-
PDF
- Split View
-
Views
-
Cite
Cite
Emmanuelle Massie, Arnaud Dominati, Sebastian Suchet, David Carballo, Elsa Hervier, Siv Fokstuen, Jöerg D Seebach, Philippe Meyer, Case report: desmoplakin cardiomyopathy presenting as an inflammatory cardiomyopathy with repeated sudden cardiac arrests, European Heart Journal - Case Reports, Volume 8, Issue 4, April 2024, ytae160, https://doi.org/10.1093/ehjcr/ytae160
Close - Share Icon Share
Abstract
Desmoplakin cardiomyopathy has been recently classified as a non-dilated left ventricular cardiomyopathy, which is characterized by inflammatory-like episodes followed by left ventricular fibrosis/dysfunction and ventricular arrhythmias. Specific management is unclear.
We report a detailed case of a 46-year-old Caucasian woman presenting with repeated sudden cardiac arrests who was diagnosed with a new variant in the desmoplakin gene. Because the initial 18F-fluorodeoxyglucose positron emission tomography scan showed significant hypermetabolism, she was treated with immunosuppressors, with only minimal improvement on imaging.
Desmoplakin cardiomyopathy should be considered in the differential diagnosis of inflammatory cardiomyopathies. Little is known about the use of immunosuppressive treatments, but it could be reasonable for some selected patients.
Clinicians should maintain a high level of clinical suspicion for DSP cardiomyopathy when a patient present with an inflammatory cardiomyopathy, particularly in the presence of ventricular arrhythmias.
Genetic testing can identify new variants (like in our case) and is important to determine appropriate management strategies.
Introduction
The cardiac desmosome complex encompasses multiple proteins, including desmoplakin (DSP), which role is to link the intercellular unit to the intermediate filaments inside the cardiomyocytes. Mutations in the DSP gene have been identified in non-dilated left ventricular cardiomyopathies (NDLVCs) and less frequently in arrhythmogenic right ventricular cardiomyopathy (ARVC) and dilated cardiomyopathy (DCM). Loss-of-function variants in DSP are known to be pathogenic and represent an established disease mechanism in this NDLVC subtype.1,2 Interestingly, some reports1,3–5 have described an inflammatory, myocarditis-like component of DSP cardiomyopathy, different from ARVC, likely responsible for the development and progression of the disease. In 2008, Sen-Chowdhry et al.6 hypothesized that these ‘hot phases’ were part of the natural history of DSP cardiomyopathy and that they were followed by repair with fibrous or fibrofatty tissue with subsequent LV systolic dysfunction and ventricular arrhythmias. Here we report a case of DSP cardiomyopathy that presented as an inflammatory cardiomyopathy with repeated episodes of sudden cardiac arrest, treated empirically with immunosuppressive treatment (Figure 1).
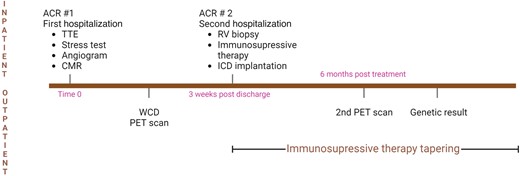
Summary figure—case timeline. The figure was created with BioRender.com.
Case presentation
A 46-year-old Caucasian woman presented to our emergency department for loss of consciousness preceded by prodromal symptoms, which occurred during exercise at home. Her husband witnessed the event and performed cardiac massage for 40 s before she regained consciousness. Past medical history was significant for a suspicion of primary Sjögren’s syndrome in 2015. Because her mother had succumbed to sudden cardiac death (SCD) at the age of 42 years, a few months earlier, the patient underwent a cardiac screening evaluation, which only revealed mild T-wave inversions in leads V4–V6 on the resting 12-lead electrocardiogram (ECG). Transthoracic echocardiogram (TTE) and exercise stress testing were completely unremarkable. A Holter monitor revealed moderate ventricular ectopy (6% of total beats over 24 h) of two morphologies, both with a superior axis of the QRS. No further investigations were performed.
