-
PDF
- Split View
-
Views
-
Cite
Cite
Ayman Helal, Mohamed Alama, Wael Ali, Mohsen Farooq, Desmoplakin cardiomyopathy: case report, European Heart Journal - Case Reports, Volume 8, Issue 9, September 2024, ytae419, https://doi.org/10.1093/ehjcr/ytae419
Close - Share Icon Share
Abstract
Desmoplakin (DSP) cardiomyopathy is a distinct form of cardiomyopathy characterized by frequent left ventricular involvement with extensive fibrosis, high arrhythmic risk, and episodes of acute myocardial injury.
We are reporting diagnosis and management of a rare case of DSP cardiomyopathy. A patient in his 70s was investigated for mild shortness of breath, recurrent chest infection, and multiple ventricular ectopic. His echocardiogram showed impaired systolic function and found to have 53% ectopic burden with run of non-sustained ventricular tachycardia on 24 h electrocardiogram. Cardiac magnetic resonance imaging showed findings highly suggestive of DSP cardiomyopathy. High-resolution computed tomography chest suggested connective tissue–related interstitial lung disease. The diagnosis of DSP cardiomyopathy was confirmed by genetic testing that demonstrated mutation in DSP protein. The patient had implantable cardioverter-defibrillator implantation for primary prevention.
Implantable cardioverter-defibrillator implantation should be considered with left ventricular ejection fraction thresholds >35%, particularly in the presence of additional risk factors.
Desmoplakin cardiomyopathy is a distinct form of cardiomyopathy characterized by a combination of frequent left ventricular (LV) involvement with extensive fibrosis, high arrhythmic risk, and episodes of acute myocardial injury. All or most of these features are usually present.
Desmoplakin cardiomyopathy is usually associated with high prevalence of ventricular arrhythmias even without significant LV dysfunction.
Implantable cardioverter-defibrillator implantation should be considered with LV ejection fraction thresholds >35%, particularly in the presence of additional risk factors.
Introduction
Desmoplakin (DSP) cardiomyopathy is a distinct form of cardiomyopathy characterized by frequent left ventricular (LV) involvement with extensive fibrosis, high arrhythmic risk, and episodes of acute myocardial injury. Implantable cardioverter-defibrillator (ICD) implantation should be considered with LV ejection fraction (LVEF) thresholds >35%, particularly in the presence of additional risk factors.
Summary figure
Case presentation
A patient in his 70s was referred to the cardiology service in our centre in early 2022 for an incidentally discovered multiple ventricular ectopic on 12-lead electrocardiogram (ECG) while he has been investigated for mild shortness of breath and chest infection. He has past medical history of transurethral resection of the prostate (TURP) for enlarged prostate, his body mass index (BMI) was 27.1, and he has no family history of heart disease. We investigated this further by blood tests, 24 h ECG, and echocardiogram.
His 12-lead ECG showed sinus rhythm (hear rate 85 b.p.m., PR 155 ms, QRS 98 ms, QTc 434) with frequent monomorphic ventricular ectopic beats. The 24 h ECG demonstrated heavy ventricular ectopic burden (52%) and episode of broad complex tachycardia lasting 8 beats at 187 b.p.m. triggered by R on T in keeping with monomorphic non-sustained ventricular tachycardia (NSVT; Figure 1); the patient reported shortness of breath correlated to this episode.
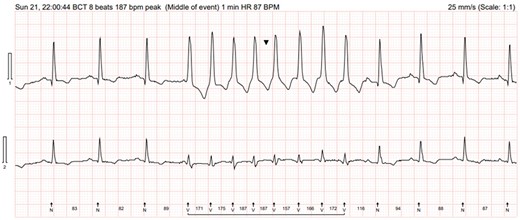
Episode of broad complex tachycardia (arrow) lasting 8 beats at 187 b.p.m. triggered by R on T on Holter electrocardiogram monitor.
His echocardiogram showed non-dilated LV with global impaired systolic function (EF 45%), impaired right ventricular (RV) radial function, and no significant valvular pathology (see Supplementary material online, Video S1).
He was commenced on dapagliflozin 10 mg, eplerenone 25 mg, bisoprolol 2.5 mg, and candesartan 4 mg.
