-
PDF
- Split View
-
Views
-
Cite
Cite
Moon-Kyung Jung, Hwan Wook Kim, Kyongmin S Beck, Yeoun Eun Sung, Mi-Hyang Jung, Managing a patient with pulmonary thromboembolic disease presenting with active haemoptysis: a case report, European Heart Journal - Case Reports, Volume 8, Issue 7, July 2024, ytae353, https://doi.org/10.1093/ehjcr/ytae353
Close - Share Icon Share
Abstract
Pulmonary thromboembolism and active haemoptysis represent distinct yet critical emergencies necessitating immediate intervention. However, the treatment protocols for these conditions—anticoagulation therapy and haemostatic therapy—often pose a dilemma.
We present the case of a 25-year-old female who presented to our emergency room with haemoptysis and a concurrent diagnosis of pulmonary thromboembolism. Due to persistent active haemoptysis, we temporarily paused anticoagulation and opted for surgical pulmonary thrombectomy, enabling the safe resumption of anticoagulation therapy.
Haemoptysis occurring in pulmonary thromboembolism is infrequently reported in the literature, and established treatment guidelines for such cases are lacking. This case could provide guidance on how to handle the intricate treatment challenges posed by concurrent haemoptysis and pulmonary thromboembolism.
Current treatment guidelines for pulmonary embolism do not provide established therapeutic strategies for cases involving active haemoptysis, relying instead on empirical approaches due to the scarcity of literature addressing this specific scenario.
This case, involving a therapeutic dilemma encompassing haemoptysis and pulmonary embolism, was successfully managed with surgical pulmonary endarterectomy followed by anticoagulation therapy, potentially aiding in decision-making for managing pulmonary embolism patients with active haemoptysis and contributing to future guideline development.
Introduction
Haemoptysis is an infrequent symptom in pulmonary thromboembolism (PTE), occurring in about 7% of all patients; haemoptysis in PTE is usually mild, often attributed to pulmonary infarction.1 In this report, we present the case of a 25-year-old female patient who presented with an ongoing large amount of haemoptysis and was subsequently diagnosed with PTE. Our case describes the management of this challenging clinical scenario where the treatment goals of bleeding control and haemostasis are in conflict.
Summary figure
Case presentation
A 25-year-old female patient presented to the emergency room with haemoptysis that had started 1 h earlier. She also complained of anterior chest and back pain. The patient had severe obesity, with a body mass index of 43.5 kg/m2, and, aside from a recent episode of pneumonia treatment 3 weeks ago, had no significant medical history. During the physical examination, coarse breath sounds were heard in both lung fields. The patient maintained stable blood pressure at 120/80 mmHg despite experiencing tachycardia at 107 b.p.m., alongside an oxygen saturation level of 97% while receiving nasal prong oxygen at 2 L/min. Laboratory tests revealed a normal haemoglobin level of 15.7 g/dL. The troponin T level was 0.009 ng/mL (reference ≤0.014 ng/mL), indicating no cardiac damage, but there was a mildly elevated D-dimer level of 1.25 mg/L (reference ≤ 0.80 mg/L) and an NT-proBNP level of 874 pg/mL (reference <300 pg/mL). Arterial blood gas analysis, conducted with 2 L/min of oxygen, showed a pH of 7.456, pCO2 of 29.5 mmHg, pO2 of 89.1 mmHg, HCO3− of 20.5 mmol/L, and lactate level of 0.7 mmol/L, suggesting hypocapnia. The chest X-ray revealed no specific abnormalities.
To further evaluate the haemoptysis, a chest computed tomography (CT) with enhancement was conducted (Figure 1). The results revealed large thrombi completely obstructing both interlobar pulmonary arteries. Additionally, small thrombi were observed in the segmental and subsegmental pulmonary arteries of both lower lobes and the left upper lobe, along with a small pulmonary infarction in the left lower lobe. Subsequent echocardiography revealed no evidence of right ventricular contractile dysfunction or pulmonary hypertension but a mobile echogenic mass (10.6 × 26 mm) at the junction of the inferior vena cava (IVC) and right atrium (RA) (Figure 2, Supplementary material online, Videos S1 and S2). Lower extremity CT did not show any deep vein thrombosis.