Upon admission, vital signs were normal, with a blood pressure of 109/70 mmHg and a heart rate of 76 b.p.m. Physical exam was unremarkable, with the absence of woolly hair or keratoderma. The ECG showed normal sinus rhythm with T-wave inversions in leads V4–V6, DII, DIII, and aVF (Figure 2). Blood tests were significant for elevated high-sensitive cardiac troponin T (hs-cTnT) at 99 ng/L (n < 14 ng/L) that peaked at 230 ng/L 2.5 h later. A TTE showed normal LV size and ejection fraction (EF), estimated at 55% with a suspected isolated apical hypokinesis (see Supplementary material online, Video S1). The right ventricle (RV) was described as of normal size and function, and no significant valvular disease was noted. After 24 h of cardiac rhythm monitoring in the intermediate care unit without significant arrhythmic event, the patient was transferred to a general ward. Because the aetiology of the exercise-induced syncope was presumed arrhythmic in origin, an exercise stress test was ordered and showed 30 polymorphic ventricular premature beats (VPBs), including three doublets (during stress and in the recovery phase). A coronary angiogram showed minor diffuse atherosclerosis. Cardiac magnetic resonance (CMR) revealed a LVEF of 52% with mid anterior and lateral hypokinesis and diffuse late gadolinium enhancement (LGE) in the mid and subepicardial parts of the myocardium without oedema, which was suggestive either of myocarditis sequelae or another chronic inflammatory process (Figure 3).
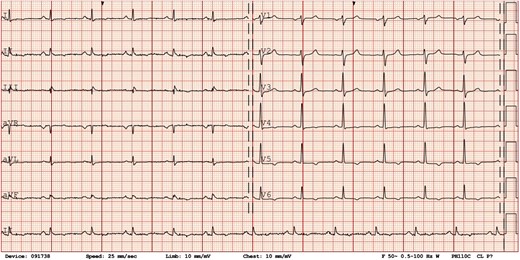
Electrocardiogram. Initial electrocardiogram showing normal sinus rhythm with T-wave inversions in leads V4–V6, DII, DIII, and aVF.
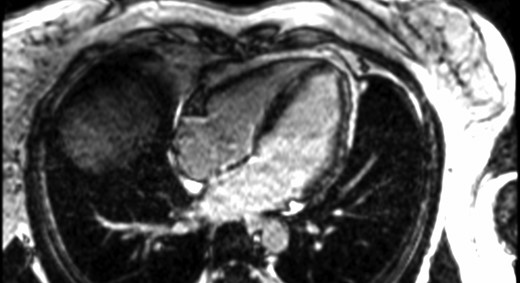
Cardiac magnetic resonance images. Cardiac magnetic resonance showing mid myocardium and subepicardial late gadolinium enhancement predominantly in the anterior and lateral walls.
She was then discharged home with a diagnosis of NDLVC of unknown origin, the mild rise in hs-cTnT being attributed to cardiac massage. An empirical decision was made to initiate metoprolol 25 mg and lisinopril 5 mg once daily as a potential aid in cardiac remodelling. Given the ongoing uncertainty surrounding the diagnosis and the absence of conclusive evidence regarding the presence of a ventricular arrhythmia in this young woman, we prescribed a wearable cardioverter defibrillator (WCD) while awaiting further outpatient testing. Sequential [13N]ammonia with regadenoson pharmacological stress and [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) scan of the heart showed a clear increase in [18F]FDG uptake on the entire LV lateral wall, with neither RV involvement nor hypermetabolism of the mediastinal lymph nodes (Figure 4B). This finding was matched with a reduced [13N]ammonia uptake in the same territory indicating hypoperfusion (Figure 4A). The overall picture was compatible with inflammatory cardiomyopathy. The immunological workup revealed positive antibodies (high titres of anti-nuclear and anti-SSA, with low-titres of anti-SSB) along with high titres of rheumatoid factor IgM, but negative Schirmer’s test and sialometry. A biopsy of accessory salivary glands was performed and showed no anomaly. Based on these findings, the patient did not fully meet the classification criteria for primary Sjögren’s syndrome.
![Perfusion positron emission tomography and consecutive [18F]fluorodeoxyglucose positron emission tomography metabolism. (A) Perfusion positron emission tomography [13N]ammonia showing reduced perfusion on the lateral wall. (B) Initial positron emission tomography with mismatched hypermetabolism by [18F]fluorodeoxyglucose, orienting towards a possible inflammatory process. (C) Follow-up positron emission tomography by [18F]fluorodeoxyglucose with slightly diminished but grossly unchanged metabolic signal.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ehjcr/8/4/10.1093_ehjcr_ytae160/9/m_ytae160f4.jpeg?Expires=1749231374&Signature=BAqehajRgftVsQUg4Z4kR2G9Nkw-dFOeUHzRTcxhDCV4Gmsl71ESsjpz0tSVB8H83Mc8EE4WTcEt7alVnOLkDm64yu6HqgZd4FI~t-YQFtw504cltsqYl0yrjUKi4fpWCZ56bV50rhdYVx3KIpugQnFSIdpUksUSRs-JnAPPB1KASKCS8cNqEYVjST47L2eC66I2pTW93BtAFC7XeRFXQp7oUvlUN8a0E-RLOmzrBuXhYIHE0bPXm8ORMMc4AWzkDWMEpmcq8PchjzvG5Na7Yv2OHqK9UT1c6R741P8DOOTLq4bC1N6TnXcZSbyVU2HulKqa~BO3sBUysHzxQ47uvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Perfusion positron emission tomography and consecutive [18F]fluorodeoxyglucose positron emission tomography metabolism. (A) Perfusion positron emission tomography [13N]ammonia showing reduced perfusion on the lateral wall. (B) Initial positron emission tomography with mismatched hypermetabolism by [18F]fluorodeoxyglucose, orienting towards a possible inflammatory process. (C) Follow-up positron emission tomography by [18F]fluorodeoxyglucose with slightly diminished but grossly unchanged metabolic signal.
