-
PDF
- Split View
-
Views
-
Cite
Cite
Krithika Krishnarao, Daniel S Yip, Rohan M Goswami, Juan C Leoni, Parag C Patel, Stiff right atrial syndrome? A complex clinical case report utilizing multimodality imaging and invasive hemodynamics, European Heart Journal - Case Reports, Volume 8, Issue 4, April 2024, ytae163, https://doi.org/10.1093/ehjcr/ytae163
Close - Share Icon Share
Abstract
Stiff left atrial syndrome is a well-established cause of heart failure symptoms. A parallel entity involving the right atrium (RA) has not previously been described. We present a case of refractory right heart failure (RHF) 12 years following orthotopic heart transplantation.
Patient underwent annuloplasty ring placement for severe tricuspid regurgitation in 2018 and kidney transplantation in 2020. The use of multimodality imaging and a multidisciplinary approach suggested a stiff RA as a potential etiology to refractory symptoms. Redo-heart and kidney transplantation in March 2021 led to the resolution of symptoms without recurrence.
We propose stiff right atrial syndrome that may need to be considered in the setting of refractory RHF primarily suggested by significant right atrial enlargement and restrictive physiology.
Case: A patient with heart transplantation in 2008 with refractory right heart failure
To create a differential diagnosis of right heart failure.
To understand the hemodynamic importance of the right atrium.
To utilize multimodality imaging and hemodynamics to differentiate restrictive and constrictive physiology.
Introduction
Atrial dysfunction has primarily been thought to occur from cardiac dysfunction or a marker for prognosis rather than a separate clinical entity.1 This has been challenged by the term atrial failure.2,3 Described mechanisms include electrical dyssynchrony, booster-pump failure, reservoir dysfunction, and conduit dysfunction.
While stiff left atrium (LA) syndrome has been well established, a parallel entity involving the right atrium (RA) has not previously been described.4,5 We propose a novel term, stiff RA syndrome, as a potential cause of refractory right heart failure.
Summary figure
Case presentation
A 57-year-old female who underwent orthotopic heart transplantation (HTx) in 2008 for ventricular arrhythmias presented with refractory right heart failure (RHF). After antibody-mediated rejection in 2013, she developed reduced functional capacity and volume overload with worsening renal function over four years. Workup revealed severe tricuspid regurgitation (TR) requiring annuloplasty ring in 2018. Post-operatively, she developed acute kidney injury, recurrent pleural effusions, and ascites. Due to progression to stage 5 chronic kidney disease, she underwent a deceased donor kidney transplantation (DDKT) in 2020. Symptoms of volume overload returned, requiring admission in December 2020. Medications included tacrolimus 6 mg twice daily, prednisone 10 mg daily, valganciclovir 450 mg twice weekly, and high-dose torsemide. Mycophenolic acid was discontinued due to leucopenia. NT-proBNP and high-sensitivity troponin were elevated without significant change from prior values.
Physical examination noted elevated jugular venous pressure with prominent v-wave, S3 gallop, hepatomegaly with pulsatility, and bilateral lower extremity oedema. Transthoracic echocardiography (TTE) demonstrated preserved biventricular function (LVEF 71%) with RA greater than LA enlargement and moderate TR (Figure 1A–E). Mitral inflow pulse wave Doppler demonstrated large E without significant A wave despite sinus rhythm at 65 b.p.m. by electrocardiography with a right bundle branch block (Figure 1F). Endomyocardial biopsy (EMBx) and renal biopsy were negative for rejection and recent coronary angiogram was unremarkable for obstructive disease or distal vessel pruning. Transesophageal and intracardiac echocardiography, and cardiac MRI quantified moderate TR but notably, MRI showed a significantly enlarged RA and abnormal atrial contraction (Figure 2). Hemodynamics showed no ventricular interdependence. Pressures supported restrictive physiology: RA 17 mmHg without significant v waves, but blunted x descent and prominent y descent, RV 35/3/12 mmHg with ‘square root’ sign, PA 37/16/24 mmHg, PCWP 15 mmHg, Fick CI 3.14 (Figure 3). RHF was not attributed to renal failure alone due to normal PCWP. With limited treatment options, she underwent redo-HTx and DDKT March 2021 with resolution of RHF symptoms. Pathologic analysis of the explanted myocardium demonstrated fibrosis without ischaemic injury. Specifically, fibrosis of the RA with a firm palpable texture was reported.
