-
PDF
- Split View
-
Views
-
Cite
Cite
Seung-Jae Joo, Song-Yi Kim, Joon-Hyouk Choi, Hyeung Keun Park, Jong Wook Beom, Jae-Geun Lee, Shung Chull Chae, Hyo-Soo Kim, Young Jo Kim, Myeong Chan Cho, Chong Jin Kim, Seung-Woon Rha, Junghan Yoon, Myung Ho Jeong, on behalf of the KAMIR-NIH registry investigators, Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 7, Issue 6, November 2021, Pages 475–482, https://doi.org/10.1093/ehjcvp/pvaa029
Close - Share Icon Share
Abstract
This observational study aimed to investigate the association between beta-blocker therapy and clinical outcomes in patients with acute myocardial infarction (AMI), especially with mid-range or preserved left ventricular systolic function.
Among 13 624 patients enrolled in the Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH), 12 200 in-hospital survivors were selected. Patients with beta-blockers showed significantly lower 1-year major adverse cardiac events (MACE), which was a composite of cardiac death, MI, revascularization, and readmission due to heart failure [9.7 vs. 14.3/100 patient-year; hazard ratio (HR) 0.84, 95% confidence interval (CI) 0.72–0.97; P = 0.022). However, this association had a significant interaction with left ventricular ejection fraction (LVEF). Beta-blocker therapy at discharge was associated with lower 1-year MACE in patients with LVEF ≤40% (HR 0.63, 95% CI 0.48–0.81; P < 0.001), and 40% <LVEF < 50% (HR 0.69, 95% CI 0.51–0.94; P = 0.020), but not in patients with LVEF ≥50% (HR 1.16, 95% CI 0.91–1.48; P = 0.234).
Beta-blocker therapy at discharge was associated with better 1-year clinical outcomes in patients with reduced or mid-range LVEF after AMI, but not in patients with preserved LVEF. These data suggested that the long-term beta-blocker therapy may be guided by LVEF.
Introduction
After acute myocardial infarction (AMI), an evidence-based, optimal medical therapy should be prescribed to improve clinical outcomes. Although oral beta-blockers have long been recommended after AMI, the evidence for their beneficial effects is based on early studies in the era of little usages of current evidence-based interventional or medical therapies. A meta-analysis of clinical trials before the reperfusion era showed that beta-blockers reduced the cardiac mortality when used as a long-term, secondary preventive therapy after AMI.1 In the reperfusion era, beta-blockers decreased a short-term re-infarction,2 but their long-term cardiovascular benefit has not been definitely shown3,4 except in patients with left ventricular ejection fraction (LVEF) ≤40%.5 There are still controversies about beta-blockers’ benefit in patients with LVEF >40%, especially undergoing successful primary percutaneous coronary intervention (PCI) after ST-elevation MI (STEMI).
The lack of contemporary clinical trials on the effect of beta-blocker therapy has resulted in the inconsistent recommendations in the guidelines for the management of patients with AMI. In patients with STEMI, both the American and the European guidelines recommend oral beta-blockers in all patients without contraindications.6,7 In case of non-ST elevation MI (NSTEMI), beta-blocker therapy is recommended only in patients with LVEF ≤40% in the European guideline,8 but in all patients irrespective of LVEF in the American guideline.9
The role of beta-blockers in patients with intermediate range of LVEF 40–49% is even more unclear because they may be classified as having either preserved or reduced LVEF. A recent guideline classified them as having mid-range LVEF,10 and one meta-analysis showed that beta-blockers improved clinical prognosis in patients with heart failure (HF) with mid-range LVEF.11
This study aimed to investigate the association between beta-blocker therapy and clinical outcomes in patients with AMI, especially with mid-range or preserved LVEF.
Methods
Study population and data collection
The Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH) is a nation-wide, prospective, observational, and online registry of South Korea from 20 university hospitals.12 Patients who were hospitalized primarily for AMI and signed informed consents were consecutively enrolled from November 2011 to October 2015. This study was conducted according to the Declaration of Helsinki. The institutional review boards of all participating hospitals approved the study protocol. Written informed consents were obtained from participating patients or legal representative. Data were collected by the attending physician with the assistance of a trained clinical research coordinator, via a web-based case report form in the Clinical Data Management System (iCReaT) of the Korea NIH. Patients who did not undergo echocardiographic study, died during index hospitalization, or had incomplete clinical data were excluded.
Acute myocardial infarction was diagnosed when there was an evidence of myocardial necrosis (a rise and/or fall in cardiac biomarker, preferably cardiac troponin), and at least one of the following: (i) symptoms of ischaemia, (ii) new or presumed new significant ST-segment-T wave changes or a new left bundle branch block, (iii) a development of pathologic Q waves in the ECG, (iv) an imaging evidence of the new loss of viable myocardium or new regional wall motion abnormality, and (v) the identification of an intracoronary thrombus by angiography.13 Coronary reperfusion included reperfusion by PCI, thrombolysis, or coronary artery bypass graft (CABG), MI with non-obstructed coronary arteries (MINOCA),7 and myocardial bridge.
