-
PDF
- Split View
-
Views
-
Cite
Cite
Johan E Larsson, Cæcilie Stilling Denholt, Jens Jakob Thune, Anna Axelsson Raja, Emil Fosbøl, Morten Schou, Lars Køber, Olav Wendelboe Nielsen, Finn Gustafsson, Søren L Kristensen, Initiation of eplerenone or spironolactone, treatment adherence, and associated outcomes in patients with new-onset heart failure with reduced ejection fraction: a nationwide cohort study, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 9, Issue 6, September 2023, Pages 546–552, https://doi.org/10.1093/ehjcvp/pvad045
Close - Share Icon Share
Abstract
The mineralocorticoid receptor antagonists (MRAs) eplerenone and spironolactone are beneficial in heart failure with reduced ejection fraction (HFrEF), but have not been prospectively compared. We compared clinical outcomes, daily dosages, and discontinuation rates for the two drugs in a nationwide cohort.
We identified all patients with HFrEF in the period 2016–2020, who were alive and had initiated MRA treatment at study start, 180 days after HF diagnosis. We estimated the 2-year risk of a composite of death and HF hospitalization, as well as each component separately, using Kaplan–Meier, cumulative incidence functions, and Cox proportional hazards models adjusted for age, sex, and comorbidities. Secondly, we assessed treatment withdrawal, cross-over, and daily drug dosage.
We included 7479 patients; 653 (9%) on eplerenone and 6840 (91%) on spironolactone. Patients in the eplerenone group were younger (median age 65 vs. 69 years), and more often men (91% vs. 68%), both P < 0.001. In adjusted analyses, with spironolactone as reference, there were no differences in the risk of the composite of all-cause death and HF hospitalization (HR 1.02, 95% CI 0.82–1.27), all-cause death (HR 0.93, 95% CI 0.67–1.30), or HF hospitalization (HR 1.10, 95% CI 0.84–1.42). Treatment withdrawal occurred in 34% in the eplerenone group and 53% in the spironolactone group (P < 0.001), treatment cross-over in 3%, and 10%, respectively. Daily dose >25 mg at 12 months, was observed in 230 patients (37%) in the eplerenone group and 771 patients (12%) in the spironolactone (P < 0.001).
In a contemporary nationwide cohort of patients with new-onset HFrEF who initiated MRA, we found no differences in clinical outcomes associated with initiation of eplerenone vs. spironolactone. Treatment was more frequently withdrawn, and daily drug dosage was lower among patients treated with spironolactone.
Introduction
Mineralocorticoid receptor antagonists (MRAs) remain a cornerstone in guideline directed medical treatment (GDMT) of heart failure with reduced ejection fraction (HFrEF), with a substantial effect on morbidity and mortality.1,2 Despite the obvious benefits, MRAs remain underused, possibly due to a perception of the drug class to be reserved for severe HF only and the risk of hyperkalemia as well as worsening of renal function.3,4
Two MRAs are available, spironolactone and eplerenone. Spironolactone was tested in severely symptomatic HFrEF, whereas eplerenone was tested in less symptomatic patients with HFrEF.1,2 Both drugs demonstrated convincing benefit on all-cause death and HF hospitalization, but have not been directly compared in randomized clinical trials. Mineralocorticoid receptor antagonists work by decreasing sodium reabsorption in the kidneys but also hold anti-fibrotic effects.5 In addition to the risk of hyperkalemia, and worsening kidney function seen with both MRAs, spironolactone (but not eplerenone) inhibits free testosterone from binding to androgen receptors, which, as a side effect, leads to more estrogen binding and potential breast tissue proliferation. As a result, gynecomastia was seen in ∼10% of patients treated with spironolactone vs. <1% among patients treated with eplerenone.1,2
Prior observational studies comparing clinical outcomes of patients treated with eplerenone and spironolactone have yielded somewhat inconsistent findings. Both drugs had comparable efficacy on cardiovascular outcomes in acute decompensated HF, whereas eplerenone was associated with lower risk of mortality in patients with chronic HFrEF.6 These comparisons may have been hampered by the considerably higher price of eplerenone and restricted subsidization available for its prescription. Until 2019, reimbursement of treatment costs for eplerenone, required that the patient had tried and could not tolerate spironolactone due to side effects. In addition to an evident underuse of MRA, discontinuation of the drug is frequent and associated with a low probability of subsequent reintroduction of MRA, although prior studies did not assess these outcomes separately for eplerenone and spironolactone.6,7
In the present study, we utilized the Danish nationwide registries to study treatment adherence, drug doses achieved, and clinical outcomes following initiation of eplerenone or spironolactone in patients with new-onset HFrEF.
Methods
Data sources
The unique personal identification number assigned to all Danish residents was used to link information between different health and administrative registries on an individual level.8 We collected clinical information, including diagnoses, prescription redemption, in- or outpatient visits, and vital status. We gathered data from the Danish National Patient Registry, The National Prescription Registry, and the National Cause of Death Registry.
