-
PDF
- Split View
-
Views
-
Cite
Cite
Gakuto Bando, Taiji Okada, Hideki Tsubota, Yutaka Furukawa, Prosthetic valve infective endocarditis with severe mitral stenosis caused by Cutibacterium acnes: a case report, European Heart Journal - Case Reports, Volume 8, Issue 5, May 2024, ytae205, https://doi.org/10.1093/ehjcr/ytae205
Close - Share Icon Share
Abstract
Infective endocarditis rarely results in mitral stenosis. This report presents a case of prosthetic valve infective endocarditis caused by Cutibacterium acnes infection, which resulted in mitral stenosis and was difficult to diagnose.
A 78-year-old Japanese man underwent aortic and mitral bioprosthetic valve replacement six years prior to the initiation of hormone therapy for prostate cancer. Three weeks after hormone therapy initiation, the patient developed exertional dyspnoea that progressively worsened and ultimately led to orthopnoea. Chest radiography revealed pulmonary congestion, and transthoracic echocardiography revealed mitral stenosis that was not present three months previously. The patient progressed to heart failure, and bicuspid valve replacement was performed. The excised aortic and mitral bioprosthetic valves were covered with vegetations, and pathological examination confirmed the presence of C. acnes. Therefore, the cause of mitral stenosis was infective endocarditis.
In patient with rapidly progressive prosthetic valve stenosis after valve replacement, infective endocarditis due to C. acnes should be suspected even if blood cultures are negative.
Mitral stenosis is rare in patients with infective endocarditis, especially in those with prosthetic valve infective endocarditis.
Infective endocarditis caused by Cutibacterium acnes frequently yields negative blood culture results. When prosthetic valve stenosis is rapidly occurring, infective endocarditis due to C. acnes should be suspected.
Introduction
Prosthetic valve endocarditis (PVE) accounts for ∼20% of all endocarditis cases and occurs in up to 6% of patients with prosthetic valve implants. Prosthetic valve endocarditis is classified into early and late PVE according to the time of onset. Late PVE is attributed to community-acquired infections and shares pathogenic similarities with native valve endocarditis (NVE). The most common pathogens are Streptococcus and Staphylococcus aureus.1 This study reports blood culture-negative infective endocarditis (IE) caused by Cutibacterium acnes, an attenuated bacterium commonly found on the skin.2 Infective endocarditis typically causes valve regurgitation, annular abscesses, and destruction. This case report presents a patient with severe mitral stenosis resulting from PVE caused by C. acnes.
Summary figure
Case presentation
A 78-year-old Japanese man presented to the emergency department with a 2-day history of exertional dyspnoea and orthopnoea. He underwent aortic and mitral bioprosthetic valve replacement [Perimount Magna-Ease™ 21 mm (Edwards Lifesciences, Irvine, CA, USA) and Epic™ 29 mm (Abbott, Abbott Park, IL, USA), respectively] for IE caused by methicillin-sensitive Staphylococcus aureus six years prior to his presentation. Three months previously, transthoracic echocardiography revealed no prosthetic valve dysfunction. A prostate biopsy was performed with antibiotic prophylaxis (single dose of piperacillin–tazobactam 4.5 g) two months prior to his presentation, and hormone therapy for prostate cancer (degarelix plus estramustine) was initiated three weeks prior to his presentation. At the time of the visit, the patient’s blood pressure was 151/100 mmHg, pulse rate was 110 beats/min, respiratory rate was 29 breaths/min, temperature was 36.4°C, SpO2 was 78% on room air, and 94% with non-invasive ventilation (NIV; 8 cm H2O, fraction of inspired oxygen 0.5). On physical examination, a diastolic murmur (Levine grade II/Ⅵ) was auscultated at the apex of the heart, and wet lung sounds were heard. There were no embolic or petechial haemorrhagic findings suggestive of IE. A chest radiograph revealed pulmonary congestion. Electrocardiography revealed sinus tachycardia (Figure 1). Blood tests showed elevated NT-proBNP levels (3460 pg/mL), a white blood cell count of 20 800/µL, and a C-reactive protein level of 5.33 mg/dL. Transthoracic echocardiography revealed thickening of the mitral valve leaflets with a transmitral blood flow velocity of 2.9 m/s and a mean pressure gradient of 20 mmHg, indicating severe mitral stenosis (Figure 2A and B). Furthermore, there was mild to moderate tricuspid regurgitation with elevated pulmonary artery systolic pressure (Figure 2C and D). Non-invasive ventilation and intravenous furosemide were administered to treat the decompensated heart failure. The aetiology of the heart failure was identified as rapidly-progressing mitral stenosis, either due to thrombosed valves secondary to hormone therapy or PVE. The patient was administered heparin and empiric antimicrobial therapy (ceftriaxone, 2 g once a day; vancomycin, 1 g once a day; and cefazolin, 2 g three times a day) after three sets of blood cultures in both aerobic and anaerobic atmospheres were obtained from three different peripheral veins.
