-
PDF
- Split View
-
Views
-
Cite
Cite
Iulia Tustiu, Sara Woods, Jennifer Lee, Orla Buckley, David Moore, Acute myocarditis and haemoptysis in an adult with human bocavirus monoinfection: a case report, European Heart Journal - Case Reports, Volume 8, Issue 10, October 2024, ytae480, https://doi.org/10.1093/ehjcr/ytae480
Close - Share Icon Share
Abstract
Bocavirus monoinfection-related acute myocarditis is an aetiology that has rarely been described in the literature.
A 36-year-old male, with no significant medical history, presented to the emergency department with a 4-day history of dyspnoea, haemoptysis, left-sided chest pain, and high-grade pyrexia. The initial investigations revealed a raised troponin T, raised C-reactive protein, and a normal electrocardiogram. A comprehensive microbiological and virological work-up (testing for 14 viruses and bacteria) detected human bocavirus (HBoV) DNA monoinfection. Cardiac magnetic resonance imaging showed left ventricular ejection fraction of 48%, with subepicardial late gadolinium enhancement. Other imaging modalities (chest X-ray, echocardiography, computed tomography pulmonary angiography, and bronchoscopy) revealed no other causative pathology. The patient was treated with anti-inflammatory medications and left ventricle remodelling therapy. He had a good clinical outcome. Moreover, a collateral history revealed that the patient’s infant had presented with a severe respiratory illness, which was felt to be of viral aetiology, several days prior to the patient’s own onset of symptoms.
To our knowledge, this is the fourth case of HBoV-related acute myocarditis in an immunocompetent adult. This case also displays new clinical features for HBoV infection—haemoptysis, high-grade pyrexia, and a potential for vertical transmission from infants.
Bocavirus monoinfection is a rare cause of acute myocarditis, previously being described in immunocompetent adults (three cases) and infants (four cases).
The particularity of this case is haemoptysis as a new clinical feature for human bocavirus (HBoV) infection and the potential transmission from infants.
Screening for HBoV DNA within multiplex molecular respiratory assays in patients with suspected acute myocarditis associating respiratory symptoms may be a justifiable extension of the diagnostic modality.
Introduction
Acute myocarditis may result from exposure to a wide spectrum of pathogens or systemic conditions, but a causal association has been most frequently demonstrated with so-called cardiotropic viruses (adenovirus, enterovirus, Epstein–Barr virus, human herpesvirus 6, parvovirus B19, and cytomegalovirus).1,2
In clinical practice, the majority of patients presenting with clinical and imaging features of myocarditis do not have viral genomes isolated, however are assumed to be viral in origin.3
In 2005, a novel human bocavirus (HBoV) was identified from nasopharyngeal aspirates in infants with respiratory tract infection.1 The bocavirus belongs to family Parvoviridae, genus Bocaparvovirus, having four species (HBoV1–HBoV4) and no geographic restrictions.4 Bocavirus infection causes predominantly respiratory tract symptoms with uncommon associated gastrointestinal features in children or immunocompromised hosts. There is limited experience of its occurrence in immunocompetent adults.4
We report the fourth case with HBoV as the causative pathogen of acute myocarditis in a young adult without significant comorbidities.
Summary figure
Case presentation
A 36-year-old male self-referred to the emergency department with flu-like symptoms, haemoptysis, dyspnoea, and chest pain.
He described a sudden onset dyspnoea, sore throat, diaphoresis, pyrexia (42°C), and productive cough with green–brown sputum. Over a period of 4 days, multiple episodes of haemoptysis containing a moderate amount of blood were reported.
Symptoms began 3 days prior to his presentation and were progressive.
His 10-month-old child, who developed upper respiratory symptoms of infection associated with vomiting, had been brought to the emergency department 7 days prior to his onset of symptoms.
The patient’s medical history included a Covid19 viral infection 5 months previously. He is a former smoker (10 packs/year) and had been working as a soldier. He has a family history of coronary artery disease.