Clinical endpoints and definition
The primary endpoint was 1-year major adverse cardiac events (MACE), which was a composite of cardiac death, MI, revascularization, and re-admission due to HF. The secondary endpoints were all-cause death, 1-year major adverse cardiac and cerebrovascular events (MACCE), which was a composite of the primary endpoint and stroke, and each component of MACCE. To avoid the potential bias in determining the cause of death without blinded adjudication, 1-year composite outcome of all-cause death, MI, revascularization, and re-admission due to HF was also analysed.
All deaths were considered to be associated with cardiac problems, unless a definite non-cardiac cause was established. Myocardial infarction included re-infarction or recurrent MI. Re-infarction, which occurred within 28 days following an incident or recurrent MI, was defined as the recurrence of ischaemic symptoms or new ECG changes of ST elevation ≥0.1 mV or new pathologic Q waves in at least two contiguous leads in association with the increase of cardiac biomarker level ≥20% if the initially elevated value was stable or decreasing. If the cardiac biomarker concentration returned to normal level, the criteria for new MI applied. If characteristics of MI occurred after 28 days following an incident MI, it was considered to be a recurrent MI.13 Revascularization included repeated PCI or CABG on either target or non-target vessels. The staged PCI was excluded from revascularization. The clinical follow-ups were routinely performed at 6, 12, and 24 months by visiting the hospital and whenever any clinical events occurred. If patients did not visit the hospitals, the outcome data were assessed by telephone interview. Clinical events were not centrally adjudicated. The patient’s physician identified all events and the principal investigator of each hospital confirmed them.
Statistical analysis
Data were expressed as mean ± standard deviation for continuous variables, and as number (percentage) for categorical variables. Data were compared using unpaired t-test for continuous variables, andχ2 test for categorical variables. Survival curves for clinical endpoints and cumulative event rates with incidence rates per 100 patient-year were generated using the Kaplan–Meier estimates. Hazard ratios (HR) and their 95% confidence interval (CI) for each clinical endpoint were calculated using Cox proportional hazard analysis. In multivariate Cox regression analysis, age, sex, body mass index (BMI), hypertension, diabetes mellitus (DM), prior angina, prior MI, prior HF, current smoker, Killip class, estimated glomerular filtration rate (eGFR) by MDRD equation, LVEF, type of MI, coronary reperfusion, and medications [aspirin, P2Y12 inhibitors, inhibitors of renin-angiotensin system (RAS), and statins] at discharge were included as covariates. Subgroups that were defined post hoc according to demographic and clinical characteristics included age (<65, ≥65–<80, and ≥80 years), sex, hypertension, DM, prior MI, prior HF, current smoker, Killip class ≥2, eGFR < 60 mL/min/1.73 m2, LVEF (≤40%, >40–<50%, and ≥50%), STEMI, coronary reperfusion, and medications (P2Y12 inhibitors, RAS inhibitors, and statins) at discharge.
Because beta-blocker therapy was not randomized, propensity score matching was done as a sensitivity analysis to minimize selection or predisposition bias. The propensity score was estimated using multiple logistic regression analysis with all variables in Table 1. Using a greedy nearest matching algorithm with 0.1 calliper width, each patient without beta-blockers was matched to a maximum of two patients in beta-blocker group. The efficacy of the propensity score model was assessed by estimating standardized differences for each covariate between groups.
| . | All patients (N = 12 200) . | With beta-blocker(N = 10 251) . | Without beta-blocker(N = 1949) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63.6 ± 12.6 | 63.2 ± 12.5 | 65.6 ± 12.9 | <0.001 |
| Male | 9067 (74.3) | 7655 (74.7) | 1414 (72.4) | 0.041 |