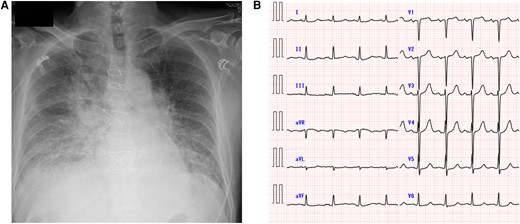
Chest X-ray and electrocardiogram. The patient’s chest radiograph revealed marked pulmonary congestion (A). Electrocardiography revealed a heart rate of 102 beats/min and ST depression in the lateral thoracic leads (B).
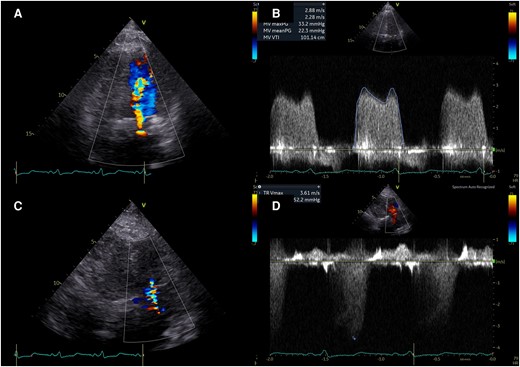
Transthoracic echocardiography in emergency room. Transthoracic echocardiography revealed massive turbulent colour Doppler signal (A), increased transmitral flow (B), mild to moderate tricuspid regurgitation (C), and increased estimated pulmonary artery systolic pressure (D).
Initially, the patient responded well to the diuretic treatment; however, his urine output decreased. The patient did not compensate properly and his tolerance to NIV decreased. Mechanical ventilation was initiated, and emergency transoesophageal echocardiography (TEE) was performed. Transoesophageal echocardiography revealed thickening of the prosthetic mitral valve, slightly increased in echogenicity, and reduced mobility with a mitral valve mean pressure gradient of 18.4 mmHg (Figure 3A–C, Supplementary material online, Videos S1–S3). No evidence of a mitral annular abscess was observed. The prosthetic aortic valve exhibited reduced mobility of the right coronary/non-coronary cusp (Figure 3D, Supplementary material online, Video S4). No aortic regurgitation was observed. The severity of tricuspid regurgitation was moderate (Figure 3E, Supplementary material online, Video S5). Despite negative blood cultures, the TEE findings strongly indicated IE, leading to a decision to proceed with urgent surgery. On the third day of hospitalization, the patient underwent revision of the aortic and mitral valve prostheses [Avalus™ 21 mm (Medtronic, Minneapolis, MN, USA) and Epic™ 27 mm (Abbott, Abbott Park, IL, USA), respectively], along with tricuspid valve annuloplasty using a Tailorband™ 29 mm (St. Jude Medical, St Paul, MN, USA). The excised mitral valve prosthesis had vegetations on all three leaflets, and the right coronary/non-coronary cusp of the excised aortic valve was also covered in vegetations (Figure 4). Cutibacterium acnes was identified in the surgical specimen, leading to a definitive diagnosis of PVE.

Pre-operative transoesophageal echocardiography. Transoesophageal echocardiography revealed a thickened prosthetic mitral valve, slightly increased echoluminance (A), massive turbulent colour Doppler signal (B), and reduced mobility of all valve leaflets (C). The prosthetic aortic valve exhibited reduced mobility of the right coronary/non-coronary cusp (D). The severity of tricuspid regurgitation was moderate (E).
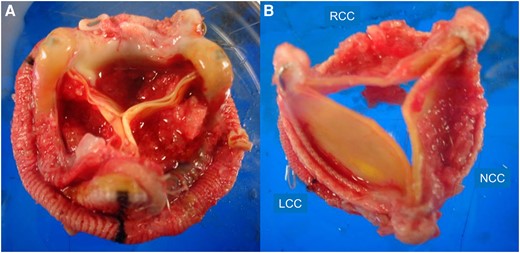
Surgical specimen. The excised mitral valve prosthesis has vegetations on all three leaflets (A). The excised aortic valve is covered with vegetations (B).
The patient was administered ceftriaxone (2 g twice daily) for two weeks and ampicillin (2 g six times daily) for four weeks post-operatively. The patient’s post-operative recovery was uneventful. Transthoracic echocardiography revealed good haemodynamics (Figure 5A–E), and there was no recurrence of infection following the discontinuation of antibiotic therapy. The patient subsequently transferred for rehabilitation.