On clinical examination, he was pale and lethargic, with no stigmata for heart failure and normal breath sounds. He was stable haemodynamically with normal oxygen saturation at room air. Clinical investigations were conducted in accordance with the guidelines for acute myocarditis and tailored to his clinical course.1,3
He had an extensive microbiological and virological panel of investigations, and only bocavirus DNA was detected by multiplex BIOFIRE™ FilmArray® respiratory panel (Table 1).
| . | Pathogen . | Results . |
|---|---|---|
| RNA viruses | Respiratory syncytial virus Influenza A (H1 and H3) Influenza B Human metapneumovirus Parainfluenza viruses 1, 2, 3, and 4 Rhino/enterovirus Coronavirus OC43 Coronavirus HKU1 Coronavirus 229E Coronavirus NL63 SARS-CoV-2 Hepatitis C | Negative |
| DNA viruses | Adenovirus Hepatitis B virus Epstein–Barr virus Parvovirus B19 | Negative |
| Bacteria | Mycoplasma pneumoniae Chlamydia pneumoniae Bordetella parapertussis Bordetella pertussis Bartonella spp. Coxiella burnetii | Negative |
| . | Pathogen . | Results . |
|---|---|---|
| RNA viruses | Respiratory syncytial virus Influenza A (H1 and H3) Influenza B Human metapneumovirus Parainfluenza viruses 1, 2, 3, and 4 Rhino/enterovirus Coronavirus OC43 Coronavirus HKU1 Coronavirus 229E Coronavirus NL63 SARS-CoV-2 Hepatitis C | Negative |
| DNA viruses | Adenovirus Hepatitis B virus Epstein–Barr virus Parvovirus B19 | Negative |
| Bacteria | Mycoplasma pneumoniae Chlamydia pneumoniae Bordetella parapertussis Bordetella pertussis Bartonella spp. Coxiella burnetii | Negative |
| . | Pathogen . | Results . |
|---|---|---|
| RNA viruses | Respiratory syncytial virus Influenza A (H1 and H3) Influenza B Human metapneumovirus Parainfluenza viruses 1, 2, 3, and 4 Rhino/enterovirus Coronavirus OC43 Coronavirus HKU1 Coronavirus 229E Coronavirus NL63 SARS-CoV-2 Hepatitis C | Negative |
| DNA viruses | Adenovirus Hepatitis B virus Epstein–Barr virus Parvovirus B19 | Negative |
| Bacteria | Mycoplasma pneumoniae Chlamydia pneumoniae Bordetella parapertussis Bordetella pertussis Bartonella spp. Coxiella burnetii | Negative |
| . | Pathogen . | Results . |
|---|---|---|
| RNA viruses | Respiratory syncytial virus Influenza A (H1 and H3) Influenza B Human metapneumovirus Parainfluenza viruses 1, 2, 3, and 4 Rhino/enterovirus Coronavirus OC43 Coronavirus HKU1 Coronavirus 229E Coronavirus NL63 SARS-CoV-2 Hepatitis C | Negative |
| DNA viruses | Adenovirus Hepatitis B virus Epstein–Barr virus Parvovirus B19 | Negative |
| Bacteria | Mycoplasma pneumoniae Chlamydia pneumoniae Bordetella parapertussis Bordetella pertussis Bartonella spp. Coxiella burnetii | Negative |
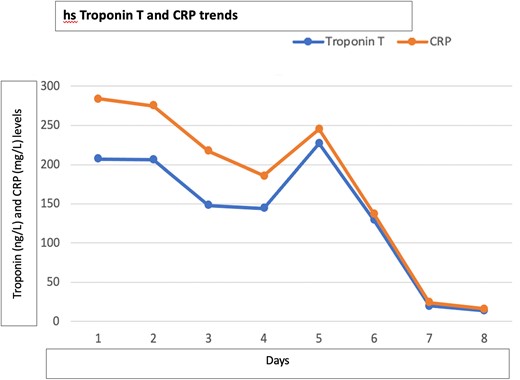
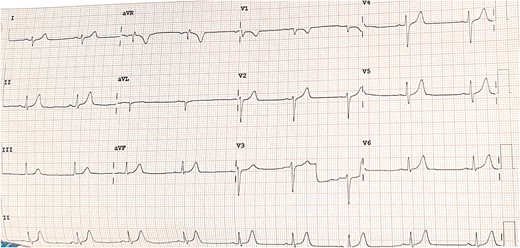
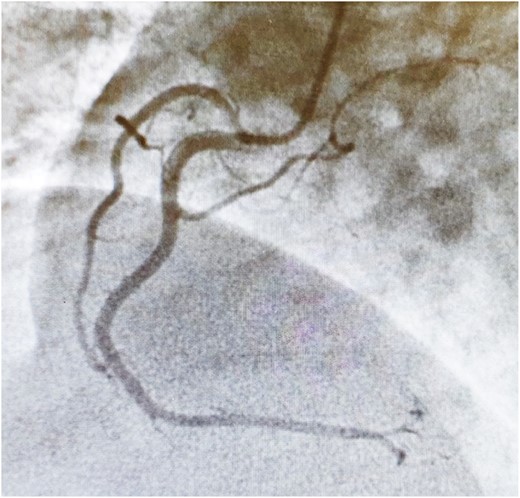
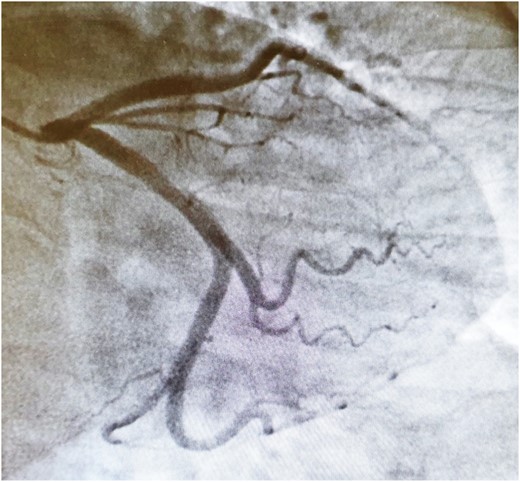