Three weeks later, while doing light strength training, the patient experienced a cardiac arrest due to ventricular tachycardia, which was successfully reverted by the WCD. The patient was then equipped with an implantable cardioverter defibrillator (ICD). A transvenous device was chosen as we thought she could benefit from anti-tachycardia pacing (ATP) if her ventricular arrhythmia was to reoccur. An endomyocardial RV biopsy (four samples) performed to rule out autoimmune diseases, sarcoidosis, or another cause of inflammatory cardiomyopathy revealed fibrosis without any evidence of granuloma, active inflammation, or infection. Because of the suspicion of an inflammatory cardiomyopathy on the PET scan, along with lethal arrhythmias and the initial suspicion of Sjögren’s syndrome, and despite a negative biopsy (possibility of sampling error), the multidisciplinary cardiology and immunology team decided to start high-dose i.v. methylprednisolone that was further switched to tapering doses of oral prednisone combined with steroid-sparing agent azathioprine, as recommended for some autoimmune myocarditis/inflammatory cardiomyopathies (e.g. cardiac sarcoidosis). The subsequent clinical outcome was uneventful. Six months later, a second [18F]FDG PET scan was performed under immunosuppressive therapy of prednisone 2.5 mg and azathioprine 150 mg o.d., revealing slightly diminished, but persistent metabolic hyperactivity in the same territory (Figure 4C). Following these results, prednisone was weaned off 2 months later, but the patient remains on a residual dose of 50 mg of azathioprine o.d.
Due to the unclear aetiology and the family history of SCD, molecular genetic analysis by targeted exome sequencing of 124 genes associated with Mendelian cardiomyopathies and/or arrhythmias was performed. A novel heterozygous nonsense variant in the DSP gene [c.2716A > T, p.(Lys906*), NM_004415] was detected. This mutation results in a premature stop codon leading to a truncated protein and was classified as pathogenic according to the American College of Medical Genetics criteria.7
Because of significant anxiety related to the diagnosis and the prior cardiac arrests, the patient followed our cardiovascular rehabilitation programme to restart light exercise under supervision. A year after the second hospitalization, she remained event free and was tolerating the medication without adverse effect.
Discussion
According to the latest ESC guidelines on cardiomyopathies, our patient received a diagnosis of DSP-related NDLVC, characterized by subepicardial scarring and a low-normal LVEF. Non-dilated left ventricular cardiomyopathy is a newly recognized cardiomyopathy subtype, defined by the presence of non-ischaemic LV scarring or fatty replacement without LV dilation. This can be either accompanied by global or regional wall motion abnormalities or present as an isolated global LV hypokinesia without scarring not solely explained by abnormal loading conditions or coronary artery disease. DSP gene mutations are a common cause of NDLVC, resulting in a distinct cardiomyopathy with a high prevalence of LV fibrosis and myocardial inflammation.2 Notably, much of the available data on natural history and risk assessment in LVNDC is derived from cohorts encompassing patients with DCM or ARVC, making specific data for LVNDC limited. Consequently, the ensuing discussion will primarily centre on DSP cardiomyopathy in comparison to ARVC and DCM.
Smith et al.1 recently described the specific features that distinguish DSP cardiomyopathy from ARVC and dilated cardiomyopathy through a multicentre cohort of 107 patients with DSP mutations. Curly hair and thick palms/soles (palmoplantar keratoderma) were found in 55% of their cohort. These patients exhibited at least some degree of LV involvement (51% had predominant LV cardiomyopathy) with a great proportion of systolic dysfunction (64%). T-wave inversions were more often seen in V4–V6 instead of in V1–V3 as observed in ARVC. Patients with DSP cardiomyopathy were also very likely to have extensive LGE on CMR (40%), with typical involvement of the subepicardial portion of the inferior segment. Interestingly, in 16 of these patients (15%), some degree of myocardial injury (elevated troponins with normal coronary angiogram, frequently associated with chest pain) was noted, while no similar episode was described in patients with ARVC. These acute episodes seem to be able to occur even in the presence of normal systolic function. In four of these patients, a [18F]FDG PET scan showed extensive inflammation.1 In a similar manner, Wang et al.8 followed 91 patients with a DSP mutation and found some degree of myocardial injury in 20 of them, with that being the initial presentation for 13 out of those 20 individuals. They described more frequent LGE in these patients (90%), which pattern was also mostly subepicardial (72%), with a higher incidence of heart failure and sustained arrhythmias.