Study population and baseline variables
The study population was comprised of all patients aged 18–85 years who had a first-time in- or outpatient hospital contact with a primary diagnosis of HF during 2016–2020 and no history of MRA use in the previous 10 years. Study start was set to 180 days after initial HF diagnosis, and patients who died during the 180 days between HF diagnosis and study start were excluded. To ensure we only included patients with HFrEF, we required patients to redeem a prescription of a renin-angiotensin system inhibitor (RASi), either an angiotensin-converting enzyme inhibitor, an angiotensin-II receptor blocker, or an angiotensin receptor-neprilysin inhibitor, as well as a beta-blocker during the first 120 days following HF diagnosis. This approach identifies patients with HFrEF with a positive predictive value of 95% in the Danish registries.9
We included patients who initiated treatment with either eplerenone or spironolactone in the period from day of HF diagnosis to study start, 180 days later (Figure 1). In patients who switched drug within the 180 days from HF diagnosis until study start (or during follow-up), patients were grouped according to first treatment initiated and remained in that group. As a supplementary analysis, we estimated propensity scores for all patients with multivariable logistic regression by using treatment with eplerenone as the outcome. We used 22 baseline variables for derivation of the propensity scores. Patients treated with eplerenone were matched 1:1 with patients treated with spironolactone on the logit of the propensity score using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score. We also assessed baseline characteristics in patients with HFrEF who did not initiate MRA within 180 days for comparison.
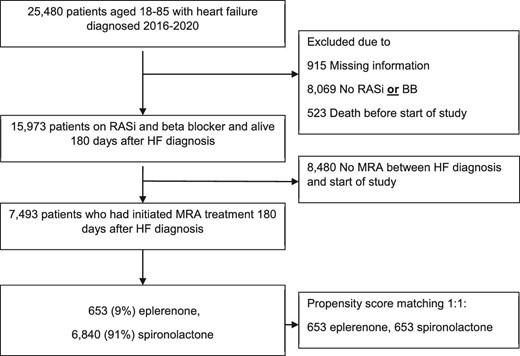
Baseline use of pharmacotherapy was defined as at least one filled prescription in the 180 days from HF diagnosis to start of study (see Supplementary material online, Table S1 for Anatomic Therapeutic Chemical codes). Baseline comorbidity was defined by hospital admission discharge diagnoses and outpatient diagnoses in the 10-year period before and including index HF hospital contact (see Table S1 for International Classification of Diseases codes). Heart failure readmission was defined as a new HF admission within the 180 days from initial HF diagnosis until start of study.
Outcome measures and follow-up
Patients were followed for 2 years, or until the time of death, or end of study on 31 December 2021. All-cause death and HF hospitalization, separately and combined were assessed. For these analyses, patients stayed in their initial treatment group regardless of drug discontinuation or cross-over to the other MRA during follow-up. For analyses of treatment adherence, we included potential treatment with the other MRA as active treatment.
We studied persistence of MRA treatment and defined withdrawal of treatment as a period longer than 90 days without any MRA. The date for withdrawal of treatment was defined as the first day after 90 days out of treatment with either MRA. Re-initiation was defined as redemption of a prescription of either MRA after withdrawal of treatment during follow-up. We used daily dosages of 25 and 50 mg for treatment with eplerenone and 12.5, 25, 37.5, and 50 mg for spironolactone to estimate the daily dose of MRA treatment (as these were the available doses in Denmark in the studied time period). Doses of MRA treatment at start of study, 6 months, and 12 months in the follow-up period were calculated.
Statistics
Differences in baseline characteristics were tested using Pearson's χ2 test for categorical variables and the Welch two-sample t-test with adjustment for unequal variances for continuous variables, and presented with standardized mean differences. The magnitude of the standardized mean difference is commonly interpreted as small if the SMD = 0.2, medium if the SMD = 0.5, and large if the SMD = 0.8.10 Continuous variables are presented as median (Q1–Q3) and categorical variables as numbers with percentages. We used multivariable Cox proportional hazards regression models to estimate the risk of a composite of death and HF hospitalization, and the two components separately. Proportional hazard assumptions was evaluated and found to be valid unless otherwise stated. We included age, sex, ischemic heart disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease (CKD), cancer, readmission with HF, use of loop diuretics, and reimbursement status for eplerenone as covariates in the multivariable models. These variables were chosen inspired by the MAGGIC risk score,11 with the addition of cancer and ischemic heart disease (Figure S1). The composite of death and HF hospitalization as well as mortality separately at up to 2 years follow-up were assessed with Kaplan–Meier estimates and differences between groups were tested with the log-rank test. The separate clinical outcome of HF hospitalization and the persistence endpoint were estimated with cumulative incidence functions with death as a competing risk (Aalen–Johansen estimator12). The withdrawal analysis was univariate with only death as competing risk. We further evaluated the hypotheses of equality of cause-specific cumulative incidence functions between the two groups with Gray's test.13 Re-initiation of MRA treatment after treatment withdrawal >90 days were tested with the χ2 test. The probability of a daily dose of an MRA >25 mg at 18 months after HFrEF diagnosis in patients with complete follow-up in that interval were modeled by multiple logistic regression with two models; (1) age- and sex-adjusted and (2) adjusted for age, sex, diabetes, chronic kidney disease, cancer, readmission with HF, use of loop diuretics, and reimbursement status for eplerenone. Two sided P-values < 0.05 were considered significant. We analyzed data with SAS version 9.4 (SAS Institute, Cary, NC, USA) and R14 (details on packages used in Table S6).