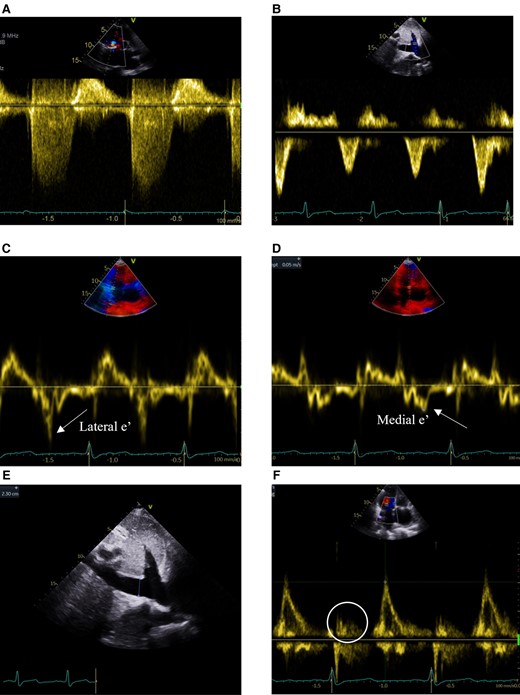
TTE continuous-wave Doppler (CWD) and pulsed-wave Doppler (PWD) evaluating TR and evidence of constrictive (CP) or restrictive (RCM) physiology. (A) CWD of TR signal without ‘dagger’-shaped appearance of severe TR. (B) Hepatic vein PWD without systolic flow reversal seen in severe TR, inspiratory diastolic flow reversals in RCM, or expiratory diastolic flow reversals in CP (respirometer not shown). (C) PWD of lateral mitral annulus (lateral > medial e’ inconsistent with annulus reversus seen in CP). (D) PWD of medial mitral annulus (medial e’=0.05 m/s not elevated inconsistent with annulus paradoxus seen in CP). (E) 2D measurement of dilated inferior vena cava (2.3 cm without inspiratory collapse). (F) Mitral inflow PWD large E without a significant A wave (circle) despite sinus rhythm.
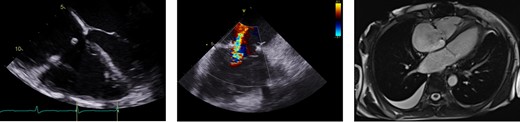
Multimodality imaging. TEE showing RA enlargement with well-seated annuloplasty ring (left). ICE revealing no more than moderate TR (middle). Cardiac MRI demonstrating significantly enlarged RA (right).
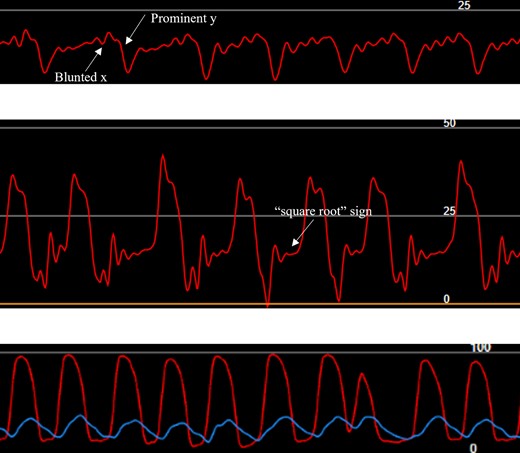
Invasive hemodynamics. RA tracing showing blunted x and prominent y descents without large v-waves suggestive of restrictive physiology (top). RV ‘square-root’ sign consistent with elevated end-diastolic pressure (middle). Simultaneous LV (red) and RV (blue) tracings without ventricular interdependence seen in constrictive physiology (bottom).
Discussion
We describe a case of RHF with suspicion for RA pathology as a contributor to refractory symptoms. The long-term effects of HTx on the structure and function of the RA are not well known. The effect of cold-ischaemic time, reperfusion at HTx, and iatrogenic injury from EMBx on the RA is unknown. RHF following HTx may occur due to pulmonary hypertension, RV injury, allograft rejection, or primary graft dysfunction.6 These factors were less likely based on our workup.