To investigate the cause of his heart failure (HF), the patient underwent cardiac magnetic resonance imaging (CMR) showing findings that may suggest DSP cardiomyopathy in the form of (i) normal indexed LV volumes [end-diastolic dimension (EDD) 48 mm; end-systolic dimension (ESD) 43 mm; end-diastolic volume (EDV) 128 mm, 68 mL/m2; end-systolic volume (ESV) 80 mL, 42 mL/m2] with global hypokinesia and impaired systolic function (EF 38%); (ii) normal RV size [EDV 118 mm, 62 mL/m2; ESV 75 mL, 39 mL/m2; stroke volume (SV) 39 mL] with impaired systolic function (EF 36%); and (iii) multiple areas of late gadolinium enhancement (LGE) seen at the mid–interventricular septum (IVS), sub-epicardium mid-wall of the lateral wall with focal thinning of the mid-lateral wall. There was some associated findings like mild tricuspid and mitral regurgitation, bilateral reticular shadowing in both lungs consistent with usual interstitial pneumonia and mildly dilated aortic root (38 mm at sinus of Valsalva; Figure 2).
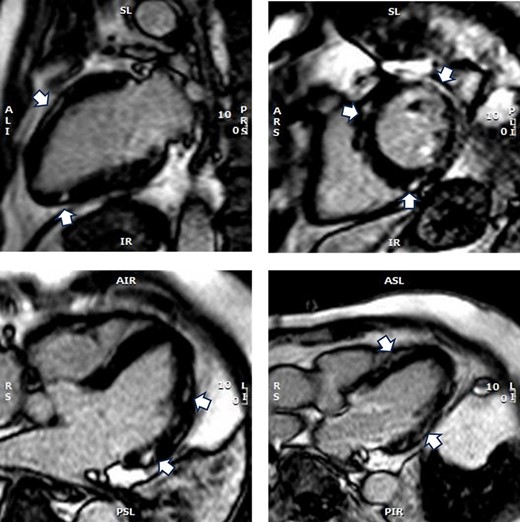
Cardiac magnetic resonance imaging showing multiple areas of late gadolinium enhancement seen at the mid–interventricular septum, sub-epicardium mid-wall of the lateral wall with focal thinning of the mid-lateral wall (arrows).
As part of the investigation of his shortness of breath, he had high-resolution chest computed tomography that showed established changes of predominant sub-pleural ground-glass opacities, sub-pleural reticulations with interlobular/intralobular septal thickening, areas of tractional bronchiectasis, and no evidence to suggest honeycombing. The appearances are suggestive of nonspecific interstitial pneumonia in keeping with connective tissue–related interstitial lung disease (Figure 3).
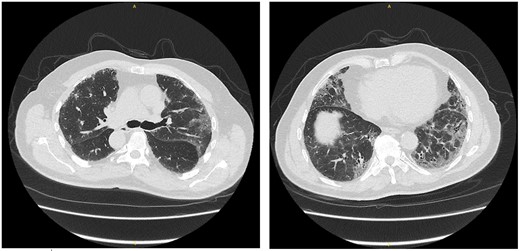
High-resolution chest computed tomography showing nonspecific interstitial pneumonia pattern of interstitial lung disease commonly related to connective tissue diseases.
The diagnosis of DSP cardiomyopathy was confirmed by genetic testing for the patient and his family as well (daughter and brother) that demonstrated mutation in the DSP protein; gene variant is c.2783_2786del p.(asn928Serfs*48).
The patient was commenced on HF guideline-directed medical therapy. There was a long discussion with the patient to explain the risks/benefits of ICD implantation in his condition, and he agreed to have ICD implantation. Implantable cardioverter-defibrillator was implanted without any complications.
After 6 months of follow-up, the patient was asymptomatic, and his exercise tolerance was remarkably improved. He had two episodes of NSVT that persisted for 12 s (Figure 4) detected by the ICD device, but no device therapy was required.
