-
PDF
- Split View
-
Views
-
Cite
Cite
Sandeep M Patel, Percutaneous repositioning of Impella RP: the Snare–Manoeuvre–Prolapse technique—a case report, European Heart Journal - Case Reports, Volume 6, Issue 4, April 2022, ytac085, https://doi.org/10.1093/ehjcr/ytac085
Close - Share Icon Share
Abstract
Impella RP (Abiomed, Danvers, MA, USA) is indicated for right ventricular failure after left ventricular assist device insertion or biventricular shock. Once the peel-away sheath is removed, Impella RP repositioning can only be achieved with manual manipulation of the catheter itself. This method does not always accomplish appropriate positioning of the catheter and can result in continued haemodynamic instability.
A young male presented to our institution with recurrent ventricular fibrillation and ST-elevation myocardial infarction that underwent emergent coronary intervention but was in progressive cardiogenic shock requiring implantation of Impella 5.0 and Impella RP. After insertion of the right ventricular support, the patient stabilized transiently then became unstable once more, and repeat fluoroscopy demonstrated that the Impella RP had ‘fallen back’ into the right ventricle. Due to continued instability, we improvised a previously undescribed method of repositioning of the Impella RP catheter with the use of a goose-neck snare.
The snare–manoeuvre–prolapse method of Impella RP repositioning is a relatively novel approach at the management of Impella RP retrograde migration into the right ventricle and prevents the need for large-bore venous closure and re-access and the use of a new Impella RP catheter while providing rapid improvement of haemodynamics.
Fluoroscopy should be the primary diagnostic modality when determining Impella RP malpositioning.
Failure to reposition the Impella RP into the appropriate position will result in poor haemodynamics, haemolysis, ventricular arrhythmias, may impair valve function, and result in worsening of right ventricular failure/shock.
The snare–manoeuvre–prolapse technique is a percutaneous interventional option that allows for repositioning of the Impella RP catheter.
Introduction
Cardiogenic shock complicates 5–10% of cases of myocardial infarction1–3 and mechanical support devices have demonstrated limited benefit with early initiation and appropriate protocols.4,5 Right ventricular failure occurs in ∼25% of patients after left ventricular assist device insertion, thus the advent of Impella RP.6,7 However, the device may migrate out of appropriate position into the right ventricle (‘fallback’) resulting in haemodynamic instability. At present, Impella RP can only be repositioned with direct torqueing of the catheter. We report a case of Impella RP ‘fallback’ and discuss a novel technique for repositioning.
Timeline
| 0 h | Recurrent ventricular fibrillation with anterior ST-elevation. |
| 1 h | Intubation, 18 biphasic defibrillations at 200–360 J, percutaneous coronary intervention of the left anterior descending, with residual left circumflex chronic total occlusion; Impella CP inserted in the same setting. |
| 2–6 h | Progressive cardiogenic shock/stunning, right ventricular haemodynamics poor, in addition to low cardiac index and low cardiac power; decision to upgrade to Impella 5.0 via surgical axillary access, and add Impella RP in the same setting. |
| 7 h | Impella RP repositioning via snare–manoeuvre–prolapse technique with stabilization of haemodynamics. |
| 8–9 h | Colonic ischaemia/infarction, family consents to emergency bowel resection. |
| 10–20 h | Patient stable from cardiac perspective, but developed progressive renal failure, septic shock, and shock liver, family elects for renal replacement therapy. |
| 20–30 h | Continues veno-venous hemofiltration ongoing, aggressive supportive care with coagulation factors due to hepatic failure. |
| 30–36 h | Multiple discussions with family regarding upgrading to extracorporeal membrane oxygenation to allow for organ recovery, however, family elects to stop all aggressive care and patient expires shortly after turning off all haemodynamic, respiratory, and renal support. |
| 0 h | Recurrent ventricular fibrillation with anterior ST-elevation. |
| 1 h | Intubation, 18 biphasic defibrillations at 200–360 J, percutaneous coronary intervention of the left anterior descending, with residual left circumflex chronic total occlusion; Impella CP inserted in the same setting. |
| 2–6 h | Progressive cardiogenic shock/stunning, right ventricular haemodynamics poor, in addition to low cardiac index and low cardiac power; decision to upgrade to Impella 5.0 via surgical axillary access, and add Impella RP in the same setting. |