While restrictive cardiomyopathy may align with the physical, hemodynamic, and echocardiography features, it does not account for significant RA enlargement, abnormal atrial contraction noted by cardiac MRI, and firm RA texture at explant. Limited atrial tissue was available for staining to evaluate higher degrees of fibrosis that may suggest atrial remodelling.1 While details of the initial HTx were limited, transection at cavo-atrial junctions with the bicaval technique minimize residual recipient atrial tissue. Electrocardiography noted relative bradycardia post HTx with heart rate of 65 b.p.m. in sinus rhythm without rate-reducing therapies. This finding in conjunction with the loss of A wave by Doppler echocardiography may suggest some degree of electromechanical dissociation. Ventricular myocardium did not show ischaemic damage by histopathological analysis to suggest ischaemic injury or under-estimated cardiac allograft vasculopathy. Although no v-waves were seen in RA tracings (unlike prominent v-waves with stiff LA), this may be related to the thinner-walled RA accommodating greater volumes without increasing pressure in conjunction with the vena cava also tolerating pressure changes well that may dampen right-sided v-waves.1
The RA may serve a more important role than previously known, particularly in RHF, which remains one of the most challenging diagnoses to manage. Assessment of LA function utilizing echocardiographic parameters has traditionally been studied.3 LA reservoir, conduit, and pump function can be measured from volumetric assessment, Doppler echocardiography, tissue Doppler imaging, and strain/strain rate.3 Utilizing volumetric assessments, LA emptying volume and emptying fraction can be calculated. Although affected by other factors, E and A velocities may allow LA function assessment. Tissue Doppler imaging profile of mitral annulus a’ has been shown to correlate with atrial function.3 These have been studied in LA function, but a similar evaluation of the RA is not described.
Cardiac MRI was used to assess RA function post-myocardial infarction.7 The study found impairment of RA reservoir and conduit functions was associated with adverse cardiovascular events. Abnormal conduit function was associated with heart failure onset and adverse cardiovascular events independent of RV function and atrial fibrillation.7
Conclusion
While our evaluation of the RA did not include the techniques described to assess LA function, this can be assessed in the future with widely available multimodality imaging techniques. Evaluation of RA mechanics in RHF and/or post-HTx may promote understanding of this forgotten chamber. RHF in the setting of significant RA enlargement and restrictive physiology may be associated with a similar entity to stiff LA syndrome. We propose a stiff RA syndrome that requires further investigation.
Patient’s perspective
Our patient was appreciative of team efforts managing her complex case which adversely affected her quality of life. She has returned to playing tennis, enjoys travelling with family, and will be completing her third annual visit March 2024.
Lead author biography

Krithika Krishnarao, D.O., F.A.C.C., is an Advanced Heart Failure and Transplantation Cardiologist. She earned her medical degree in 2013 from LECOM in Erie, Pennsylvania and completed her Internal Medicine Residency at Allegheny General Hospital in Pittsburgh, where she stayed on to complete a year as Chief Resident. After moving to Jacksonville, Florida for General Cardiology Fellowship at Mayo Clinic, she developed a particular interest in cardiogenic shock and pursued a fellowship in Advanced Heart Failure and Transplantation in Mayo Clinic Florida. She helped develop the Cardiogenic Shock Quality Initiative Program at Mayo Clinic Florida in 2019 and remains active with involvement of cardiogenic shock quality improvement.
Acknowledgements
We thank our teams for their multidisciplinary approach to this challenging case. Also a thanks to the pathology department for reviewing explanted heart tissue and Melissa Lyle for the details of imaging reports.
Consent: Information submitted is de-identified. The authors confirm that written consent for submission and publication of this case report including the images and associated text have been obtained from the patient in line with COPE guidance.
Disclosure: An abstract of this case was published with a virtual poster presentation at the American College of Cardiology in 2022. However, we have no prior cases discussing our topic and believe this concept is novel to be considered for publication as a case report.
Funding: No research grants or financial support was used.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Parag C Patel senior author.
Conflict of interest: R.K.G. received speaker payment for HFSA from Abiomed. No financial disclosures for any of the other listed authors.





Comments