Ethics
Register-based studies that are conducted for the sole purpose of statistics and scientific research do not require ethical approval in Denmark. Individual patients were not identifiable as the personal identification numbers were encrypted. The study was approved by the data responsible institute (Capital Region of Denmark—Approval number: P-2019-191) in accordance with the General Data Protection Regulation.
Results
Baseline characteristics
During the period 1 January 2016 to 3 July 2020, 25.480 patients aged 18–85 were diagnosed with HF and 15.973 patients were alive after 6 months, and redeemed prescriptions of beta blocker and a RASi, within 120 days. Of these 15.973 eligible patients, 7493 (47%) were prescribed an MRA between HF diagnosis and start of study, 180 days later (Figure 1), of whom 653 (9%) did initially redeem a prescription of eplerenone and 6840 of spironolactone (91%). Baseline characteristics for the two groups are presented in Table 1. Patients treated with eplerenone were younger (median age 65 vs. 69 years), more often males (91% vs. 68%) and more frequently treated with sacubitril/valsartan (9% vs. 5%), all P < 0.001.
| Variable . | Eplerenone, (n = 653) . | Spironolactone, (n = 6840) . | P-value1 . | SMD . |
|---|---|---|---|---|
| Age, median (Q1–Q3) | 65 (55, 74) | 69 (59, 76) | <0.001 | 0.233 |
| Male | 591 (90.5%) | 4651 (68.0%) | <0.001 | 0.578 |
| Readmitted with HF before study start | 66 (10.1%) | 1164 (17.0%) | <0.001 | 0.203 |
| Pharmacotherapy | ||||
| RASi2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Beta blockers2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Sacubitril/valsartan | 59 (9.0%) | 348 (5.1%) | <0.001 | 0.155 |
| SGLT2-inhibitors | 36 (5.5%) | 253 (3.7%) | 0.021 | 0.087 |
| Loop diuretics | 441 (67.5%) | 4980 (72.8%) | 0.004 | 0.115 |
| Statins | 418 (64.0%) | 3971 (58.1%) | 0.003 | 0.122 |
| Antidepressants | 54 (8.3%) | 848 (12.4%) | 0.002 | 0.136 |
| Antiplatelet therapy | 331 (50.7%) | 3260 (47.7%) | 0.14 | 0.061 |
| Oral anticoagulants | 241 (36.9%) | 2847 (41.6%) | 0.019 | 0.097 |
| Opioids | 29 (4.4%) | 404 (5.9%) | 0.13 | 0.066 |
| Digoxin | 49 (7.5%) | 884 (12.9%) | <0.001 | 0.180 |
| Comorbidity | ||||
| Ischemic heart disease | 296 (45.3%) | 2805 (41.0%) | 0.032 | 0.087 |
| Peripheral vascular disease | 56 (8.6%) | 652 (9.5%) | 0.4 | 0.033 |
| Cerebrovascular disease | 49 (7.5%) | 617 (9.0%) | 0.2 | 0.055 |
| Cancer | 79 (12.1%) | 905 (13.2%) | 0.4 | 0.034 |
| Cardiac dysrhythmias | 244 (37.4%) | 2750 (40.2%) | 0.2 | 0.058 |
| Chronic renal failure | 21 (3.2%) | 290 (4.2%) | 0.2 | 0.054 |
| COPD | 47 (7.2%) | 776 (11.3%) | 0.001 | 0.143 |
| Rheumatic disease | 20 (3.1%) | 175 (2.6%) | 0.4 | 0.031 |
| Diabetes | 134 (20.5%) | 1481 (21.7%) | 0.5 | 0.028 |
| Variable . | Eplerenone, (n = 653) . | Spironolactone, (n = 6840) . | P-value1 . | SMD . |
|---|---|---|---|---|
| Age, median (Q1–Q3) | 65 (55, 74) | 69 (59, 76) | <0.001 | 0.233 |
| Male | 591 (90.5%) | 4651 (68.0%) | <0.001 | 0.578 |
| Readmitted with HF before study start | 66 (10.1%) | 1164 (17.0%) | <0.001 | 0.203 |
| Pharmacotherapy | ||||
| RASi2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Beta blockers2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Sacubitril/valsartan | 59 (9.0%) | 348 (5.1%) | <0.001 | 0.155 |