| Body mass index (kg/m2) | 24.05 ± 3.31 | 24.15 ± 3.29 | 23.53 ± 3.38 | <0.001 |
| Hypertension | 6156 (50.5) | 5230 (51.0) | 926 (47.5) | 0.005 |
| Diabetes mellitus | 3411 (28.0) | 2902 (28.3) | 509 (26.1) | 0.051 |
| Prior angina pectoris | 1159 (9.5) | 908 (8.9) | 251 (12.9) | <0.001 |
| Prior myocardial infarction | 934 (7.7) | 768 (7.5) | 166 (8.5) | 0.124 |
| Prior heart failure | 180 (1.5) | 137 (1.3) | 43 (2.2) | 0.004 |
| Prior stroke | 795 (6.5) | 674 (6.6) | 121 (6.2) | 0.580 |
| Current smoker | 4849 (39.7) | 4123 (40.2) | 726 (37.2) | 0.014 |
| Killip Class ≥II | 2436 (20.0) | 1977 (19.3) | 459 (23.6) | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 2217 (18.2) | 1791 (17.5) | 426 (21.9) | <0.001 |
| Left ventricular EF (%) | 52.2 ± 11.0 | 52.2 ± 10.8 | 52.5 ± 12.1 | 0.309 |
| STEMI | 5,805 (47.6) | 5,065 (49.4) | 740 (38.0) | <0.001 |
| Coronary reperfusiona | 11 661 (95.6) | 9844 (96.0) | 1817 (93.2) | <0.001 |
| Medications at discharge | ||||
| Aspirin | 12 160 (99.7) | 10 235 (99.8) | 1925 (98.8) | <0.001 |
| P2Y12 inhibitor | 11 666 (95.6) | 9986 (97.4) | 1680 (86.2) | <0.001 |
| RAS inhibitors | 9729 (79.7) | 8663 (84.5) | 1066 (54.7) | <0.001 |
| Statins | 11 415 (93.6) | 9725 (94.9) | 1690 (86.7) | <0.001 |
| . | All patients (N = 12 200) . | With beta-blocker(N = 10 251) . | Without beta-blocker(N = 1949) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63.6 ± 12.6 | 63.2 ± 12.5 | 65.6 ± 12.9 | <0.001 |
| Male | 9067 (74.3) | 7655 (74.7) | 1414 (72.4) | 0.041 |
| Body mass index (kg/m2) | 24.05 ± 3.31 | 24.15 ± 3.29 | 23.53 ± 3.38 | <0.001 |
| Hypertension | 6156 (50.5) | 5230 (51.0) | 926 (47.5) | 0.005 |
| Diabetes mellitus | 3411 (28.0) | 2902 (28.3) | 509 (26.1) | 0.051 |
| Prior angina pectoris | 1159 (9.5) | 908 (8.9) | 251 (12.9) | <0.001 |
| Prior myocardial infarction | 934 (7.7) | 768 (7.5) | 166 (8.5) | 0.124 |
| Prior heart failure | 180 (1.5) | 137 (1.3) | 43 (2.2) | 0.004 |
| Prior stroke | 795 (6.5) | 674 (6.6) | 121 (6.2) | 0.580 |
| Current smoker | 4849 (39.7) | 4123 (40.2) | 726 (37.2) | 0.014 |
| Killip Class ≥II | 2436 (20.0) | 1977 (19.3) | 459 (23.6) | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 2217 (18.2) | 1791 (17.5) | 426 (21.9) | <0.001 |
| Left ventricular EF (%) | 52.2 ± 11.0 | 52.2 ± 10.8 | 52.5 ± 12.1 | 0.309 |
| STEMI | 5,805 (47.6) | 5,065 (49.4) | 740 (38.0) | <0.001 |
| Coronary reperfusiona | 11 661 (95.6) | 9844 (96.0) | 1817 (93.2) | <0.001 |
| Medications at discharge | ||||
| Aspirin | 12 160 (99.7) | 10 235 (99.8) | 1925 (98.8) | <0.001 |
| P2Y12 inhibitor | 11 666 (95.6) | 9986 (97.4) | 1680 (86.2) | <0.001 |
| RAS inhibitors | 9729 (79.7) | 8663 (84.5) | 1066 (54.7) | <0.001 |
| Statins | 11 415 (93.6) | 9725 (94.9) | 1690 (86.7) | <0.001 |
Values are mean ± standard deviation or number (%).
EF, ejection fraction; eGFR, estimated glomerular filtration rate by MDRD equation; RAS, renin-angiotensin system; STEMI, ST-elevation myocardial infarction.
Included reperfusion by percutaneous coronary intervention, thrombolysis, or coronary artery bypass graft, myocardial infarction with non-obstructed coronary arteries, and myocardial bridge.
| . | All patients (N = 12 200) . | With beta-blocker(N = 10 251) . | Without beta-blocker(N = 1949) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63.6 ± 12.6 | 63.2 ± 12.5 | 65.6 ± 12.9 | <0.001 |
| Male | 9067 (74.3) | 7655 (74.7) | 1414 (72.4) | 0.041 |
| Body mass index (kg/m2) | 24.05 ± 3.31 | 24.15 ± 3.29 | 23.53 ± 3.38 | <0.001 |
| Hypertension | 6156 (50.5) | 5230 (51.0) | 926 (47.5) | 0.005 |
| Diabetes mellitus | 3411 (28.0) | 2902 (28.3) | 509 (26.1) | 0.051 |
| Prior angina pectoris | 1159 (9.5) | 908 (8.9) | 251 (12.9) | <0.001 |
| Prior myocardial infarction | 934 (7.7) | 768 (7.5) | 166 (8.5) | 0.124 |
| Prior heart failure | 180 (1.5) | 137 (1.3) | 43 (2.2) | 0.004 |
| Prior stroke | 795 (6.5) | 674 (6.6) | 121 (6.2) | 0.580 |
| Current smoker | 4849 (39.7) | 4123 (40.2) | 726 (37.2) | 0.014 |
| Killip Class ≥II | 2436 (20.0) | 1977 (19.3) | 459 (23.6) | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 2217 (18.2) | 1791 (17.5) | 426 (21.9) | <0.001 |