Connective tissue disease screening was negative, and no pathogens were isolated in blood cultures or sputum samples. Troponin (ng/L) and C-reactive protein (mg/L) level trends are described in Figure 1. Other biomarkers including NT-proBNP were within normal range. Electrocardiogram showed sinus bradycardia, at 54 b.p.m., with no acute ischaemic features (Figure 2). Chest X-ray and transthoracic echocardiogram were unremarkable. A computed tomography pulmonary angiogram showed no pulmonary embolism, with minimal subsegmental bilateral lower lobe atelectasis noticed. Coronary angiogram revealed only minor coronary atheroma (Figures 3 and 4).
Cardiac magnetic resonance imaging (MRI) (Figure 5) revealed short-axis T1 post-contrast magnitude image acquired 10 min following a weight-based dose of IV gadolinium (Gadovist™) demonstrating subepicardial myocardial late gadolinium enhancement (LGE) in the mid-ventricular anterolateral and inferolateral segments. No corresponding myocardial oedema was seen on T2 spectral adiabatic inversion recovery oedema-sensitive short-axis images. No wall motion abnormality noticed.
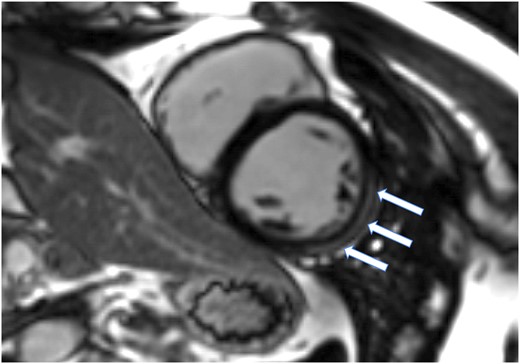
Selected image from cardiac magnetic resonance imaging acquired on 3T Siemens Lumina magnetic resonance imaging scanner. LGE evidence in the mid ventricular antero-lateral and infero-lateral segments (arrows). For more cardiac MRI and ecocardiographic imaging, please find in the Supplementary material online, available at European Heart Journal – Case Reports online.
Bronchoscopy was performed after 3 months, and no endobronchial lesions were seen up to subsegmental levels to explain the source of haemoptysis. Bocavirus was not isolated from sputum or bronchial aspirates.
Although a myocardial biopsy (EMBx) would have added diagnostic value, it was not performed in this case due to invasiveness and high sampling error rate. Moreover, the patient remained stable haemodynamically throughout his hospitalization, presented no features of acute heart failure or arrhythmia, and his response to standard therapy was satisfactory. We considered that the EMBx would not have added benefits to therapy or prognosis.1,3
The patient received aspirin 300 mg in the emergency department as the initial diagnosis was thought to be acute coronary syndrome. He received colchicine 0.5 mg b.i.d. for 3 months, ibuprofen was added for 2 weeks due to severe chest pain, pantoprazole 40 mg o.d., bisoprolol 2.5 mg o.d., ramipril 2.5 mg o.d., and paracetamol as PRN. On Day 5 during hospitalization, the patient developed a productive cough with brown–green sputum, pyrexia, worsening inflammatory markers, and mild troponin rise. This was thought to be bacterial bronchitis for which he received a 7-day course of intravenous amoxicillin/clavulanic acid with resolution of symptoms and biomarkers.