| 7 h | Impella RP repositioning via snare–manoeuvre–prolapse technique with stabilization of haemodynamics. |
| 8–9 h | Colonic ischaemia/infarction, family consents to emergency bowel resection. |
| 10–20 h | Patient stable from cardiac perspective, but developed progressive renal failure, septic shock, and shock liver, family elects for renal replacement therapy. |
| 20–30 h | Continues veno-venous hemofiltration ongoing, aggressive supportive care with coagulation factors due to hepatic failure. |
| 30–36 h | Multiple discussions with family regarding upgrading to extracorporeal membrane oxygenation to allow for organ recovery, however, family elects to stop all aggressive care and patient expires shortly after turning off all haemodynamic, respiratory, and renal support. |
| 0 h | Recurrent ventricular fibrillation with anterior ST-elevation. |
| 1 h | Intubation, 18 biphasic defibrillations at 200–360 J, percutaneous coronary intervention of the left anterior descending, with residual left circumflex chronic total occlusion; Impella CP inserted in the same setting. |
| 2–6 h | Progressive cardiogenic shock/stunning, right ventricular haemodynamics poor, in addition to low cardiac index and low cardiac power; decision to upgrade to Impella 5.0 via surgical axillary access, and add Impella RP in the same setting. |
| 7 h | Impella RP repositioning via snare–manoeuvre–prolapse technique with stabilization of haemodynamics. |
| 8–9 h | Colonic ischaemia/infarction, family consents to emergency bowel resection. |
| 10–20 h | Patient stable from cardiac perspective, but developed progressive renal failure, septic shock, and shock liver, family elects for renal replacement therapy. |
| 20–30 h | Continues veno-venous hemofiltration ongoing, aggressive supportive care with coagulation factors due to hepatic failure. |
| 30–36 h | Multiple discussions with family regarding upgrading to extracorporeal membrane oxygenation to allow for organ recovery, however, family elects to stop all aggressive care and patient expires shortly after turning off all haemodynamic, respiratory, and renal support. |
| 0 h | Recurrent ventricular fibrillation with anterior ST-elevation. |
| 1 h | Intubation, 18 biphasic defibrillations at 200–360 J, percutaneous coronary intervention of the left anterior descending, with residual left circumflex chronic total occlusion; Impella CP inserted in the same setting. |
| 2–6 h | Progressive cardiogenic shock/stunning, right ventricular haemodynamics poor, in addition to low cardiac index and low cardiac power; decision to upgrade to Impella 5.0 via surgical axillary access, and add Impella RP in the same setting. |
| 7 h | Impella RP repositioning via snare–manoeuvre–prolapse technique with stabilization of haemodynamics. |
| 8–9 h | Colonic ischaemia/infarction, family consents to emergency bowel resection. |
| 10–20 h | Patient stable from cardiac perspective, but developed progressive renal failure, septic shock, and shock liver, family elects for renal replacement therapy. |
| 20–30 h | Continues veno-venous hemofiltration ongoing, aggressive supportive care with coagulation factors due to hepatic failure. |
| 30–36 h | Multiple discussions with family regarding upgrading to extracorporeal membrane oxygenation to allow for organ recovery, however, family elects to stop all aggressive care and patient expires shortly after turning off all haemodynamic, respiratory, and renal support. |
Case presentation
A 49-year-old Caucasian male without prior medical history presented with recurrent ventricular fibrillation and anterior ST-segment elevation myocardial infarction. Physical exam demonstrated an intubated and sedated male, who was hypotensive and tachycardic with cool, non-cyanotic extremities. Patient was started on amiodarone, lidocaine, norepinephrine, epinephrine, and vasopressin infusions and taken to the cardiac catheterization laboratory. An Impella CP and Swan-Ganz were emergently inserted. Percutaneous intervention of the left anterior descending artery was performed with a single drug-eluting stent. Over the next 6 h, right atrial to wedge pressure ratios began to rise (0.80), pulmonary artery (PA) pulsatility index dropped (0.75), mixed venous oxygen saturation was 40%, cardiac index was 1.7 L/min/m2 (cardiac power output = 0.6 W), and multiple suction alarms were noted on the Impella CP console. Transthoracic echocardiography demonstrated severe biventricular failure with appropriately positioned Impella CP (Videos 1 and 2).