| Left ventricular EF (%) | 52.2 ± 11.0 | 52.2 ± 10.8 | 52.5 ± 12.1 | 0.309 |
| STEMI | 5,805 (47.6) | 5,065 (49.4) | 740 (38.0) | <0.001 |
| Coronary reperfusiona | 11 661 (95.6) | 9844 (96.0) | 1817 (93.2) | <0.001 |
| Medications at discharge | ||||
| Aspirin | 12 160 (99.7) | 10 235 (99.8) | 1925 (98.8) | <0.001 |
| P2Y12 inhibitor | 11 666 (95.6) | 9986 (97.4) | 1680 (86.2) | <0.001 |
| RAS inhibitors | 9729 (79.7) | 8663 (84.5) | 1066 (54.7) | <0.001 |
| Statins | 11 415 (93.6) | 9725 (94.9) | 1690 (86.7) | <0.001 |
| . | All patients (N = 12 200) . | With beta-blocker(N = 10 251) . | Without beta-blocker(N = 1949) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63.6 ± 12.6 | 63.2 ± 12.5 | 65.6 ± 12.9 | <0.001 |
| Male | 9067 (74.3) | 7655 (74.7) | 1414 (72.4) | 0.041 |
| Body mass index (kg/m2) | 24.05 ± 3.31 | 24.15 ± 3.29 | 23.53 ± 3.38 | <0.001 |
| Hypertension | 6156 (50.5) | 5230 (51.0) | 926 (47.5) | 0.005 |
| Diabetes mellitus | 3411 (28.0) | 2902 (28.3) | 509 (26.1) | 0.051 |
| Prior angina pectoris | 1159 (9.5) | 908 (8.9) | 251 (12.9) | <0.001 |
| Prior myocardial infarction | 934 (7.7) | 768 (7.5) | 166 (8.5) | 0.124 |
| Prior heart failure | 180 (1.5) | 137 (1.3) | 43 (2.2) | 0.004 |
| Prior stroke | 795 (6.5) | 674 (6.6) | 121 (6.2) | 0.580 |
| Current smoker | 4849 (39.7) | 4123 (40.2) | 726 (37.2) | 0.014 |
| Killip Class ≥II | 2436 (20.0) | 1977 (19.3) | 459 (23.6) | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 2217 (18.2) | 1791 (17.5) | 426 (21.9) | <0.001 |
| Left ventricular EF (%) | 52.2 ± 11.0 | 52.2 ± 10.8 | 52.5 ± 12.1 | 0.309 |
| STEMI | 5,805 (47.6) | 5,065 (49.4) | 740 (38.0) | <0.001 |
| Coronary reperfusiona | 11 661 (95.6) | 9844 (96.0) | 1817 (93.2) | <0.001 |
| Medications at discharge | ||||
| Aspirin | 12 160 (99.7) | 10 235 (99.8) | 1925 (98.8) | <0.001 |
| P2Y12 inhibitor | 11 666 (95.6) | 9986 (97.4) | 1680 (86.2) | <0.001 |
| RAS inhibitors | 9729 (79.7) | 8663 (84.5) | 1066 (54.7) | <0.001 |
| Statins | 11 415 (93.6) | 9725 (94.9) | 1690 (86.7) | <0.001 |
Values are mean ± standard deviation or number (%).
EF, ejection fraction; eGFR, estimated glomerular filtration rate by MDRD equation; RAS, renin-angiotensin system; STEMI, ST-elevation myocardial infarction.
Included reperfusion by percutaneous coronary intervention, thrombolysis, or coronary artery bypass graft, myocardial infarction with non-obstructed coronary arteries, and myocardial bridge.
All statistical analyses were performed with the statistical package SPSS version 23 (IBM Co, Armonk, NY, USA) and R version 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria). Clinical significance was defined as P < 0.05.
Results
Total 13 624 consecutive patients were enrolled. After excluding 1153 patients without echocardiographic data, 252 patients who died during index hospitalization, and 19 patients with incomplete data, final 12 200 patients were analysed in this study (Figure 1). Beta-blockers were prescribed at the discretion of attending physicians. Total 10 251 patients (84%) were taking beta-blockers at discharge.
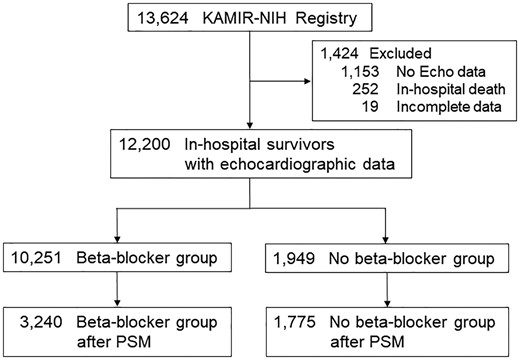
Patients selection for analysis. KAMIR-NIH, Korean Acute Myocardial Infarction Registry-National Institute of Health; PSM, propensity score-matching.
Patients without beta-blockers were older, and had lower BMI, more hypertension, more prior angina and heart failure, more Killip Class ≥II, more eGFR <60 mL/min/1.73 m2, more NSTEMI, and less coronary reperfusion. They were taking less aspirin, P2Y12 inhibitors, RAS inhibitors, and statins (Table 1). Percutaneous coronary intervention with drug-eluting stents was the main method of coronary reperfusion. Patients without beta-blockers received less PCI, but had more MINOCA, especially in cases of NSTEMI (Supplementary material online, Tables S1–S3). After propensity score matching, baseline characteristics of 3240 patients with beta-blockers and 1775 patients without beta-blockers were well balanced (Supplementary material online, Table S4).