| SGLT2-inhibitors | 36 (5.5%) | 253 (3.7%) | 0.021 | 0.087 |
| Loop diuretics | 441 (67.5%) | 4980 (72.8%) | 0.004 | 0.115 |
| Statins | 418 (64.0%) | 3971 (58.1%) | 0.003 | 0.122 |
| Antidepressants | 54 (8.3%) | 848 (12.4%) | 0.002 | 0.136 |
| Antiplatelet therapy | 331 (50.7%) | 3260 (47.7%) | 0.14 | 0.061 |
| Oral anticoagulants | 241 (36.9%) | 2847 (41.6%) | 0.019 | 0.097 |
| Opioids | 29 (4.4%) | 404 (5.9%) | 0.13 | 0.066 |
| Digoxin | 49 (7.5%) | 884 (12.9%) | <0.001 | 0.180 |
| Comorbidity | ||||
| Ischemic heart disease | 296 (45.3%) | 2805 (41.0%) | 0.032 | 0.087 |
| Peripheral vascular disease | 56 (8.6%) | 652 (9.5%) | 0.4 | 0.033 |
| Cerebrovascular disease | 49 (7.5%) | 617 (9.0%) | 0.2 | 0.055 |
| Cancer | 79 (12.1%) | 905 (13.2%) | 0.4 | 0.034 |
| Cardiac dysrhythmias | 244 (37.4%) | 2750 (40.2%) | 0.2 | 0.058 |
| Chronic renal failure | 21 (3.2%) | 290 (4.2%) | 0.2 | 0.054 |
| COPD | 47 (7.2%) | 776 (11.3%) | 0.001 | 0.143 |
| Rheumatic disease | 20 (3.1%) | 175 (2.6%) | 0.4 | 0.031 |
| Diabetes | 134 (20.5%) | 1481 (21.7%) | 0.5 | 0.028 |
Variables are presented as numbers with percentages unless otherwise specified.
RASi—Renin angiotensin system inhibitors, COPD—Chronic obstructive pulmonary disease, SGLT-2 inhibitor—Sodium Glucose Co-transport 2 inhibitor, and SMD—standardized mean difference
Statistical test performed: Welch two-sample t-test with adjustment for unequal variances; Pearson's Chi-squared test,
2By study design, all patients were on RASi and Beta blockers.
| Variable . | Eplerenone, (n = 653) . | Spironolactone, (n = 6840) . | P-value1 . | SMD . |
|---|---|---|---|---|
| Age, median (Q1–Q3) | 65 (55, 74) | 69 (59, 76) | <0.001 | 0.233 |
| Male | 591 (90.5%) | 4651 (68.0%) | <0.001 | 0.578 |
| Readmitted with HF before study start | 66 (10.1%) | 1164 (17.0%) | <0.001 | 0.203 |
| Pharmacotherapy | ||||
| RASi2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Beta blockers2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Sacubitril/valsartan | 59 (9.0%) | 348 (5.1%) | <0.001 | 0.155 |
| SGLT2-inhibitors | 36 (5.5%) | 253 (3.7%) | 0.021 | 0.087 |
| Loop diuretics | 441 (67.5%) | 4980 (72.8%) | 0.004 | 0.115 |
| Statins | 418 (64.0%) | 3971 (58.1%) | 0.003 | 0.122 |
| Antidepressants | 54 (8.3%) | 848 (12.4%) | 0.002 | 0.136 |
| Antiplatelet therapy | 331 (50.7%) | 3260 (47.7%) | 0.14 | 0.061 |
| Oral anticoagulants | 241 (36.9%) | 2847 (41.6%) | 0.019 | 0.097 |
| Opioids | 29 (4.4%) | 404 (5.9%) | 0.13 | 0.066 |
| Digoxin | 49 (7.5%) | 884 (12.9%) | <0.001 | 0.180 |
| Comorbidity | ||||
| Ischemic heart disease | 296 (45.3%) | 2805 (41.0%) | 0.032 | 0.087 |
| Peripheral vascular disease | 56 (8.6%) | 652 (9.5%) | 0.4 | 0.033 |
| Cerebrovascular disease | 49 (7.5%) | 617 (9.0%) | 0.2 | 0.055 |
| Cancer | 79 (12.1%) | 905 (13.2%) | 0.4 | 0.034 |
| Cardiac dysrhythmias | 244 (37.4%) | 2750 (40.2%) | 0.2 | 0.058 |
| Chronic renal failure | 21 (3.2%) | 290 (4.2%) | 0.2 | 0.054 |
| COPD | 47 (7.2%) | 776 (11.3%) | 0.001 | 0.143 |
| Rheumatic disease | 20 (3.1%) | 175 (2.6%) | 0.4 | 0.031 |
| Diabetes | 134 (20.5%) | 1481 (21.7%) | 0.5 | 0.028 |
| Variable . | Eplerenone, (n = 653) . | Spironolactone, (n = 6840) . | P-value1 . | SMD . |