Since respiratory virus in adults can usually be considered a trigger for an inflammatory response, rather than virus-mediated myocarditis with viral replication, a corticosteroid therapy could have been considered as next-line treatment option. However, in this case, the viral illness was of short duration and the clinical response to non-steroidal anti-inflammatory therapy was satisfactory from both a cardiac and respiratory clinical perspective.
The patient had favourable progress as an inpatient and had no further haemoptysis after a 4-day period. He was advised to avoid strenuous exertion on discharge until follow-up in the outpatient clinic.
At 3-month follow-up, he reported having atypical brief episodes of left-sided chest discomfort. It was decided to continue on colchicine 0.5 mg o.d. until a further follow-up after 3 months and to discontinue the ramipril and the beta-blocker.
At 18 months, a follow-up cardiac MRI was completed and revealed normal ventricular volumes and function with the only finding being of stripe of lateral and inferolateral epicardial fibrosis consistent with previous myocarditis. These findings suggest complete resolution of the myocarditis episode, and the patient was allowed to resume full duties in the army.
Discussion
The HBoV, thought to be an innocuous viral agent previously, is rarely identified as monoinfection in immunocompetent adults. However, it has been reported to range in severity from mild infection to life-threatening illness. It has also been reported to cause prolonged shedding respiratory tract infections, as well being linked to serious infections of the gastrointestinal tract.5–9
Interestingly, several authors mentioned that HBoV has been detected in myocardium biopsy samples. At a post-mortem of a 69-year-old male with respiratory failure, the only other site where the HBoV DNA was detected, apart from his lungs, was the myocardial tissue from the right and left ventricles.10
In a study from South Africa, where patients undergoing open-heart surgery had also harvested samples from the left atrium, 5% of patients demonstrated the presence of HBoV DNA in myocytes, but none were DNA serum positive. Moreover, 96% of these individuals demonstrated seropositivity of IgG HBoV. Although this may be noted as a small ability for latency of HBoV within the heart tissue, it is actually revealing its cardiac tropism potential.11
This finding suggests a hitherto unreported predilection for HBoV localization and latency in human myocardium.
Clinical evidence for human bocavirus myocarditis
Among the paediatric population, four cases of viral myocarditis have been reported where HBoV was considered the culprit pathogen, three of which had fatal outcomes.8,9,12,13
The first case of HBoV-related myocarditis in adults was described in an 85-year-old immunocompetent male with pneumonia and acute myocarditis. Human bocavirus monoinfection was identified on an oropharyngeal swab by polymerase chain reaction (PCR).5
A single isolated positive at histological and viral PCR for HBoV was reported in a South African study, which enrolled acute myocarditis cases between August 2017 and January 2021.14
The third case of HBoV acute myocarditis was recently described in a 22-year-old pregnant woman who developed severe pneumonia and dilated cardiomyopathy, complicated by multiorgan failure and stillbirth.15
We present the fourth case of HBoV-related acute myocarditis in an immunocompetent adult. Although we did not perform an EMB due to the risks associated with the invasiveness and the insensitivity of the technique in the context of favourable clinical outcome, a diagnosis of acute myocarditis was confirmed by myocardial tissue characterization on cardiac MRI demonstrating LGE of classical myocarditis pattern, complementing the clinical and laboratory findings.
The concomitant respiratory viral illness and the evidence of HBoV monoinfection, in addition to a paediatric family member having suffered respiratory and gastroenterological symptoms in the proximity of his illness debut, all suggest the possible relationship of HBoV with acute myocarditis.
In this case, where HBoV is most likely the culprit pathogen for acute myocarditis, the particularities are haemoptysis and high-grade pyrexia as new clinical features of HBoV infection and potential for vertical transmission.
With increasing reports of HBoV monoinfection associated with severe infections in children and adults,6 screening for HBoV DNA within multiplex molecular respiratory assays in patients with suspected myocarditis may be a justifiable extension of the diagnostic modality.
Lead author biography

Cardiology registrar and specialist doctor in internal medicine, MD, MRCPI, MSc.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of the case report has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
Author notes
Conflict of interest: None.





Comments