trial. Patients with HF post-MI were enrolled in the EPHESUS trial; therefore, eplerenone may be more effective in patients in this specific population. We endeavoured to enroll the AMI patients with AHF; however, it was difficult to enroll AMI patients with low LVEF, and we only enrolled 35 AMI patients. Consequently, the sub-analysis stratified by the presence of AMI did not explain the difference between the EARLIER and EPHESUS trials.
The components of the primary endpoints of the EARLIER and EPHESUS trials were compared and analysed as follows. The primary outcome of the EPHESUS trial comprised death from cardiovascular causes or first hospitalization for a cardiovascular event, including HF, recurrent AMI, stroke, or ventricular arrhythmia. Nevertheless, the primary outcome of the EARLIER trial consisted of cardiovascular death or first re-hospitalization for any cardiovascular disease, including interventions with PCI or CABG. The sub-analysis of the EPHESUS trial showed no differences between the eplerenone and placebo groups in the events related to PCI or CABG, such as recurrence of angina, unstable angina, and AMI.22 We included hospitalization due to the incidence of either PCI and CABG as a part of the primary endpoint in the EARLIER trial and found that the numbers of events related to coronary artery disease (CAD) such as PCI and CABG were 16 and 9 in the eplerenone and placebo groups, respectively. This deviation could have occurred by chance because eplerenone should not cause the progression of CAD; however, larger coronary events in the eplerenone group may have affected the results of primary endpoint in the present study. This outcome may naturally lead us to the idea that the incidence of hospitalization for HF is predominantly reduced in patients with AHF.
ATHENA-HF trial23 assessed the effect of the 96 h administration of high-dose spironolactone (100 mg) on N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in 360 patients with AHF. The high-dose spironolactone reduced neither NT-proBNP levels nor the incidence in death or hospitalization for HF. The sample size and study patients were similar in ATHENA-HF and our EARLIER trials, but the timing and duration was different between the two groups. Both results represented that the addition of MRA treatment to standard of care for AHF was well tolerated but failed to improve the prognosis of patients with AHF.
Effects of eplerenone on hospitalization for HF
In the EPHESUS trial, hospitalization for HF was significantly decreased, culminating in the positive results for the primary endpoint of death from cardiovascular causes or hospitalization for cardiovascular events. The EARLIER trial showed no significant difference in the incidence of hospitalization for HF between in the eplerenone group and in the placebo group. Eplerenone administration at the acute phase improved biomarkers for the assessment of the severity of HF at 6 months after enrolment, observation in the eplerenone group compared with that in the placebo group showed the tendency of decreasing plasma BNP levels and left ventricular systolic/diastolic dimension and increases in LVEF at 6 months after entry. The results of the biomarkers for HF are in consistency with the results of the hospitalization for HF for 6 and 16 months in the EARLIER and EMPHASIS-HF trials, respectively.
We continued to administer eplerenone at the stable phase after AHF for 6 months; the average hospitalization periods were 18 and 19 days in the eplerenone and placebo groups, respectively. Other studies using MRA, such as eplerenone, have shown beneficial results for HFrEF16,19,24; the administration of eplerenone at the chronic phase may largely contribute to the improvement of the outcome of hospitalization for HF in the present study. When we checked the hospitalization for HF in our study, the incidences of hospitalization for HF at 30 and 60 days after enrolment were 0 and 2 in the eplerenone group and 2 and 5 in the placebo group.
Safety of eplerenone in patients with AHF
Eplerenone therapy may produce several adverse effects such as hypotension, hyperkalaemia, and renal dysfunction; however, eplerenone had a favourable safety profile in patients with AHF in Japan. There was no noticeable difference in the incidence of adverse events between the eplerenone and the placebo groups (79.1% and 79.2%, respectively).
Hyperkalaemia is the most essential adverse effect of eplerenone. In this trial, hyperkalaemia occurred more frequently in the eplerenone group, whereas hypokalaemia was observed in the placebo group. Particularly when combined with renal function impairment, diabetes, and advanced age, hyperkalaemia frequently occurs in patients with cardiovascular diseases.25 Severe hyperkalaemia is an independent predictor of all-cause and in-hospital mortality and hospitalizations. Therefore, renin–angiotensin–aldosterone system inhibitor therapy should be started at a low dosage and titrated to the maximum tolerated evidence-based doses.
Hypertension and hypotension occurred 0% and 2% in the eplerenone group and 2% and 0% in the placebo group. The prevalence of renal dysfunction was 6.8% and 6.0% in the eplerenone and the placebo groups, respectively.
Because of high incidence of hyperkalaemia due to eplerenone treatment, adherence is an important issue. A recent study explained that approximately 20% of patients with HF were partially or completely non-adherent to prescribed drugs including diuretics, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, beta-blockers, and MRAs.26 In our study, we did not observe the difference in adherence between the eplerenone and placebo groups.
Limitations
There were several limitations of our trial. The sample size of our trial was 300 patients, which seems small compared with that in the EPHESUS trial; this sample size was calculated to show the consistency of the results with the EPHESUS trial for the drug approval for AHF in Japan. A realistic enrolment number of patients with AHF to be enrolled for several years in Japan was 300. Indeed J-EMPHASIS-HF trial for approval of eplerenone for chronic ejection fraction in Japan enrolled 221 HFrEF patients for more than 5 years in Japan.27
The second limitation of the EARLIER trial was that enrolled patients were all Japanese. The pathophysiology of AHF seems to be identical worldwide, so one may argue that the present results can be extended to patients in Western countries, suggesting that ethnicity has little effect on the present conclusion.
Lastly, it would have been useful to refer to the EMPHASIS-HF trial19; the results of the EMPHASIS-HF trial were not available during the planning stages of the EARLIER trial. The study was designed using information from the EPHESUS trial. The EARLIER trial demonstrated the beneficial effect of eplerenone on the reduction in the incidence of cardiovascular death or first re-hospitalization for HF, which was a primary endpoint of the EMPHASIS-HF trial. This result suggests the possibility of a benefit of eplerenone regarding the secondary outcome of re-hospitalization for HF.
Conclusion
The EARLIER trial evaluated the effect of early initiation of eplerenone in patients with AHF. Because of inadequate power, this trial could not reach a conclusion that eplerenone reduced the incidence of cardiovascular death or first re-hospitalization for HF within 6 months. Further trial is needed to confirm these results of this trial. The occurrence of adverse events was comparable in the two groups, and the safety of eplerenone, including hyperkalaemia, was similar to that seen in other eplerenone studies in HF.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Acknowledgements
We acknowledge the great help from Ms Y. Miyawaki, M. Ono and C. Takayama, A. Yokoe, M. Takahashi, and Mr T. Uraki for the secretarial works. Mr M. Yamamoto, Mr S. Fukunaga, and Ms K. Terasawa at the Center for Clinical Trial, the Japan Medical Association (JMACCT) advised us how to appropriately plan and organize the EARLIER study for study implementation.
Funding
This work was supported by a clinical trial promotion project of Japan Medical Association, which is funded by a Health Labor Sciences Research Grant from the Ministry of Health, Labor, and Welfare.
Conflict of interest: M.K., K.A., K.H., K.K., Y.K., Y.S., M.A., and A.Y. received honorarium form Pfizer Japan. M.K., A.H., and Y.S. received the research funding from Pfizer Japan. M.K. and T.Y. are advisory board members of clinical trial on eplerenone in chronic heart failure (J-EMPHASIS-HF Study). The drugs are generously provided by Pfizer USA based on the policy of Japanese-GCP.