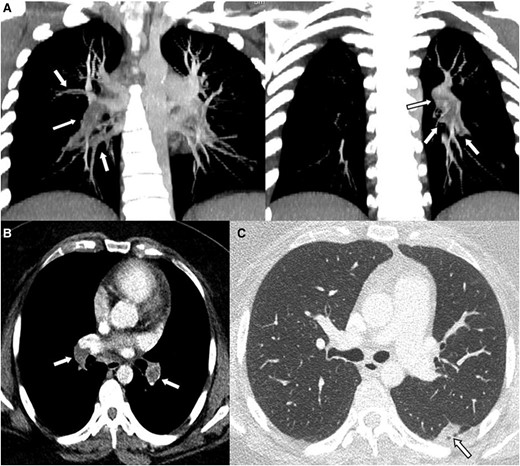
Computed tomography findings suggestive of thrombi involving both interlobar pulmonary arteries. Enhanced chest cardiac tomography revealed large thrombi causing complete obstruction in both interlobar pulmonary arteries. Additionally, smaller thrombi were observed in the segmental and subsegmental pulmonary arteries of both lower lobes and the left upper lobe (A and B). (C) also displayed evidence of a small pulmonary infarction in the left lower lobe. The arrows in panels A and B indicate thrombus, while the arrow in panel C points to the area of pulmonary infarction.

Gross findings of haemoptysis at (A) initial and (B) after surgery. After surgery, the colour of the haemorrhage gradually lightened, and eventually, only old blood clots were discharged, even after the resumption of anticoagulation therapy.
Following the 2019 European Society of Cardiology (ESC) guidelines, the severity of pulmonary embolism was assessed using the simplified Pulmonary Embolism Severity Index, which scored 0 points. However, the NT-proBNP level was elevated at 874 pg/mL (≥600), indicating an intermediate–low risk.2 After carefully considering risks of bleeding and haemostasis, we initiated a reduced dose of low molecular weight heparin (enoxaparin 100 mg = 10 000 IUs, equivalent to 0.7 mg/kg or 70 IU/kg). Nevertheless, a significant amount (400 cc) of fresh haemoptysis persisted continuously for 8 h (Figure 3A), and haemoglobin levels dropped by 2 mg/dL overnight from 15.7 to 13.6 mg/dL. After multidisciplinary consultation, we decided to perform an emergency pulmonary artery thrombectomy with RA thrombus removal. This decision was based on the likelihood that obstructive thrombosis in the interlobar pulmonary arteries might abruptly elevate the pulmonary artery pressure and lead to the rupture of small vessels, thereby being the underlying cause of the ongoing large amount of haemoptysis. Although we expected to find fresh thrombus, the surgical finding revealed chronic organized thrombi in the pulmonary arteries and at the IVC–RA junction, making the exact operation akin to pulmonary endarterectomy (Figure 4A). The final histology also confirmed the presence of chronic organized thrombi (Figure 4B).
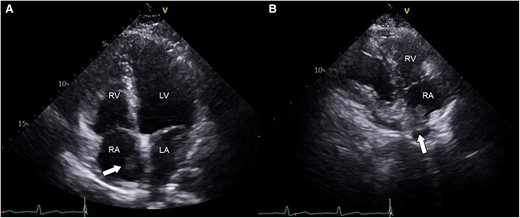
Echocardiographic findings. (A) Apical four-chamber view. (B) Right ventricular inflow view. Note the presence of an oscillating echogenic mass (presented as arrows) at the junction of the inferior vena cava and right atrium (A and B). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
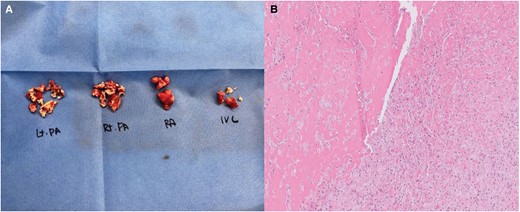
Surgical specimen (A) and histologic findings (B). (A) exhibits the surgical specimen, revealing organized white thrombi present in the pulmonary artery, right atrium, and inferior vena cava. In (B), the histological analysis of this specimen revealed that the specimens primarily consisted of thrombi composed of accumulated fibrin, some displaying characteristic lines of Zahn. These lines, indicative of thrombus formation in rapid arterial blood flow, exhibit laminations resulting from successive platelet and fibrin deposition (pale layers) alternating with red blood cells (dark layers). Sections from the left pulmonary artery and inferior vena cava thrombi displayed signs of fibroblast infiltration and focal recanalization, signifying organization. Initially, the right atrium and right pulmonary artery thrombi appeared fibrin rich. However, upon complete slide preparation of the remaining specimens, localized signs of organization were also observed. IVC, inferior vena cava; PA, pulmonary artery; RA, right atrium.