Given the worsening haemodynamics, an Impella RP was inserted and suction alarms stopped; however, cardiac power did not increase, and thus we upgraded to Impella 5.0. The patient stabilized and fluoroscopy demonstrated appropriate pump positions.
As the patient was being transferred, suction alarms were noted on the Impella 5.0 console and a dampened placement curve similar to a right ventricular pressure tracing was seen on the Impella RP console. Fluoroscopy demonstrated that the Impella RP catheter had ‘fallen back’ into the right ventricle. Multiple attempts at manual manipulation were unsuccessful, and we elected to use snare-assistance to manoeuvre the pump.
A 6-Fr sheath was already in place in the left femoral vein and thus we advanced a 6-Fr multi-purpose (MP) coronary guiding catheter with a 25-mm Amplatz GooseNeck Snare (Medtronic, Minneapolis, MN, USA) into the right atrium. The snare captured the Impella RP pigtail, and then we were able to advance both into the right ventricular outflow tract (RVOT). With the cinched snare, we intentionally prolapsed the MP catheter against the pulmonic valve (PV) and advanced both the Impella RP and the MP catheter across the valve and into the left PA (Video 3). The snare was then released and removed. Thereafter, we had re-established stable haemodynamics without suction alarms. Unfortunately, the patient developed colonic ischaemia and necrosis and family elected for emergent bowel resection. Despite resection, the patient sustained progressive renal and hepatic failure along with septic shock. Due to the poor prognosis, the family elected to withdraw all life-sustaining measures ∼36 h after the initial arrest.
Discussion
The Impella RP catheter is currently indicated for right ventricular support following left ventricular assist device implantation or myocardial infarction.8 The catheter requires a venous 23-French peel-away sheath with the pump positioned in the distal left PA for optimal flows.8 Fluoroscopy is recommended to ensure appropriate catheter position. Due to torqueing of the catheter and tortuosity of the RVOT, the pump may ‘fallback’ into the right ventricle. In the inappropriate position, the Impella RP may cause haemolysis, ventricular arrhythmias, tricuspid/pulmonic regurgitation, and result in worsening of right ventricular failure/shock. At times, this may occur after the peel-away is already removed and the manufacturer suggest attempt at manual manipulation and if unsuccessful, to restart the process and place a new Impella RP pump. The obvious cons to this approach include needing a second large bore venous access and the need for a new Impella RP access kit/unit.
Percutaneous interventional technique
Due to the above disadvantages, we devised a novel method for Impella RP repositioning: the snare–manoeuvre–prolapse technique. Operators have described the use of snares to help reposition the Impella CP device, but none have demonstrated similar efficacy with Impella RP repositioning.9 The steps for percutaneous Impella RP repositioning are described as follows:
Snaring of the Impella RP: Turn the Impella RP to P2 while manipulating. Obtain contralateral 6-French femoral venous access. Ensure the Impella RP pigtail portion is pulled back into the right atrium/inferior vena cava to avoid snare entanglement in the tricuspid valve (TV). Advance a 6-Fr MP coronary guiding catheter into the right atrium. We advocate for the use of a 20–30 mm 90° GooseNeck snare advanced into the guiding catheter to cinch the pigtail portion of the pump (Supplementary material online, Video S1; Figure 1).
Manoeuvringof the Impella RP: Once the pump is securely grasped, gently advance the Impella RP and the snare-guide apparatus (SGA) across the TV. Once across the TV, clockwise torque on just the Impella RP with continued forward pressure on the SGA in the region of the RVOT allows for advancement upwards towards the PV (Figure 2). The goal is to align the body curve of the Impella RP catheter with the RVOT. A Swan-Ganz catheter in the PA may provide further guidance with respect to pump trajectory.