The utility of [18F]FDG PET scan to guide diagnosis and management remains unclear. Protonotarios et al.4 analysed [18F]FDG PET scan in 14 patients with ARVC, of whom 5 came back positive (2 of them had a DSP mutation). As none of the cardiac biopsies showed inflammatory infiltrate, they hypothesized that these positive [18F]FDG PET scans may represent abnormal glucose metabolism within the myocardium rather than true inflammation. Moreover, in a patient with DSP cardiomyopathy, a repeated [18F]FDG PET scan after 1.5 years of immunosuppressive therapy still demonstrated ongoing hypermetabolism despite treatment, which was then tapered down without any symptom recurrence.1 Thus, this disease might be an inflammatory cardiomyopathy mimicker. Yet, a genuine myocardial inflammatory response remains a possibility. Indeed, in mice carrying desmoglein mutations (another protein part of the desmosome complex), cardiomyocyte necrosis led to an inflammatory response involving predominantly neutrophils but also macrophages and T cells.9 Currently, there is no recommendation to use [18F]FDG PET in the diagnostic workup of NDLVC, except when cardiac sarcoidosis is suspected.2
Management of DSP cardiomyopathy associated with inflammatory-like episodes is still controversial due to a lack of data and the unclear pathophysiology of the disease. According to multiple retrospective studies, intense and prolonged exercise can lead to progression of the disease and increase the risk of sustained ventricular arrhythmias in patients with arrhythmogenic cardiomyopathies.10 However, recreational exercise activity does not appear to confer a higher risk compared to sedentary patients and can therefore be advised for these patients.11 Our patient never had a clear diagnosis of myocarditis, and as her stress test did not reveal any sustain ventricular arrhythmia, she was therefore not restrained from mild-to-moderate physical activities. As to immunosuppressive therapy, it is uncertain whether it leads to improved outcomes compared to watchful monitoring. Nevertheless, it may be reasonable to treat selected patients according to clinical judgment. A combination of high-dose steroids and mycophenolate mofetil led to clinical improvement and a drastic reduction of the troponin levels in a patient suffering from recurrent myocarditis and DSP cardiomyopathy.5 In patients where troponins are not markedly elevated, such treatment should be discussed and debated by a multidisciplinary team.
Finally, regarding SCD risk assessment, current guidelines propose a unified approach for both NDLVC and DCM. In cases involving a history of cardiac arrest or VT with haemodynamic compromise, ICD placement is recommended (class I). For primary prevention, an ICD must be considered in patients with LVEF ≤35% (class IIa). When LVEF exceeds 35%, two other criteria are taken into account: (i) the presence of high-risk genes, such as DSP, and (ii) additional risk factors specific to each genotype, which include LVEF <45% and the extent of LGE on CMR for DSP mutations. If both criteria are met, ICD implantation should be considered (class IIa). Where only one criterion is met, an ICD may be considered (class IIb).2 Interestingly, the location of the DSP variant was recently found to be a novel independent risk factor for ventricular arrhythmias (VA). A mutation in the constitutive nonsense-mediated decay (NMD) competent, like in our case, was associated with a 2.8–3.2 times increased risk of VA compared to NMD incompetent mutations.12
Conclusion
This case emphasizes the importance of clinical suspicion of a DSP-related NDLVC in the context of unclear inflammatory-like cardiomyopathy. In our case, we found a new heterozygous nonsense variant in the DSP gene. Typically, these patients present with some degree of LV involvement, ventricular arrhythmias, subepicardial LGE enhancement on CMR, and sometimes myocarditis-like episodes. As these patients carry a higher risk of SCD, it is important to make the appropriate diagnosis and to identify potential high-risk features to guide adequate management including ICD implantation. Little is known regarding the role of immunosuppressive treatment, but further studies are needed to specify its potential benefits in selected patients.
Lead author biography

Dr Emmanuelle Massie is a cardiologist trained at the University of Montreal in Quebec, Canada. She then completed a 1-year fellowship in echocardiography at McGill and is now completing another year in heart failure at Geneva University Hospitals in Switzerland.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article, in compliance with COPE guidelines.
Funding: None declared.
Data availability
The data that support the findings of this case report are available upon request from the corresponding author.
References
Author notes
Conflict of interest: None declared.




Comments