One-year follow-up rate was 98% and 97% in patients with and without beta-blockers respectively. Among patients who survived at 1 year, only 4% of patients with beta-blockers at discharge discontinued beta-blockers, but 72% of patients without beta-blockers at discharge were taking beta-blockers at 1 year.
Patients with beta-blockers showed significantly lower 1-year MACE (9.7 vs. 14.3/100 patient-year), and HR was 0.84 (95% CI 0.72–0.97; P = 0.022) after full adjustment (Table 2, Figure 2A). This result was mainly caused by lower cardiac deaths in patients with beta-blockers (2.5 vs. 5.5/100 patient-year; HR 0.74, 95% CI 0.57–0.96; P = 0.022) (Table 2, Figure 2B). Likewise, in the propensity score-matched patients, 1-year MACE and cardiac deaths were significantly lower in patients with beta-blockers (Supplementary material online, Table S5, Figure S1).
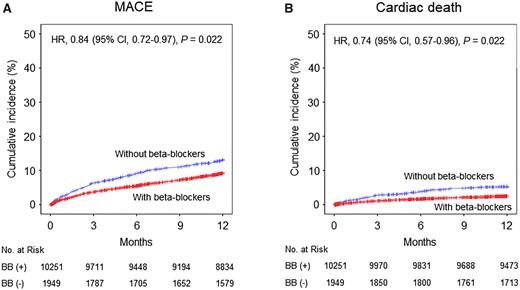
Unadjusted Kaplan–Meier curves and adjusted hazard ratios (HR) for 1-year clinical events in the entire cohort with vs. without beta-blockers (BB). (A) Major adverse cardiac events (MACE). (B) Cardiac death. CI, confidence interval.
Multivariate Cox-proportional hazard analysis of 1-year events in the entire cohort
| Events . | With beta-blocker (N = 10 251) . | Without beta-blocker (N = 1949) . | Hazard ratioa (95% CI) . | P-value . | ||
|---|---|---|---|---|---|---|
| . | No. of patients with events . | Rate per 100 patient-yearb . | No. of patients with events . | Rate per 100 patient-yearb . | . | . |
| MACE | 919 | 9.7 | 247 | 14.3 | 0.84 (0.72–0.97) | 0.022 |
| Cardiac death | 248 | 2.5 | 99 | 5.5 | 0.74 (0.57–0.96) | 0.022 |
| All-cause death | 384 | 3.9 | 134 | 7.4 | 0.81 (0.65–1.00) | 0.053 |
| MI | 190 | 2.0 | 35 | 2.0 | 1.10 (0.75–1.63) | 0.620 |
| Revascularization | 448 | 4.7 | 84 | 4.8 | 0.99 (0.77–1.28) | 0.958 |
| Heart failurec | 250 | 2.6 | 76 | 4.3 | 0.82 (0.62–1.08) | 0.157 |
| Stroke | 110 | 1.1 | 16 | 0.9 | 1.37 (0.79–2.36) | 0.259 |
| MACCE | 1,009 | 10.7 | 259 | 15.1 | 0.87 (0.75–1.01) | 0.067 |
| Events . | With beta-blocker (N = 10 251) . | Without beta-blocker (N = 1949) . | Hazard ratioa (95% CI) . | P-value . | ||
|---|---|---|---|---|---|---|
| . | No. of patients with events . | Rate per 100 patient-yearb . | No. of patients with events . | Rate per 100 patient-yearb . | . | . |
| MACE | 919 | 9.7 | 247 | 14.3 | 0.84 (0.72–0.97) | 0.022 |
| Cardiac death | 248 | 2.5 | 99 | 5.5 | 0.74 (0.57–0.96) | 0.022 |
| All-cause death | 384 | 3.9 | 134 | 7.4 | 0.81 (0.65–1.00) | 0.053 |
| MI | 190 | 2.0 | 35 | 2.0 | 1.10 (0.75–1.63) | 0.620 |
| Revascularization | 448 | 4.7 | 84 | 4.8 | 0.99 (0.77–1.28) | 0.958 |
| Heart failurec | 250 | 2.6 | 76 | 4.3 | 0.82 (0.62–1.08) | 0.157 |
| Stroke | 110 | 1.1 | 16 | 0.9 | 1.37 (0.79–2.36) | 0.259 |
| MACCE | 1,009 | 10.7 | 259 | 15.1 | 0.87 (0.75–1.01) | 0.067 |
CI, confidence interval; MACCE, major adverse cardiocerebral event; MACE, major adverse cardiac event; MI, myocardial infarction.
Adjusted for age, sex, body mass index, hypertension, diabetes mellitus, prior angina, prior MI, prior heart failure, current smoker, Killip class, estimated glomerular filtration rate, left ventricular ejection fraction, type of myocardial infarction, coronary reperfusion, and medications (aspirin, P2Y12 inhibitors, inhibitors of renin-angiotensin system, and statins) at discharge.
Unadjusted event rate.
Re-hospitalization due to heart failure.