Prolapsing of the SGA: Once the Impella RP and the SGA are facing cephalad on antero-posterior fluoroscopy, we advocate no further pushing of the Impella RP. At this point, forward advancement of the pump will typically result in retrograde movement and ‘fallback’ into the right ventricle (Figure 3A). Instead, hold the Impella RP stationary, and begin advancing the SGA. The cinched pigtail portion becomes a fulcrum and the SGA begins to prolapse across the PV, splaying it open. Once the tip of the guide is facing caudal on the fluoroscopy and the body of the guide is in the PA, further advancement of the tightly cinched SGA, will actually pull the Impella RP forward into the PA (Figure 3B). At that moment, simultaneously continue to advance the prolapsed SGA system into the PA while advancing the Impella RP forward. The combined forward pressure from the back of the pump and pulling of the pigtail portion from the front of the pump ultimately results in the forward inertia that allows the Impella RP to advance across the PV into the PA (Video 3). Once the pump is in the correct position, the snare can be recaptured (Figure 4, Supplementary material online, Video S2). Final position of the Impella RP (and Impella 5.0) is then checked to be appropriate on fluoroscopy (Figure 5).
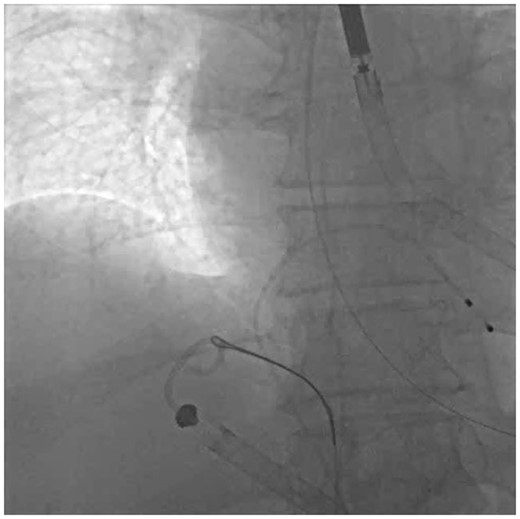
Step 1—Snare: 25 mm GooseNeck snare tightly cinched around the Impella RP pigtail portion in the inferior vena cava–right atrium junction.
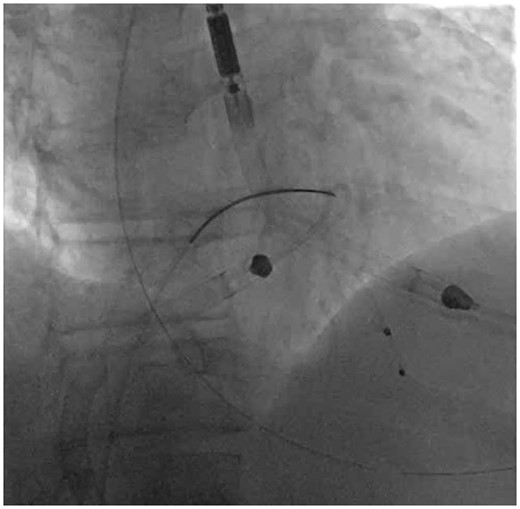
Step 2—Manoeuvre: The snare-guide apparatus and the Impella RP are both manoeuvred across the tricuspid valve and are positioned in the proximal portion of the right ventricular outflow tract.
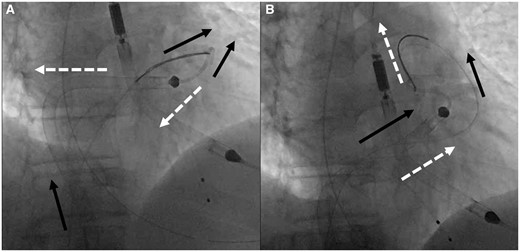
Step 3—Prolapse: (A) Black solid arrows demonstrate the vector forces with continued pushing from the femoral vein and the white dashed arrows demonstrate the motion vectors that the Impella RP-SGA will follow due to the natural course of the right ventricular outflow tract and the geometry of the Impella RP; continued pushing results in the main body of the Impella RP being pushed back into the right atrium and the pigtail portion falling out of the right ventricular outflow tract and into the right ventricle due to opposing vectors of force and motion. (B) After prolapse of the snare-guide apparatus across the pulmonic valve, the vector forces (black solid arrows) now change as the tip of the snare-guide apparatus is now facing 180° in opposition to the path of motion which results in pulling of the Impella RP up into the pulmonary artery while pushing the Impella RP and the snare-guide apparatus from the femoral position, thus creating parallel vectors of force and motion (white dashed arrows).