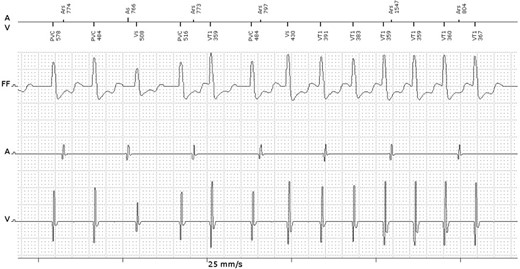
Episode of non-sustained ventricular tachycardia detected by the implantable cardioverter-defibrillator device.
Discussion
Desmoplakin is a protein that plays an essential role for cell-to-cell adhesion within the cardiac cells. Desmoplakin cardiomyopathy is a distinct form of cardiomyopathy characterized by frequent LV involvement with extensive fibrosis, high arrhythmia risk, and episodes of acute myocardial injury. Clinical presentation involves a degree of LV dysfunction, in association with frequent ventricular ectopic beats up to life-threatening ventricular arrhythmias. It can also present with recurrent myocarditis like episodes.1 The presence of thick callused skin (palmoplantar keratoderma) and curly hair further heightens the likelihood of DSP cardiomyopathy, as the DSP protein is also present in the skin and hair and plays an important role in their integrity.2 The typical ECG findings may include low QRS voltages and inverted T-wave in inferior or lateral leads. However, in some patients, the ECG can be unremarkable.1
The echocardiographic evaluation can be unremarkable in DSP carriers, in spite of a coexisting extensive scar, and these changes may not present until advanced stages as the subendocardial layer is usually spared. Therefore, CMR is the most sensitive test for phenotype assessment in DSP carriers. The classic CMR finding is myocardial fibrosis that is usually confined to the epicardial layer; LV dimensions and systolic function are frequently normal. A distinctive feature of DSP cardiomyopathy is the disproportionate involvement of the LV extensive fibrosis and arrhythmic propensity that largely exceed the degree of ventricular dysfunction.1
Desmoplakin cardiomyopathy is usually associated with high prevalence of ventricular arrhythmias even without significant LV dysfunction. There is no specific risk stratification for sudden cardiac death (SCD) in such patients.1 Smith et al. (2020) stated that the standard dilated cardiomyopathy (DCM) LVEF threshold of <35% was an insensitive marker in DSP cardiomyopathy, with many events occurring in an EF range of 35–55%, and occasionally at an EF > 55%. Their study provides compelling evidence that LV systolic function <55% is associated with increased risk regardless of RV involvement. They also suggest that frequent premature ventricular ectopic beats (PVCs) and LV fibrosis may contribute independently to risk prediction in DSP cardiomyopathy.2 In contrary, Wang et al. (2022) found that LVEF < 35% and RV dysfunction were independently associated with higher risk of sustained ventricular arrhythmia.3 According to the recently published meta-analysis by Brandão et al. (2023), LVEF < 55% was strongly associated with malignant ventricular arrhythmias regardless of RV involvement.1
A recent report stated that arrhythmogenic cardiomyopathy phenotypes involving predominantly the LV are associated with HF complications, and on the contrary patients with RV involvement such as arrhythmogenic right ventricular cardiomyopathy (ARVC) had higher incidence of life-threatening ventricular arrhythmias.4
According to the 2023 ESC Guidelines for the management of cardiomyopathies, DCM-causing variants in high-risk genes (LMNA, EMD, TMEM43, DSP, RBM20, PLN, FLNC-truncating variants) and patients with a high-risk genetic background for SCD, primary prevention ICD implantation should be considered with LVEF thresholds >35%, particularly in the presence of additional risk factors (e.g. NSVT, increased ventricular ectopic beats, male sex, significant LGE, specific gene variant), Class IIb recommendation. DSP gene mutation is associated with 3–5% annual SCD rate, the predictors of SCD is LGE on CMR and LVEF < 45%.5
Lead author biography
Dr Ayman Helal is an interventional cardiologist at Kettering General Hospital.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: Consent for publication has been obtained from the patient being reported on, in line with the COPE best practices guidelines, and he is aware of the possible consequences of reporting.
Funding: None declared.
Data availability
The data underlying this article are available to use for all readers.
References
Author notes
Conflict of interest: None declared.





Comments