Multivariate Cox-proportional hazard analysis of 1-year events in the entire cohort
| Events . | With beta-blocker (N = 10 251) . | Without beta-blocker (N = 1949) . | Hazard ratioa (95% CI) . | P-value . | ||
|---|---|---|---|---|---|---|
| . | No. of patients with events . | Rate per 100 patient-yearb . | No. of patients with events . | Rate per 100 patient-yearb . | . | . |
| MACE | 919 | 9.7 | 247 | 14.3 | 0.84 (0.72–0.97) | 0.022 |
| Cardiac death | 248 | 2.5 | 99 | 5.5 | 0.74 (0.57–0.96) | 0.022 |
| All-cause death | 384 | 3.9 | 134 | 7.4 | 0.81 (0.65–1.00) | 0.053 |
| MI | 190 | 2.0 | 35 | 2.0 | 1.10 (0.75–1.63) | 0.620 |
| Revascularization | 448 | 4.7 | 84 | 4.8 | 0.99 (0.77–1.28) | 0.958 |
| Heart failurec | 250 | 2.6 | 76 | 4.3 | 0.82 (0.62–1.08) | 0.157 |
| Stroke | 110 | 1.1 | 16 | 0.9 | 1.37 (0.79–2.36) | 0.259 |
| MACCE | 1,009 | 10.7 | 259 | 15.1 | 0.87 (0.75–1.01) | 0.067 |
| Events . | With beta-blocker (N = 10 251) . | Without beta-blocker (N = 1949) . | Hazard ratioa (95% CI) . | P-value . | ||
|---|---|---|---|---|---|---|
| . | No. of patients with events . | Rate per 100 patient-yearb . | No. of patients with events . | Rate per 100 patient-yearb . | . | . |
| MACE | 919 | 9.7 | 247 | 14.3 | 0.84 (0.72–0.97) | 0.022 |
| Cardiac death | 248 | 2.5 | 99 | 5.5 | 0.74 (0.57–0.96) | 0.022 |
| All-cause death | 384 | 3.9 | 134 | 7.4 | 0.81 (0.65–1.00) | 0.053 |
| MI | 190 | 2.0 | 35 | 2.0 | 1.10 (0.75–1.63) | 0.620 |
| Revascularization | 448 | 4.7 | 84 | 4.8 | 0.99 (0.77–1.28) | 0.958 |
| Heart failurec | 250 | 2.6 | 76 | 4.3 | 0.82 (0.62–1.08) | 0.157 |
| Stroke | 110 | 1.1 | 16 | 0.9 | 1.37 (0.79–2.36) | 0.259 |
| MACCE | 1,009 | 10.7 | 259 | 15.1 | 0.87 (0.75–1.01) | 0.067 |
CI, confidence interval; MACCE, major adverse cardiocerebral event; MACE, major adverse cardiac event; MI, myocardial infarction.
Adjusted for age, sex, body mass index, hypertension, diabetes mellitus, prior angina, prior MI, prior heart failure, current smoker, Killip class, estimated glomerular filtration rate, left ventricular ejection fraction, type of myocardial infarction, coronary reperfusion, and medications (aspirin, P2Y12 inhibitors, inhibitors of renin-angiotensin system, and statins) at discharge.
Unadjusted event rate.
Re-hospitalization due to heart failure.
The association between beta-blocker therapy at discharge and 1-year MACE appeared to be consistent across a series of subgroups in the entire cohort (Figure 3) or in the propensity score-matched patients (Supplementary material online, Figure S2). The only significant interaction term was LVEF [P for interaction (Pint) = 0.002]. Beta-blocker therapy at discharge was associated with lower 1-year MACE in patients with LVEF ≤40% (HR 0.63, 95% CI 0.48–0.81; P < 0.001), and 40 < LVEF < 50% (HR 0.69, 95% CI 0.51–0.94; P = 0.020), but not in patients with LVEF ≥50% (HR 1.16, 95% CI 0.91–1.48; P = 0.234) (Figure 4). This association was mainly driven by lower cardiac deaths in patients with LVEF ≤40%, but lower MI in patients with 40 < LVEF < 50% (Supplementary material online, Tables S6–S8). The same results were observed in the propensity score-matched patients (Supplementary material online, Figure S3, Table S9–S11). Beta-blocker therapy at discharge was also associated with lower 1-year composite outcome of all-cause death, MI, revascularization, and re-admission due to HF with a significant interaction with LVEF in the entire cohort (Pint = 0.002) or the propensity score-matched patients (Pint = 0.004) (Supplementary material online, Tables S12 and S13).