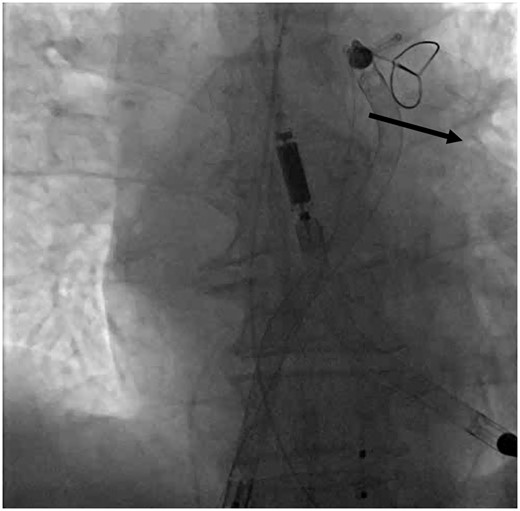
Snare disengagement with black solid arrow demonstrating the direction in which the snare should be advanced after releasing to help facilitate recapture and withdrawal.
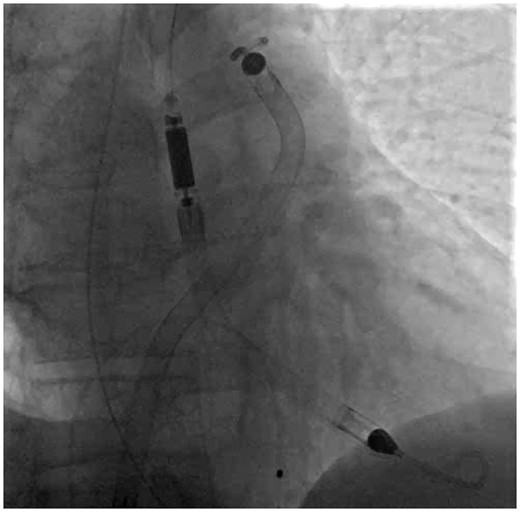
The final correct position of the Impella RP and Impella 5.0 catheters on fluoroscopy.
Standard parasternal long axis demonstrating Impella CP in the left ventricle and biventricular failure.
Standard subcostal view demonstrating severe biventricular failure.
Continuous fluoroscopy of the Snare-Maneuver-Prolapse technique for Impella RP repositioning.
Limitations
Limitations of the technique include the need for a second femoral venous access. The operator also needs to have a thorough understanding of available snare technology. Finally, we have not attempted the technique from the jugular venous approach, thus it is unclear whether the Impella RP catheter could be repositioned from this access site.
Conclusion
Impella RP is a significant technological advance in the management of right ventricular failure/shock and requires a position across the PV towards the left PA. This optimal position may be lost due to ‘fallback’ phenomenon. If feasible, manual manipulation should be attempted, however, if the Impella RP does not advance, we advocate for the use of the snare–manoeuvre–prolapse technique (Supplementary material online, Figure) to avoid the need for a second large bore access, a new Impella RP catheter/kit, and to quickly re-establish haemodynamic support.
Lead author biography
Dr Sandeep M. Patel, MD is a practicing board-certified interventional cardiologist at the St.Rita’s Medical Center in Lima, OH, USA, where he is currently the director of the Structural Heart and Interventional Center. His interests include structural heart disease interventions, high-risk coronary interventions, mechanical circulatory support, and peripheral arterial disease interventions.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Acknowledgements
The author would like to acknowledge Dr John Sirak, MD, Mr Bill Carder, RT(R)(CV), and Mrs Sarah Kallay, RT(R) who were present during the index case and assisted in the active management of the patient. The author would also like to acknowledge the assistance of Mr Andrew Macke, RT(R)(CV) who was pivotal in the figure and video generation for the manuscript.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirms that witnessed verbal consent for submission and publication of this case report including images and associated text has been obtained from the patient's next of kin. This has been discussed with the editors.
Conflictofinterest: None declared.
Funding: None declared.





Comments