There were no identified triggering factors for PTE such as malignancy, oral contraceptive use, or immobility. However, the presence of a consecutively high titre of anti-β2-glycoprotein I antibody and anti-cardiolipin antibody, at both baseline and 3 months later, confirmed the diagnosis of antiphospholipid syndrome, necessitating lifelong anticoagulation therapy. The detailed results of autoimmune tests are provided in Supplementary material online, Table S1. There was no evidence of other combined autoimmune diseases, such as systemic lupus erythematosus. Low molecular weight heparin (enoxaparin 1 mg/kg twice a day) was resumed on the second day post-surgery and subsequently switched to warfarin. Following this change, there were no events of fresh bleeding; only old blood clots were detected (Figure 3B). Post-operative CT revealed almost complete removal of the main pulmonary artery thrombi, with only small residual thrombi in the lobar, segmental, and subsegmental pulmonary arteries. She was safely discharged and followed up in the outpatient clinic.
Discussion
Pulmonary thromboembolism is a severe condition that can result in various complications, such as hypoxia necessitating ventilator therapy, hypotension, and even death. Haemoptysis is an infrequent complication, occurring in ∼7% of cases.1,3,4 In the literature, there is limited data regarding the management of PTE with haemoptysis.5–9
In this case, we weighed three treatment options. The first was to use a reduced dose of heparin while awaiting clot resolution. The second was to perform bronchial artery embolization and start heparin once active bleeding ceased. The third option was surgical thrombectomy with a delayed start of anticoagulation therapy. Initially, we leaned towards the first option, opting for a reduced dose of low molecular weight heparin as we awaited resolution of the thrombi. We chose this approach due to the critical location of the thrombi (in both interlobar pulmonary arteries), which showed complete obstruction on imaging, coupled with the patient’s stable vital signs and normal haemoglobin levels, indicating intermediate risk. However, the patient unexpectedly experienced ongoing significant fresh haemoptysis, leading us to reassess our options. We deliberated between two other options: bronchial artery embolization and surgical thrombectomy. According to the literature, bronchial artery embolization was the predominant approach.7,8 However, we determined that relying solely on bronchial artery embolization might not provide a fundamental treatment approach. This consideration arose from the potential for the thrombi obstructing interlobar pulmonary arteries to elevate vascular pressure in the lungs, thereby exacerbating haemoptysis. Surgical thrombectomy was deemed the definitive treatment directly addressing the root cause of haemoptysis, although we acknowledged the possibility of scarring, especially in a young woman’s chest. While the literature suggests performing an IVC filter insertion, followed by a waiting period before initiating anticoagulant therapy,9 as recommended in the ESC guidelines, in our case, there was no evidence of deep vein thrombosis, thus obviating the need for IVC filter insertion. Additionally, transcatheter aspiration thrombectomy may serve as an alternative option in some countries, but unfortunately, it was not available in our institution.10,11
Regarding the cause of haemoptysis, we hypothesized that the obstructing material in the interlobar artery abruptly elevated the pulmonary artery pressure, potentially leading to a rupture somewhere in the pulmonary artery. This scenario warrants consideration of either surgical thrombectomy or bronchial embolization, as previously discussed.7,8 Although the exact explanation for the unexpected surgical or histologic finding of chronic thrombus remains unclear, our presumed cause is a superimposed acute thrombus in the presence of subchronic thrombus. The exact timing of thrombus development is uncertain, but it likely represents a subchronic stage thrombus showing features of chronicity (such as fibroblast infiltration and focal recanalization indicating organization) without being too old (lacking calcification or other evidence of chronic thrombus on CT scan, such as webs or bands, retraction, atrophy, post-stenotic dilatation of pulmonary arteries, or enlarged bronchial artery as collateral vessel formation).12
In summary, when managing a young woman with PTE presenting with ongoing active haemoptysis, we encountered challenges in selecting the appropriate treatment strategy. The best treatment option between the conflicting strategies of anticoagulation and haemostasis can vary based on individual clinical scenarios. As in our case, surgical thrombectomy with delayed anticoagulation therapy might be considered a potentially effective approach in cases where bleeding risk outweighs thrombosis risk.
Lead author biography

I am a fellow in the Department of Cardiology at Seoul St. Mary’s Hospital in South Korea. After completing 6 years of medical school, 1 year of internship, and 3 years of internal medicine residency, I am currently serving as a fellow in cardiology, undergoing specialized training in various subspecialties.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors affirm obtaining written consent from the patient for submitting and publishing this case report in accordance with COPE guidance.
Funding: None declared.
Data availability
Upon a reasonable request, the corresponding author will share the data underlying this article.
References
Author notes
Conflict of interest: None declared.





Comments