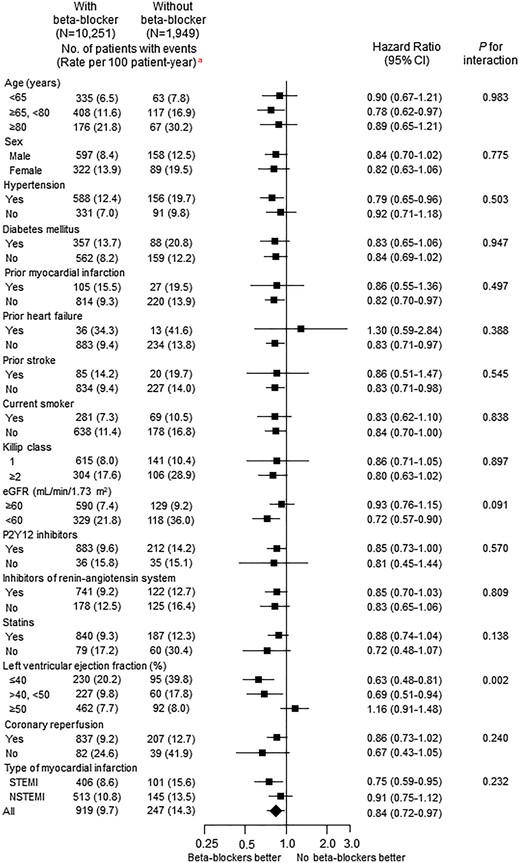
Adjusted hazard ratios of 1-year major adverse cardiac events for subgroups in the entire cohort with vs. without beta-blockers. CI, confidence interval; eGFR, estimated glomerular filtration rate by MDRD equation; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST-elevation myocardial infarction. aUnadjusted event rate.
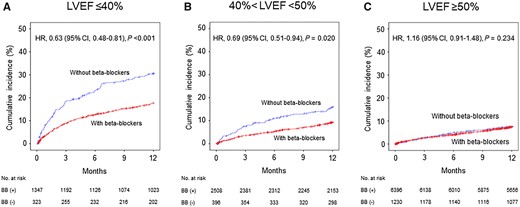
Unadjusted Kaplan–Meier curves and adjusted hazard ratios (HR) for 1-year major adverse cardiac events in the entire cohort with vs. without beta-blockers (BB) according to left ventricular ejection fraction (LVEF). (A) LVFE ≤40%; n = 1670 patients. (B) 40% <LVEF <50%; n = 2904 patients. (C) LVEF ≥50%; n = 7626 patients. CI, confidence interval.
Overall success rate of primary PCI in patients with STEMI was 99.7%. Drug-eluting stents were used in 92.4% of patients with beta-blockers and 88.6% of those without beta-blockers (Supplementary material online, Table S14). In STEMI patients undergoing successful primary PCI, beta-blocker therapy at discharge was equally associated with lower 1-year MACE (HR 0.73, 95% CI 0.57–0.94; P = 0.015), but an interaction with LVEF was not noted (Supplementary material online, Figure S4).
Bisoprolol (47.8%), carvedilol (44.8%), and nebivolol (5.0%) were the major beta-blockers that prescribed at discharge (Supplementary material online, Table S15). All beta-blockers were used in lower doses than those recommended in the guidelines. Bisoprolol, carvedilol, and nebivolol were all associated with lower 1-year MACE (Figure 5). Bisoprolol and carvedilol also showed comparable clinical outcomes in all subgroups of LVEF (Supplementary material online, Figure S5).
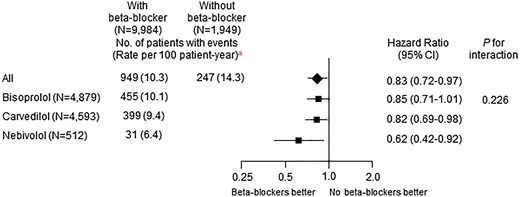
Adjusted hazard ratios of 1-year major adverse cardiac events in the entire cohort with vs. without beta-blockers according to generic names of beta-blockers. CI, confidence interval. aUnadjusted event rate.
Discussion
The main result of this study is that beta-blockers are still associated with lower cardiac events at least until 1 year after AMI, even in the era of early coronary reperfusion and the contemporary optimal medical therapy with antiplatelet agents and statins in patients with LVEF <50%, but not in those with LVEF ≥50%.
The guidelines for the management of AMI recommend oral beta-blockers only based on the clinical studies published in the early 1980s when the current effective treatment for AMI other than beta-blockers was not introduced and the imaging modalities to evaluate LV systolic function were not widely used. A meta-analysis of studies in 1970s and 1980s showed that beta-blockers reduced all-cause mortality of 23% when used as a long-term therapy.1 However, in the reperfusion era, the association between beta-blockers and long-term cardiovascular outcomes was not shown in a propensity score-matched analysis of registry data. 3 Furthermore, a significant interaction of beta-blockers’ effect between pre- and post-reperfusion era was noted in a meta-analysis of randomized clinical trials. In the pre-reperfusion era, beta-blockers reduced cardiovascular events but, in the reperfusion era, a long-term benefit was not shown.4
In our study, beta-blocker therapy at discharge was associated with lower MACE and cardiac mortality, but a significant interaction with LVEF was observed. Previously, the role of beta-blocker therapy was more clearly shown in patients with LVEF ≤40%. Beta-blockers’ benefit in patients with HF with reduced EF (HFrEF) has been unequivocally demonstrated, and evidence-proven beta-blockers are strongly recommended.10 Likewise, their clinical effect among MI patients with EF ≤40% was demonstrated.5
In the era of the prompt primary PCI or thrombolytic therapy for STEMI or an early invasive strategy for NSTEMI, a number of patients with preserved EF after AMI becomes increasing. Our registry data showed that more than 60% of patients had LVEF ≥50%. However, the role of beta-blockers in patients with HF with preserved EF (HFpEF) has not been clearly proven yet. Likewise, no randomized, long-term clinical data of beta-blockers in AMI patients with preserved EF are available so far. Only registry data showed no association between beta-blocker therapy and lower long-term mortality among survivors of AMI without HF or LV systolic dysfunction.14,15 Our registry data also showed that beta-blockers were not associated with either lower MACE or cardiac mortality in patients with LVEF ≥50%.