|---|---|---|---|---|
| Age, median (Q1–Q3) | 65 (55, 74) | 69 (59, 76) | <0.001 | 0.233 |
| Male | 591 (90.5%) | 4651 (68.0%) | <0.001 | 0.578 |
| Readmitted with HF before study start | 66 (10.1%) | 1164 (17.0%) | <0.001 | 0.203 |
| Pharmacotherapy | ||||
| RASi2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Beta blockers2 | 653 (100%) | 6840 (100%) | NA | 0 |
| Sacubitril/valsartan | 59 (9.0%) | 348 (5.1%) | <0.001 | 0.155 |
| SGLT2-inhibitors | 36 (5.5%) | 253 (3.7%) | 0.021 | 0.087 |
| Loop diuretics | 441 (67.5%) | 4980 (72.8%) | 0.004 | 0.115 |
| Statins | 418 (64.0%) | 3971 (58.1%) | 0.003 | 0.122 |
| Antidepressants | 54 (8.3%) | 848 (12.4%) | 0.002 | 0.136 |
| Antiplatelet therapy | 331 (50.7%) | 3260 (47.7%) | 0.14 | 0.061 |
| Oral anticoagulants | 241 (36.9%) | 2847 (41.6%) | 0.019 | 0.097 |
| Opioids | 29 (4.4%) | 404 (5.9%) | 0.13 | 0.066 |
| Digoxin | 49 (7.5%) | 884 (12.9%) | <0.001 | 0.180 |
| Comorbidity | ||||
| Ischemic heart disease | 296 (45.3%) | 2805 (41.0%) | 0.032 | 0.087 |
| Peripheral vascular disease | 56 (8.6%) | 652 (9.5%) | 0.4 | 0.033 |
| Cerebrovascular disease | 49 (7.5%) | 617 (9.0%) | 0.2 | 0.055 |
| Cancer | 79 (12.1%) | 905 (13.2%) | 0.4 | 0.034 |
| Cardiac dysrhythmias | 244 (37.4%) | 2750 (40.2%) | 0.2 | 0.058 |
| Chronic renal failure | 21 (3.2%) | 290 (4.2%) | 0.2 | 0.054 |
| COPD | 47 (7.2%) | 776 (11.3%) | 0.001 | 0.143 |
| Rheumatic disease | 20 (3.1%) | 175 (2.6%) | 0.4 | 0.031 |
| Diabetes | 134 (20.5%) | 1481 (21.7%) | 0.5 | 0.028 |
Variables are presented as numbers with percentages unless otherwise specified.
RASi—Renin angiotensin system inhibitors, COPD—Chronic obstructive pulmonary disease, SGLT-2 inhibitor—Sodium Glucose Co-transport 2 inhibitor, and SMD—standardized mean difference
Statistical test performed: Welch two-sample t-test with adjustment for unequal variances; Pearson's Chi-squared test,
2By study design, all patients were on RASi and Beta blockers.
Composite of death and HF hospitalization
The event rate for the composite endpoint of death or HF hospitalization was 9.6 per 100 person-years in the eplerenone group and 11.1 per 100 person-years in the spironolactone group. Adjusted hazard ratios for the risk of the composite endpoint and its components are presented in Table 2. There were no significant differences between patients initially treated with eplerenone vs. spironolactone (HR 1.02 95% CI 0.82–1.27, P = 0.86). Kaplan–Meier curves estimating the probability of freedom from the combined endpoint are presented in Figure 2a. The absolute 2-year freedom from the combined endpoint was 84% (95% CI 81–87%) in the eplerenone group and 81% (80–82%) in the spironolactone group (P = 0.09). Importantly, we found no interaction between eplerenone initiation and the changes in reimbursement status that occurred during the study period (P = 0.50).
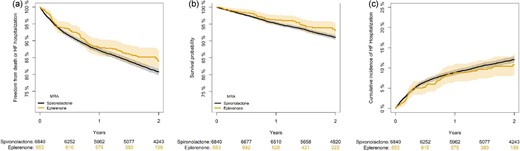
Kaplan–Meier analysis of the probability of freedom from the composite endpoint of all-cause mortality and HF hospitalization (2a), all-cause mortality (2b), and cumulative incidence of heart failure hospitalization with death as a competing risk (2c) among patients with new-onset HFrEF according to initiation of eplerenone vs. spironolactone.