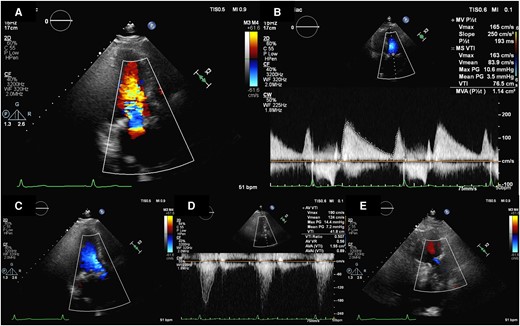
Post-operative transthoracic echocardiography. Transthoracic echocardiography revealed good prosthetic mitral valve function (A and B), prosthetic aortic valve function (C and D), and trivial TR (E).
Discussion
Prosthetic valve dysfunction due to IE is associated with regurgitation. Although stenosis due to vegetation has been reported,3,4 few studies have focused on stenosis associated with valve leaflet thickening.5 This report presents a patient with mitral valve prosthesis stenosis caused by C. acnes.
Cutibacterium acnes is a gram-positive anaerobic rod that grows mainly in human pores. It also acts as an opportunistic pathogen, leading to various post-operative and device-related infections.6 Cutibacterium acnes has been reported as the causative agent of late-stage PVE,7 is more frequently the causative agent of PVE than NVE, and occurs more often in males than in females.7,8 Due to its low virulence and slow growth, PVE is thought to develop several years post-operatively.8 On the other hand, there is a case of prosthetic valve that developed IE early after pacemaker implantation.9 The patient’s subacute PVE might have been caused by subcutaneous injections and thrombogenicity in hormone therapy for prostate cancer, however, this could not be proved. The blood culture period was 7 days; however, the culture results were negative, making the suspicion of IE low. Although C. acnes was identified in the surgical specimen, numerous reports have described negative blood cultures for this microorganism. Cases in which patients remained afebrile and exhibited normal C-reactive protein levels have also been reported.8 Extending the incubation period of delayed blood culture is necessary to detect microorganisms. Among male patients with prosthetic heart valves exhibiting signs of peripheral embolization or heart failure, the presence of C. acnes should raise suspicion, even in negative blood cultures, as it may be associated with IE. Cutibacterium acnes forms biofilms, and PVE tends to recur after medical treatment. Surgical treatment is generally recommended.8,10 Nevertheless, some patients have responded to antibiotic therapy without recurrence. Further studies are needed to assess the effectiveness of medical therapy.11
The patient in this report presented with IE and mitral stenosis. Mitral stenosis in IE is rare; few cases of obstruction due to extensive vegetation, and only one case of diffuse thickening of the prosthetic valve leaflet (similar to the present case) have been reported previously.5 Mitral stenosis, which progresses rapidly, is typically reminiscent of a thrombosed valve. The patient in this report also had a predisposition to thrombosis as he was receiving hormonal therapy for prostate cancer. Therefore, it is important to differentiate between thrombus and pannus formation during TEE. The distinction between a thrombus and pannus is based on morphology, echo density, and localization. Thrombi were larger, mostly extension into the left atrium, lower echo intensity than pannuses.12 In this patient, the MV prosthesis had a flattened and thickened morphology, suggestive of a thrombus. Furthermore, the echoluminance was slightly elevated, indicating the presence of vegetation. Therefore, we deduced that the patient’s mitral stenosis was caused by a prosthetic valve infection. The diagnosis of IE is based on the modified Duke criteria.13 The criteria includes findings suggestive of endocarditis such as agitated vegetation, abscesses, prosthetic valve rupture, and new valve regurgitation, but not valve stenosis. Even the 2023 European Society of Cardiology modified diagnostic criteria of infective endocarditis14 do not mention valve stenosis as part of the diagnostic criteria. Although valve stenosis is a relatively rare finding, IE should be considered the differential diagnosis of mitral stenosis.
In conclusion, this case highlights the necessity of suspecting IE due to C. acnes in patients with rapidly progressive prosthetic valve stenosis after valve replacement, even when blood cultures are negative.
Lead author biography

Gakuto Bando worked as a resident in the Department of Cardiology at Kobe City Medical Center General Hospital and Kurashiki Central Hospital. He’s studying to become a top-notch cardiologist.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
We would like to thank Editage for English language editing.
Consent: The authors confirm that witnessed verbal consent for submission and publication of this case report including images and associated text has been obtained from the patient. This has been discussed with the editors.
Funding: None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: None declared.





Comments