There are plausible explanations why beta-blockers did not reduce the clinical events in patients with LVEF ≥50%. Acute myocardial infarction patients with reduced LVEF had more scarred or non-viable myocardium, and in this clinical setting, beta-blocker therapy is effective in reducing fatal arrhythmia, myocardial ischaemia, or recurrent MI. Patients with LVEF ≥50% are expected to have lesser infarcted myocardium, and in these patients, the clinical benefit of beta-blockers will inevitably decrease.4,15 Another beneficial mechanism of beta-blockers in AMI is the reduction of myocardial oxygen consumption through heart rate slowing. The clinical importance of heart rate reduction in patients with HFrEF was shown in the study of ivabradine, an inhibitor of the If current in the sinoatrial node.16 However, ivabradine did not improve clinical outcomes despite heart rate reduction in stable coronary artery disease,17 which suggested that slowing of heart rate itself may not be sufficient to decrease the clinical events in MI patients with LVEF ≥50%.18
In most clinical trials of MI or HF, reduced LVEF was defined as less than 35–40%. Left ventricular ejection fraction between 40% and 50% has been a grey zone, because it may be considered as reduced or preserved EF. It is now proposed to be classified as mid-range EF in the ESC guideline on HF.10 Because patients with HF with mid-range EF (HFmrEF) were usually included in clinical trials of HFpEF, they are recommended to be managed similarly to those with HFpEF until new evidences become available. One recent meta-analysis of randomized clinical trials showed that beta-blockers reduced all-cause and cardiac mortality in patients with HFmrEF.11 But this meta-analysis had a pitfall that the median LVEF was 40% (interquartile range 40–43%) due to the inclusion criteria of LVEF ≤40% in four trials and ≤45% in one trial. This skewed distribution of LVEF could not afford the sufficient evidence about beta-blockers’ benefit in patients with HFmrEF, especially when LVEF is 45–49%. Until now, no clinical data are available about beta-blockers’ role in patients with AMI in mid-range EF. In this regard, registry data may provide an evidence about optimal medical therapy in these patients despite the inherent limitations. In our registry, a quarter of patients had mid-range EF, and in these patients, beta-blocker therapy at discharge was also associated with lower 1-year MACE, and this association was mainly driven by reduction of MI. Our data suggest that AMI patients in mid-range EF be managed similarly to those with reduced EF.
The role of beta-blockers in patients with STEMI undergoing successful primary PCI is even more unclear. In this clinical situation, our data showed beta-blocker therapy at discharge was also associated with lower 1-year MACE, which was compatible with a study of the previous Korean AMI Registry.19 However, an interaction with LVEF was not observed in these patients.
The guidelines recommend evidence-proven beta-blockers in patients with reduced systolic function.9,10 In our registry, bisoprolol, carvedilol, or nebivolol was prescribed in almost all patients. They were all associated with lower MACE without a significant interaction. Two major beta-blockers, bisoprolol and carvedilol showed a comparable association in all subgroups of LVEF.
Limitations
First, this study analysed a non-randomized, observational registry data. Beta-blockers were prescribed at the discretion of an attending physician. The information why physicians did not prescribe beta-blockers to some patients at discharge was not available. Although we tried to adjust the potential confounding factors by multivariable and a propensity score-matched analysis, other unmeasured, residual variables as well as selection bias could not be completely controlled. Second, because patients’ medications were recorded only at discharge and 12 month, we could not ascertain whether patients actually obtained them, took them as prescribed, and adhered for 1 year. Third, beta-blockers at discharge were prescribed at only a quarter of maximal dose recommended in the guidelines, and the individual doses at the time of clinical events were not available. However, also in an American registry, 60% of patients with AMI were prescribed less than a quarter of maximal dose at discharge, and the lowest mortality was observed in >12.5–25% dose group.20 The lower than maximal recommended dose may be an usual prescription pattern in ‘real-world’ registries, and the optimal dose of beta-blockers in patients with AMI needs to be confirmed in a randomized clinical trial. Fourth, a large cross-over was observed in patients without beta-blockers at discharge, and 72% of those patients were taking beta-blockers at 1 year. However, despite this cross-over, taking beta-blockers from the hospital discharge was associated with improved clinical outcomes. Fifth, only 1 year follow-up may not be long enough to evaluate clinical effects of beta-blockers in patients with AMI.
Conclusions
Patients with beta-blockers after AMI showed lower 1-year MACE, but a significant interaction with LVEF was noted. Beta-blocker therapy at discharge was associated with lower 1-year MACE in patients with reduced LVEF (≤40%) and mid-range LVEF (>40%, <50%), but not in patients with preserved LVEF (≥50%). These data suggested that long-term beta-blocker therapy may be guided by LVEF.
Funding
Korea Centers for Disease Control and Prevention (2016-ER6304-01).
Conflict of interest: none declared.
References
The CAPRICON Investigators.