Multivariate Cox proportional hazards models for all-cause death or HF hospitalization, all-cause death, and HF hospitalization
| . | No. of events (%) . | Age- and sex-adjusted HR (95% CI) . | P-value . | Adjusted* HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| All-cause death or HF Hospitalization | |||||
| Eplerenone | 95 (15%) | 0.86 (0.70–1.06) | 0.17 | 1.02 (0.82–1.27) | 0.86 |
| Spironolactone | 1263 (18%) | 1.00 (ref.) | 1.00 (ref.) | ||
| All-cause death | |||||
| Eplerenone | 38 (6%) | 0.87 (0.62–1.21) | 0.41 | 0.93 (0.67–1.30) | 0.68 |
| Spironolactone | 579 (8%) | 1.00 (ref.) | 1.00 (ref.) | ||
| HF hospitalization | |||||
| Eplerenone | 66 (10%) | 0.87 (0.68–1.13) | 0.30 | 1.10 (0.84–1.42) | 0.50 |
| Spironolactone | 802 (12%) | 1.00 (ref.) | 1.00 (ref.) |
| . | No. of events (%) . | Age- and sex-adjusted HR (95% CI) . | P-value . | Adjusted* HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| All-cause death or HF Hospitalization | |||||
| Eplerenone | 95 (15%) | 0.86 (0.70–1.06) | 0.17 | 1.02 (0.82–1.27) | 0.86 |
| Spironolactone | 1263 (18%) | 1.00 (ref.) | 1.00 (ref.) | ||
| All-cause death | |||||
| Eplerenone | 38 (6%) | 0.87 (0.62–1.21) | 0.41 | 0.93 (0.67–1.30) | 0.68 |
| Spironolactone | 579 (8%) | 1.00 (ref.) | 1.00 (ref.) | ||
| HF hospitalization | |||||
| Eplerenone | 66 (10%) | 0.87 (0.68–1.13) | 0.30 | 1.10 (0.84–1.42) | 0.50 |
| Spironolactone | 802 (12%) | 1.00 (ref.) | 1.00 (ref.) |
Abbreviations: HR = Hazard Ratio; CI = confidence interval
Adjusted for age, sex, ischemic heart disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, cancer, readmission for heart failure, loop diuretics, and reimbursement status for eplerenone.
Multivariate Cox proportional hazards models for all-cause death or HF hospitalization, all-cause death, and HF hospitalization
| . | No. of events (%) . | Age- and sex-adjusted HR (95% CI) . | P-value . | Adjusted* HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| All-cause death or HF Hospitalization | |||||
| Eplerenone | 95 (15%) | 0.86 (0.70–1.06) | 0.17 | 1.02 (0.82–1.27) | 0.86 |
| Spironolactone | 1263 (18%) | 1.00 (ref.) | 1.00 (ref.) | ||
| All-cause death | |||||
| Eplerenone | 38 (6%) | 0.87 (0.62–1.21) | 0.41 | 0.93 (0.67–1.30) | 0.68 |
| Spironolactone | 579 (8%) | 1.00 (ref.) | 1.00 (ref.) | ||
| HF hospitalization | |||||
| Eplerenone | 66 (10%) | 0.87 (0.68–1.13) | 0.30 | 1.10 (0.84–1.42) | 0.50 |
| Spironolactone | 802 (12%) | 1.00 (ref.) | 1.00 (ref.) |
| . | No. of events (%) . | Age- and sex-adjusted HR (95% CI) . | P-value . | Adjusted* HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| All-cause death or HF Hospitalization | |||||
| Eplerenone | 95 (15%) | 0.86 (0.70–1.06) | 0.17 | 1.02 (0.82–1.27) | 0.86 |
| Spironolactone | 1263 (18%) | 1.00 (ref.) | 1.00 (ref.) | ||
| All-cause death | |||||
| Eplerenone | 38 (6%) | 0.87 (0.62–1.21) | 0.41 | 0.93 (0.67–1.30) | 0.68 |
| Spironolactone | 579 (8%) | 1.00 (ref.) | 1.00 (ref.) | ||
| HF hospitalization | |||||
| Eplerenone | 66 (10%) | 0.87 (0.68–1.13) | 0.30 | 1.10 (0.84–1.42) | 0.50 |
| Spironolactone | 802 (12%) | 1.00 (ref.) | 1.00 (ref.) |
Abbreviations: HR = Hazard Ratio; CI = confidence interval
Adjusted for age, sex, ischemic heart disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, cancer, readmission for heart failure, loop diuretics, and reimbursement status for eplerenone.
Mortality
During follow-up, the rate of death was 3.6 and 4.7 per 100 person-years in the eplerenone and spironolactone group, respectively. In adjusted analyses, no significant difference in the risk of death between patients initially treated with eplerenone vs. spironolactone was found (HR 0.93 95% CI 0.67–1.30, P = 0.68). As the proportional hazard assumption was violated in this analysis (global P-value 0.03), we split the analysis into the periods 0–6 months, and a landmark analysis from 6 months until end of study at 24 months. These analyses yielded similar associations with mortality for the eplerenone group vs. spironolactone group (HR 0.85 (95% CI 0.45–1.58, P = 0.60) in the first period and 0.97 (95% CI 0.65–1.44, P = 0.88) in the latter period. The absolute 2-year survival probability was 93% (95% CI 91–95%) in the eplerenone group and 91% (95% CI 90–92%) in the spironolactone group, Figure 2b (P = 0.10).
HF hospitalization
The rate of HF hospitalization was 6.7 and 7.0 per 100 person-years in the eplerenone and spironolactone group, respectively. There were no significant differences in the risk of HF hospitalization between the two groups (HR 1.10 95% CI 0.84–1.42, P = 0.50, Figure 2c). The cumulative incidences of HF hospitalization with death as a competing risk were not significantly different between the two groups (Gray's test, P = 0.45).
Treatment withdrawal, cross-over, and daily doses
During the 180 days run-in period from date of HF diagnosis to start of study, 38 (6%) patients in the eplerenone group and 629 (9%) in the spironolactone group stopped treatment with an MRA. During the follow up period, withdrawal from treatment, defined as >90 days off treatment, occurred in 220 patients (34%) in the eplerenone group and 3641 patients (53%) in the spironolactone group when considering the competing risk of death (Figure 3). Initiation with eplerenone vs. spironolactone was associated with a significantly lower risk of withdrawal (Gray's test, P < 0.0001). Rates of death following drug discontinuation were 7.1 per 100 person-years for eplerenone users, and 6.3 per 100 person-years for spironolactone users. Later re-initiation of MRA therapy among patients with treatment withdrawal >90 days was observed in 104 patients (47%) in the eplerenone group and 1737 patients (48%) in the spironolactone group (P = 0.96). Cross-over, that is, redemption of a prescription of the other MRA than initially prescribed occurred in 18 patients (3%) in the eplerenone group and 678 patients (10%) in the spironolactone group.
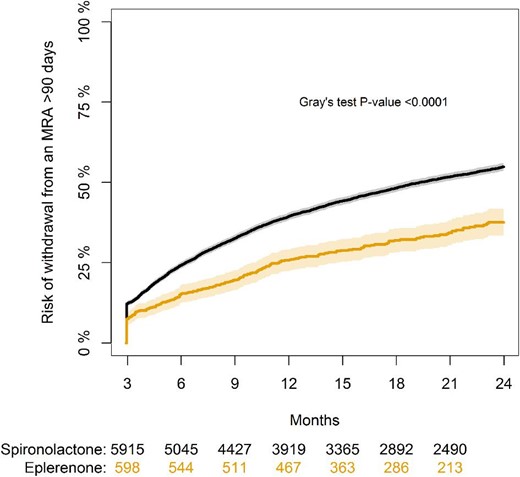
Cumulative incidence of withdrawal from MRA therapy during follow-up with death as a competing risk.
At study start (180 days after HF diagnosis), the mean estimated daily dose was 28.3 mg ± 17.6 in the eplerenone group and 21.7 mg ± 15.6 in the spironolactone group. A daily MRA dose of >25 mg was observed in 210 (32%) patients in the eplerenone group and 1433 patients (21%) in the spironolactone group.
At 12 months after start of study, 628 patients (96%) in the eplerenone group and 6510 patients (95%) in the spironolactone group were alive and still in follow-up. The mean estimated daily dose was 26.7 mg ± 20.3 in the eplerenone group and 16.6 mg ± 15.4 in the spironolactone group. At this time point, daily doses >25 mg were observed in 230 patients (37%) in the eplerenone group and 771 patients (12%) in the spironolactone group (Figure 4). Eplerenone was associated with a significantly higher probability of a daily dosage > 25 mg (odds ratio 3.59 [95% CI 2.96–4.35], P < 0.001), Table S2).
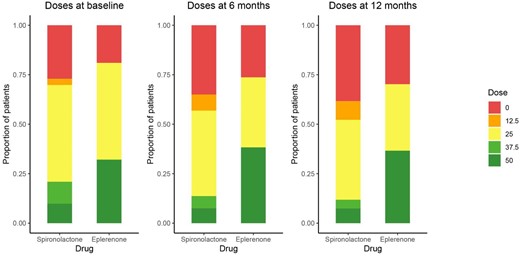
Estimated daily doses of treatment with MRA at study start, 6 months, and 12 months follow-up, in patients with new-onset HFrEF who were initiated with either eplerenone or spironolactone.
Sensitivity analyses
First, we repeated analyses in a 1:1 propensity score matched population including all 653 patients on eplerenone and 653 patients on spironolactone. Groups were well balanced (all standardized mean differences <0.02) and baseline characteristics are presented in Table S3. In line with the primary analysis, we found no differences in the risk of the composite outcome, all-cause mortality or HF hospitalization (Table S4). At 1-year follow-up, initiation of eplerenone vs. spironolactone was associated with a higher probability of an estimated daily dosage >25 mg (odds ratio 3.57 [95% CI 2.70–4.71], P < 0.001, Table S5) and a lower risk of treatment discontinuation (34% vs. 46%, P < 0.001), Table S5 and Figure S2. Second, we restricted the study population to male patients only (n = 5242) due to restrictions on eplerenone prescription, related to gynecomastia, early in the study period. Baseline characteristics for male patients are presented in supplementary Table S6. Hazard ratios for the combined endpoint, death, and HF hospitalization are presented in Table S7. Consistent, with the primary analysis, we found no differences in the clinical outcomes, and that eplerenone was associated with a lower risk of treatment discontinuation in both men and women (Gray's test, P < 0.001, Figure S3 + S4). Finally, when we compared the study population with the 8340 (53.1%) HFrEF patients who were alive at study start but did not initiate an MRA, we found those patients to be older, less likely to have been diagnosed as inpatients, with lower use of loop diuretics, but notably also with higher prevalence of CKD (11% vs. 4%, P < 0.001), supplementary Table S8. Among those patients, 1596 (18.8%) initiated an MRA after 180 days and within follow-up of up to 24 months, whereas 897 patients (10.6%) died during follow-up without initiating an MRA.
Discussion
In this contemporary nationwide dataset of all-comer patients with HFrEF who were alive and had initiated MRA treatment at study start, 180 days after HFrEF diagnosis, we found no significant difference in the associated risk of the composite endpoint of death or HF hospitalization nor each of the components separately when comparing patients who initiated eplerenone vs. spironolactone. We did, however, find that initiation of eplerenone was associated with lower risk of treatment withdrawal, and higher likelihood of a daily dose >25 mg of an MRA at 12 months after study start when compared to patients who initiated spironolactone.
Patients in the eplerenone group were somewhat younger, with some indications of milder HF—less likely to be on loop diuretics, and lower prevalence of HF readmission between diagnosis of HF and study start, but more likely to be on sacubitril/valsartan as compared to patients in the spironolactone group. Patients excluded from the study population due to not starting an MRA within 180 days of HF diagnosis were somewhat older, were less likely on loop diuretics, but had a higher prevalence of CKD. This could indicate that reasons for abstaining from MRA use could both be advanced age or poor kidney function but also some patients being less symptomatic.
An important finding in the present study is lower risk of MRA discontinuation in those treated with eplerenone, irrespective of sex. Discontinuation of MRA is a well-known challenge, also previously shown in Danish data.3,7 The lower risk of discontinuation with eplerenone did not translate to the clinical endpoints in our study, but this may have been different given longer follow-up had been available. In the propensity score matched analysis, matched on 22 baseline variables, which included all patients from the primary cohort who initiated eplerenone, and 1:1 matched patients on spironolactone, we found consistent results, including higher average daily dose at 1 year and lower risk of treatment discontinuation associated with eplerenone vs. spironolactone.
One potential reason for the lower risk of discontinuation in the eplerenone group is the signal of lower potency of eplerenone vs. spironolactone in equivalent doses, that has been seen in hypertension studies.15–17 This may translate to lower risk of hyperkalemia and worsening renal function in the eplerenone group, but whether it also extends to less potency in terms of the beneficial effects in HFrEF needs further study.
Our results are in line with the recently updated HF guidelines that recommend use of MRA in symptomatic HFrEF patients but does not specifically recommend neither eplerenone nor spironolactone.18 To fully elucidate whether outcomes differ between the two MRAs, a prospective randomized trial is needed. Currently, a cluster randomized trial (NCT03984591) is conducted in Denmark, which randomizes HFrEF patients at hospital level to either eplerenone or spironolactone if an MRA is deemed indicated. The study aims at including 7200 patients and is estimated to be completed in 2026. Moreover, a new non-steroidal MRA, Finerenone with a perceived higher potency and selectivity, has been tested against eplerenone in a phase 2 HFrEF trial and is currently being tested against placebo in HF patients with LVEF ≥40% (clinical trials.gov NCT04435626).
Limitations
The registries lack information on important clinical variables, including systolic blood pressure, heart rate, natriuretic peptides, New York Heart Association functional class, and left ventricular ejection fraction. While we used a previously validated approach to identify patients with HFrEF, it is possible that a small proportion of the population may have had hypertension and/or HF with preserved ejection fraction rather than HFrEF. We had data on MRA discontinuation, however, we lacked information on the underlying reason. Despite adjustments there might still be unmeasured residual confounding as treatment allocation was not randomized, but rather at the discretion of a treating clinician. For example, we cannot fully refute that severity of HF may vary between patients in the two treatment groups. Further, the use of eplerenone in patients with HFrEF in Denmark has up until recently been marginal and public subsidization restricted to patients who experienced adverse events on spironolactone. To minimize this selection bias issue, we chose to study a very recent time-period.
Conclusions
In a nationwide cohort of all-comer patients with new-onset HFrEF, initiation of eplerenone, and spironolactone were associated with similar risks of death or HF hospitalization. Initiation of eplerenone, was associated with lower risk of treatment discontinuation and higher likelihood of having a daily dose >25 mg at 12 months follow-up.
Conflict of interest: None declared.
Data availability
The data are stored at Statistics Denmark and research environments can apply for access.



